SUMMARY
Background
The Ethiopian mountain adder (Bitis parviocula) is a viperid known only from a few locations in southwestern Ethiopia.
Methods
a total of 30 μg of B. arietans and B. parviocula venoms were run on a 10–20% Tricine gel. To assay lethality dose fifty (LD50), five groups of eight mice for each venom were used. Hemorrhagic activity for crude venom was tested. Fibrinogenolytic activity of crude venom was measured using (2.5 mg/mL) of fibrinogen solution and (0.03 mg/mL) of crude venom. Gelatinase activity of the venom was tested on a Kodak X-OMAT™ film. Crude venoms of B. parviocula and B. arietans were tested for their abilities to affect clotting time, clotting rate and platelet function on whole human blood.
Results
The (SAIMR) antivenom was confirmed in this study to neutralize the lethal activity of venom from Bitis parviocula. The ED50s of SAIMR antivenom on B. parviocula and B. arietans neutralized half of 18.2 and 66.7 mg of venom, respectively. The hemorrhagic activities (MHDs) of B. parviocula and B. arietans were 0.88 and 1.7 μg, respectively. Bitis arietans and B. parviocula venoms degradated α and β chains at different times. The γ chains remained unaffected. Bitis parviocula venom did not exhibit gelatinase activity, while B. arietans had a MGD of 6.9 μg. At 3 mg/mL, the crude venoms of B. parviocula and B. arietans did not significantly affect clotting time or clotting rate.
Conclusions
The SAIMR antivenom is very effective in neutralizing the venom of B. parviocula and should be considered in treating envenomations by these snakes.
Keywords: Bitis parviocula, Bitis arietans, South African Vaccine Producers (SAVP), South African Institute of Medical Research (SAIMR) antivenom, Venom
INTRODUCTION
The Ethiopian mountain adder (Bitis parviocula) is a medium sized viperid known only from a few locations in southwestern Ethiopia1. Until recently only two road-killed specimens and a single live animal had been acknowledged and the species remained in relative scientific obscurity. In 2007, through an agreement with an exporter, twenty of these animals were brought to the United States from Africa10. One of the females imported produced young shortly after and this species began to make its way into the hobbyist trade as a high-dollar species.
Given the rarity of this snake, little is known about the composition or toxicity of the venom8,14. Indigenous people of southern Ethiopia consider this snake highly dangerous14. Epidemiology is virtually nonexistent although given the human population in southern Ethiopia; it is thought to inflict a reasonable number of bites each year14. The venom has traditionally been presumed to be cytotoxic similar to congenerics. Some cross-reactivity with polyvalent antivenoms produced against Bitis was suspected4. In 2009, an amateur herpetologist in San Antonio, Texas was envenomated by a young B. parviocula while attempting to ready the animal for shipment. Attending physicians began to administer the South African polyvalent antivenom SAIMR (South African Institute of Medical Research). However, after ¾ of the initial vial was given the patient showed signs of anaphylaxis and the antivenom was stopped at that point. Given the negligible dose, it remained unclear if the SAIMR would be an effective treatment for this species5,8. In this study, SAIMR was tested for its neutralizing ability on the lethal toxicity of B. pariviocula venom. The lethal and proteolytic activities of this venom were also compared to those of the closely related African Puff adder (Bitis arietans)6.
MATERIALS AND METHODS
Snake collection and husbandry
The B. parviocula were collected in SE Ethiopia, Africa (8° 27′ S 1.54′ N 36° 21′ 4.99′E; Fig. 1) and shipped to a private individual in the United States. These animals were housed in the private collection of Al Coritz. The snakes were held in Vision® snake enclosures and kept at 18–25° C. White laboratory mice (Mus musculus) were offered as food once every seven days. Fresh water was available ad libitum. B. arietans used in this study were collected between Arusha and Dar es Salaam, Tanzania, Africa. (5° 02′ 54.91S 37° 3.74684E; Fig. 1). Given that the venom profiles vary in B. arietans across their range10 the B. arietans selected for this study originated from a single location. The snakes were held as part of the private collection maintained by Douglas L. Hotle. The snakes were held in Neodesha® enclosures with an ambient temperature of 27 °C. Rodents (Mus musculus and Rattus norvegicus) were offered as food once every seven days. Water was available ad libitum. Both species were wild caught animals and were considered adults.
Fig. 1.
Top Map Collection location of B. parviocula (8° 27′ S 1.54′ N 36° 21′ 4.99′ E; elevation 1516 meters). Bottom Map: Collection location of B. arietans collected (5° 02′ 54. 91′ S 37° 3.74684 E; elevation 1194 meters). Dots indicate approximate locality of collected specimens.
Venom collection
Venom was extracted by allowing the snake to bite into a sterile disposable beaker covered with para-film. The venom sample was centrifuged 500 g for 10 min at 4 °C, filtered through a 0.45 μm filter, and frozen at −80 °C until lyophilized.
SDS PAGE
A total of 30 μg of B. arietans and B. parviocula venoms were run on a 10–20% Tricine gel at 150 V for 90 min using a SureXCell system (Invitrogen). The gel was stained with SimplyBlue (Invitrogen) for one hour and distained overnight with 18 megaOhm water. SeeBlue Plus2 markers were used as controls.
Lethality Dose (LD50)
Five groups of eight mice for each venom were housed in cages and observed throughout the quarantine period and experiments. The endpoint of lethality of the mice was determined after 48 hr. The venom was dissolved in 0.85% saline at the highest test dose per mouse. Serial dilutions of 2-fold using saline were made to obtain four additional concentrations. All solutions during the experiment were stored at 0 °C and warmed to 37 °C just before being injected into mice. The lethal toxicity was determined by injecting 0.2 mL of venom (containing dosages ranging between 220 to 13.75 μg/mouse) into the tail veins of 18–20 g female BALB/c mice. The injections were administered using a 1-mL syringe fitted with a 30-gauge, 0.5-inch needle. Saline controls were used. The LD50 was calculated by the Spearman-Karber method15 (n = 3 ± SD).
Antivenom efficacy dose (ED50)
Five groups of eight mice were challenged with a mixture of antivenom containing 3 LD50 of venom. SAIMR antivenom (Lot #: TO1946; South African Vaccine Producers) was diluted with sterile 0.85% saline. A stock venom solution was freshly prepared at 0 °C prior to use. For each group of mice, a set concentration of venom was mixed with five different antivenom concentrations and incubated at 37 °C for 30 min. Each mouse was injected into the tail vein with 0.2 mL of venom/antivenom mixture in which 3 X LD50s were administered per mice. The mice were observed for 48 h and the percent survival and ED50 was calculated by the Spearman-Karber method15 (n = 3 ± SD).
Hemorrhagic assay
Hemorrhagic activity for crude venom and the collected fractions followed the procedure of OMORI-SATOH et al. (1972)11. To test for activity, 0.1 mL of each of the fractions was injected subcutaneously into the backs of depilated New Zealand rabbits (Oryctolagus cuniculus). After 24 h, the rabbits were sacrificed and hemorrhagic spots measured (mm). The minimal hemorrhagic dose (MHD) was defined as the amount of protein (μg) that causes a 10 mm hemorrhagic spot.
Fibrinogenolytic assay
Fibrinogenolytic activity of crude venom was measured using a procedure modified from SALAZAR et al. (2007)12. Two hundred microliters of fibrinogen solution (2.5 mg/mL) and 100 μL of crude venoms (0.03 mg/mL) were incubated together at 37 °C for 30 min, 1, 2, 4 and 24 h. The samples were run on a 4–12% Bis-Tris gels under reducing conditions using a SureXCell system (Invitrogen). The gels were stained with SimplyBlue (Invitrogen) for one hour and distained with 18 megaOhm water overnight.
Gelatinase assay
Gelatinase activity of the venom fractions was tested using a method modified from HUANG & PÉREZ (1980)7. Fifty microliters of each venom dilution and fractions were placed on a Kodak X-OMAT™ scientific imaging film having a gelatin coating. Hydrolysis of gelatin on the X-ray film was determined by washing the film with tap water following incubation at 37 °C for four hours in a moist incubator. A resulting clear spot on the X-ray film indicated positive activity. The assay was repeated three times.
Sonoclot Assay
Crude venoms of B. parviocula and B. arietans were tested for their abilities to affect activated clotting time (ACT), clotting rate (CR) and platelet function (PF) on whole human blood according to the method of SÁNCHEZ et al. (2010)13.
RESULTS
SDS PAGE
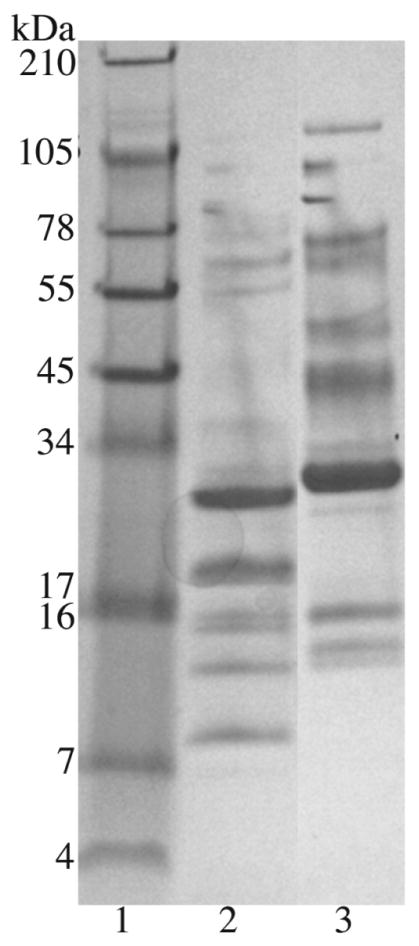
Pooled venoms of both B. parviocula and B. arietans were compared by SDS gel electrophoresis and 10 visible proteins bands were apparent between ~104 and 8 kDa for B. arietans venom while B. parviocula had 13 visible bands in the range ~128 and 11 kDa (Fig. 2).
Fig. 2.
A SDS gel electrophoresis of B. arietans and B. parviocula venoms. A total of 30 μg of protein of venoms was run on a 10–20% Tricine gel under non-reducing conditions at 150 V for 90 min using a SureXCell system. Lanes: 1) SeeBlue Plus2 markers; 2) B. arietans 3) B. parviocula.
Lethality Dose (LD50)
The LD50s of B. parviocula and B. arietans venoms were 1.6 and 1.4 mg/kg body weight, respectively (Table 1).
Table 1.
Proteolytic and lethal activities of Bitis arietans and B. parviocula venoms
| Venoms | MHD (μg) | MGD (μg) | Fibrinogenolytic 30 min 4 h 24 h α, β, γ |
LD50 (mg/kg) | ED50 (mL/mg)† | Sonoclot‡ ACT, CR, PF |
|---|---|---|---|---|---|---|
| Bitis arietans | 1.7±0.5 | 6.9±1 |
+, −, − +, −, − +, +, − |
1.35 | 1/67 | 230±15, 21±10, 0±0.1 |
| Bitis parviocula | 0.88±0.6 | NA |
−, −, − +, +, − +, +, − |
1.56 | 1/18 | 280±10, 13±5, 0±0.1 |
MHD: The minimal hemorrhagic dose is the minimal amount of venom that will cause a 10 mm hemorrhagic spot according to the method of OMORI-SATOH et al. (1972)11. MGD: The minimal gelatinase dose is the minimal amount of venom that will cause a clearing area on an X-ray film according to the method of HUANG & PEREZ (1980)7. NA: No activity. LD50: The lethal dose 50 is the amount of venom that will kill 50% of a population according to the method of SÁNCHEZ et al. (2010)13. ED50: The effective dose 50 is the titer of anitvenom to venom that will protect 50% of the population according to the method of SÁNCHEZ et al. (2010)13. Antivenom-SAIMR Polyvalent Snake Antivenom. South African Vaccine Producers (PTY) LTD. 1 Modderfontien Rd. Edenvale, Gauteng Lot # TO1946.
: mL/mg: 1 mL of antivenom incubated with venom (mg) protects 50% of the population. +: degradation, −: no degradation. Human fibrinogen was used according to the method of SALAZAR et al. (2007)12.
ACT: activated clot time (s); CR: clot rate (clot signals/min); PF: platelet function. Normal blood control ACT: 189±15, CR: 30±5, and PF: 2.0±1.5. A Sonoclot® Coagulation & Platelet Function Analyzer was used (SIENCO®, Inc., Arvada, CO). The method of SÁNCHEZ et al., (2010)13 was followed.
Antivenom efficacy dose (ED50)
The ED50s of SAIMR antivenom on B. parviocula and B. arietans were 1/18.2 and 1/66.7, in which 1 mL of SAIMR antivenom incubated with 18.2 and 66.7 mg of venom, respectively can protect 50% of the population (Table 1).
Hemorrhagic assay
The hemorrhagic activities (MHDs) of B. parviocula and B. arietans were 0.88 and 1.7 μg, respectively. Western diamondback rattlesnake (Crotalus atrox) venom was used as a control giving a MHD of 2.5 μg (Table 1).
Fibrinogenolytic assay
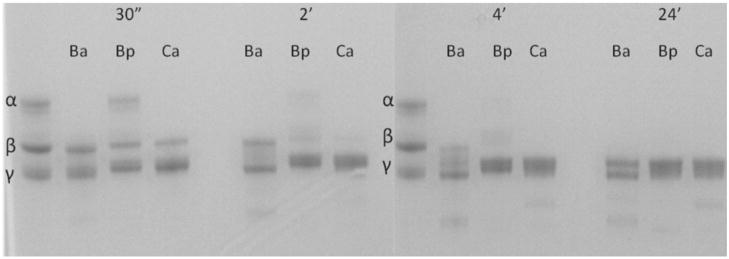
Bitis arietans was able to completely degrade the α chain of human fibrinogen by 30 min while B. parviocula took four hours to completely degrade the α chain (Table 1). However, B. parviocula venom was able to degrade the β chain much faster (four hours) than B. arietans (24 h). The venom of Crotalus atrox was used as a control and had activity similar to B. parviocula venom in the fibrinogenolytic assay. Neither venom affected the γ chain (Fig. 3).
Fig. 3.
Fibrinogenolytic activity of B. arietans (Ba), B. parviocula (Bp) and Crotalus atrox (Ca) venoms. Two hundred microliters of fibrinogen (2.5 mg/mL) was incubated with 100 μL of venom sample (0.03 mg/mL) and incubated at 30 min, 2, 4 and 24 h. The samples were run on a 4–12% Bis-Tris gel under reducing conditions using a SureXCell system (Invitrogen). The gel was stained with SimplyBlue for 1 h and distained in 18 mega ohm water overnight.
Gelatinase assay
Bitis parviocula venom did not exhibit gelatinase activity, while B. arietans had a MGD of 6.9 μg (Table 1).
Sonoclot assay
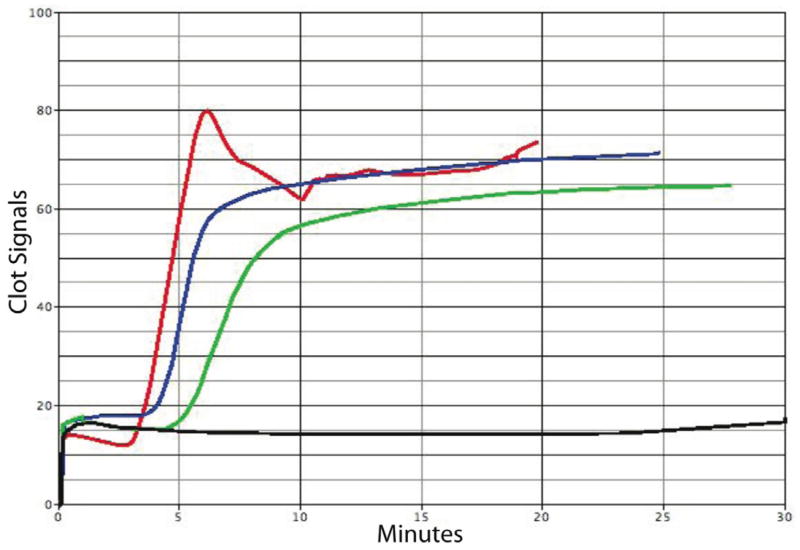
The ACTs and CRs were slightly delayed for the venoms of B. arietans and B. parviocula as compared to the normal blood control (Table 1, Fig. 4), while the PFs for both Bitis ssp. venoms were significantly affected, meaning that these venoms had a strong effect on blood platelets.
Fig. 4.
The effects of activated clot time (ACT), clot rate (CR) and platelet function (PF) by Bitis venoms. A total of 10 μL of venom sample (3 mg/mL) was added to glass bead activated cuvettes (gbACT+ KIT) containing 350 μL of 10% citrated whole human blood. The ACT, CR and PF were analyzed on a Sienco Sonoclot® Analyzer System. Red: normal blood control, blue: B. arietans venom + blood, green: B. parviocula venom + blood, and black: C. atrox venom + blood.
DISCUSSION
Bitis parviocula has a close taxonomic relationship with other Macrocerastes such as the Puff adder (Bitis arietans), Gaboon vipers (Bitis gabonica and B. rhinoceros), Rhinoceros viper (Bitis nasicornis) among others1; and therefore, the presumption was that antivenoms traditionally used for other Bitis would have cross-reactivity against envenomation from B. parviocula8,14. However, cross-reactivity does not always equal cross-protection3. In many cases usage of antivenoms against untested species can be dangerous, or at the least non-beneficial8. The addition of the B. parviocula into many private and zoological collections increases the likelihood of accidental envenomations by this species. Emergency physicians and snakebite consultants are increasingly faced with treating venomous snakebites inflicted by non-native snakes10. An understanding of the cross-protection of specific antivenoms is crucial in the treatment of these emergencies.
Prior to antivenom neutralization, the venoms of B. parviocula and B. arietans were compared by SDS electrophoresis in which there were differences in the quantity of proteins in that B. parviocula had three more proteins bands than B. arietans (Fig. 2). The venoms were also compared with a series of biological assays.
These venoms were analyzed using a Sonoclot® Coagulation & Platelet Function Analyzer, in which the measurements are based on the detection of viscoelastic changes of whole blood or plasma6. The Sonoclot® provides qualitative (Sonoclot Signature graph) and quantitative (ACT, CR and PF) results on the entire hemostasis process. The activated clotting time (ACT) is the time in which fibrin formation begins, the clotting rate (CR) is the kinetic measurement of fibrin formation and clot development, which is the maximum slope of the Sonoclot Signature during initial fibrin polymerization and clot development, and platelet function (PF) is obtained from the timing and quality of the clot retraction. The values for PF range from 0–5, where 0 represents no clot retraction. A PF higher than 1 represents normal clot retraction and varies from patient to patient. A normal PF contains a sharp peak in the Sonoclot Signature after fibrin formation, as seen on the control sample in Figure 4. The ACTs and CRs were slightly delayed for the venoms of B. arietans and B. parviocula as compared to the normal blood control (Table 1, Fig. 4), while the PFs for both Bitis venoms were significantly affected, meaning that these venoms had a strong effect on blood platelets. Whether there are components in these venoms that bind to the receptors (e.g. αIIbβ3) of platelets thus inhibiting platelet aggregation, or if the venom components degrade the receptors or the platelets themselves is yet to be determined. Crotalus atrox venom, used as a control, rendered the blood sample unclottable (Fig. 4).
The ability to degrade human fibrinogen was also tested. Bitis arietans was able to completely degrade the α chain of human fibrinogen by 30 min while B. parviocula took 4 h to completely degrade the α chain (Table 1; Fig. 3). However, B. parviocula venom was able to degrade the β chain much faster (4 h) than B. arietans (24 h). The venom of Crotalus atrox was used as a control and had activity similar to B. parviocula venom in the fibrinogenolytic assay. Neither venom affected the γ chain.
Both Bitis venoms were very hemorrhagic with minimal hemorrhagic doses (MHDs) for B. arietans and B. parviocula venoms of 1.7 and 0.87 μg, respectively (Table 1), signifying B. parviocula to be twice as hemorraghic. The hemorrhagic activity of B. arietans is comparable to the hemorrhagic activity of C. atrox and C. oreganus helleri venoms (~2.5–2.3 μg), while that of B. parviocula venom hemorrhagic activity is comparable to the venoms of Crotalus viridis (~0.7 μg)13. In contrast, B. parviocula did not contain gelatinase activity when tested on an X-ray film, while B. arietans had a minimal gelatinase dose (MGD) of 6.9 μg (Table 1). These results could imply the absence or low abundance of collagenases in the venom of B. parviocula.
The LD50 for B. arietans and B. parviocula venoms were 1.35 and 1.56 mg/kg, respectively, and the SAIMR antivenom was capable of neutralizing both venoms quite well (Table 1). The ED50 for the SAIMR antivenom against B. arietans was 1/67, which translates to 66 mg of venom incubated with 1 mL of antivenom can protect 50% of the BALB/c mouse population. The ED50 with B. parviocula was 1/18, thus the SAIMR antivenom neutralizes ~ 4 times more venom of B. arietans, which is not surprising since this venom is used in the production of this antivenom (Table 1). Even though SAIMR only protected 50% of the population with less B. parviocula venom, it still protected significantly considering that this venom was not used for the manufacture of the antivenom. According to MALLOW et al. (2003)9, the average B. arietans venom yield ranges between 100–350 mg, with a maximum of 750 mg, while the average venom yield for B. parviocula is 100 mg (personal communication by Doug Hotle). Therefore, theoretically, two to three 10 mL vials of SAIMR antivenom can neutralize the maximum amount of venom from a single extraction of either snake. It is apparent that there exist components in B. parviocula venom that are not as easily neutralized as those of B. arietans. It is well documented that variation in venom composition exists among Bitis species2.
Nonetheless, in this study we have shown that the South African polyvalent antivenom SAIMR did produce paraspecific neutralization of lethality with B. parviocula venom in vivo, and should be considered in emergency treatment.
Acknowledgments
Financial support was provided by the NIH/NCRR viper resource grant (#5 P40 RR018300-07) and Texas A&M University-Kingsville at Texas A&M University-Kingsville, Kingsville, TX. Thanks to Al Coritz for donating the crude B. parviocula venom, Justin Bennet for his technical assistance, and the faculty and staff at the National Natural Toxins Research Center.
Footnotes
The authors affirm that this work has never been presented in any scientific reunion and these data has never been published elsewhere.
References
- 1.Böhme W. Eine neue Art der Gattung Bitis (Serpentes, Viperidae) aus Äthiopien. Monit Zool Ital. 1977;(Suppl 9):59–68. [Google Scholar]
- 2.Calvete JJ, Escolano J, Sanz L. Snake venomics of Bitis species reveals large intragenus venom toxin composition variation: application to taxonomy of congeneric taxa. J Proteome Res. 2007;6:2732–45. doi: 10.1021/pr0701714. [DOI] [PubMed] [Google Scholar]
- 3.Chippaux JP. Snake venoms and envenomations. Malabar: Krieger; 2006. [Google Scholar]
- 4.Currier RB, Harrison RA, Rowley PD, Laing GD, Wagstaff SC. Intra-specific variation in venom of the African Puff Adder (Bitis arietans): differential expression and activity of snake venom metalloproteinases (SVMPs) Toxicon. 2010;55:864–73. doi: 10.1016/j.toxicon.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Fernández MC, González A. Ethiopian Mountain viper envenomation in South Texas. North American Congress of Clinical Toxicology Annual Meeting; September 21–26, 2009; San Antonio, Texas. p. 712. [Google Scholar]; Clin Toxicol. 2009;47(7) doi: 10.1080/15563650903158888. [DOI] [PubMed] [Google Scholar]
- 6.Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106:1366–75. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 7.Huang SY, Perez JC. Comparative study on hemorrhagic and proteolytic activities of snake venoms. Toxicon. 1980;18:421–6. doi: 10.1016/0041-0101(80)90049-5. [DOI] [PubMed] [Google Scholar]
- 8.Keyler D. Venomous snake species commonly displayed in zoos for which specific antivenoms are not available. Association of Zoos and Aquarium’s Antivenom Index. Available from: http://www.aza.org.
- 9.Mallow D, Ludwig D, Nilson G. True vipers: natural history and toxinology of Old World vipers. Malabar: Krieger; 2003. [Google Scholar]
- 10.Minton SA. Bites by non-native venomous snakes in the United States. Wilderness Environ Med. 1996;7:297–303. doi: 10.1580/1080-6032(1996)007[0297:bbnnvs]2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Omori-Satoh T, Sadahiro S, Ohsaka A, Murata R. Purification and characterization of an antihemorrhagic factor in the serum of Trimeresurus flavoviridis, a crotalid. Biochim Biophys Acta. 1972;285:414–26. doi: 10.1016/0005-2795(72)90328-5. [DOI] [PubMed] [Google Scholar]
- 12.Salazar AM, Rodríguez-Acosta A, Girón ME, Aguilar I, Guerrero B. A comparative analysis of the clotting and fibrinolytic activities of the snake venom (Bothrops atrox) from different geographical areas in Venezuela. Thromb Res. 2007;120:95–104. doi: 10.1016/j.thromres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez EE, Lucena SE, Reyes S, Soto JG, Cantu E, Lopez-Johnston JC, et al. Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the mohave rattlesnake (Crotalus scutulatus scutulatus) Thromb Res. 2010;126:e211–9. doi: 10.1016/j.thromres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spawls S, Branch B. The dangerous snakes of Africa. Sanibel Island, FL: Ralph Curtis; 1998. [Google Scholar]
- 15.Spearman C, Karber G. Alternative methods of analysis for quantal responses. In: Finney DJ, editor. Statistical methods in biological assays. London: Charles Griffin; 1978. pp. 1–78. [Google Scholar]