Abstract
The coral snake Micrurus tener tener (Mtt) from the Elapidae family inhabits the southwestern United States and produces severe cases of envenomations. Although the majority of Mtt venom components are neurotoxins and phospholipase A2s, this study demonstrated, by SDS-PAGE and molecular exclusion chromatography (MEC), that these venoms also contain high-molecular-weight proteins between 50 and 150 kDa that target the hemostatic system. The biological aspects of other Micrurus venoms were also studied, such as the LD50s of Micrurus isozonus (from 0.52 to 0.61 mg/kg). A pool from these venoms presented a LD50 of 0.57 mg/kg, Micrurus f. fulvius (Mff) and Mtt had LD50s of 0.32 and 0.78 mg/kg, respectively. These venoms contained fibrino(geno)lytic activity, they inhibited platelet aggregation, as well as factor Xa and/or plasmin-like activities. M. isozonus venoms from different Venezuelan geographical regions inhibited ADP-induced platelet aggregation (from 50 to 68%). Micrurus tener tener venom from the United States was the most active with a 95.2% inhibitory effect. This venom showed thrombin-like activity on fibrinogen and human plasma. Fractions of Mtt showed fibrino(geno)lytic activity and inhibition on plasmin amidolytic activity. Several fractions degraded the fibrinogen Aα chains, and fractions F2 and F7 completely degraded both fibrinogen Aα and Bβ chains. To our knowledge, this is the first report on thrombin-like and fibrino(geno)lytic activity and plasmin or factor Xa inhibitors described in Micrurus venoms. Further purification and characterization of these Micrurus venom components could be of therapeutic use in the treatment of hemostatic disorders.
Keywords: Coral snakes, Fibrino(geno)lytic activity, Hemostasis, Micrurus tener tener, Plasmin inhibitors, Platelets, Venom
1. Introduction
Venoms of the Elapidae family could produce hemorrhagic effects, which have not been thoroughly studied since these bites rapidly produce severe symptoms of neurotoxicity when humans are envenomated. Snake venoms are a diverse mixture of enzymatic and non-enzymatic toxins with an ample range of molecular masses between 15 and 380 kDa (Kini and Evans, 1992). Proteases are commonly found in venom at high concentrations and can be classified into two groups: serine proteases and metalloproteases. Several serine and metalloproteases have been well characterized, especially from viperid and crotalid venoms (Maruyama et al., 1992; Zaganelli et al., 1996; Aguilar et al., 2001; Salazar et al., 2007). A number of metalloproteases from the Elapidae venom are fibrin(ogen)ases (Jagadeesha et al., 2002) or factor V activators (Gerads et al., 1992). Proteases are not the only type of venom molecules that affect hemostatic components; for instance, the three-finger toxins from the Ringhals cobra venom inhibits factor VIIa (Banerjee et al., 2005). Furthermore, various venom phospholipase A2 (PLA2s) have also been described as anticoagulants (Kini, 2005).
Coral snakes represent a taxonomic assembly of more than 120 species and subspecies found throughout the Southern United States, Central and South America with their maximum diversity close to the equator (Roze, 1996). The active components of only a few species have been investigated (Gutiérrez et al., 1991; de Roodt et al., 2004; Suntravat et al., 2010). The Texas coral snake, Mtt is a small venomous snake inhabiting the southwestern United States which contains potent neurotoxic venom. Even though most of the venom components are neurotoxins and/or PLA2s, our study has demonstrated that it also contains high-molecular-weight (between 40 and 150 kDa) proteins targeting the hemostatic system.
This work demonstrated several hemostatic and biological activities found in Mtt venom, one of the venoms of important medical interest in the United States of America. To our knowledge, severe hemostatic activities in Mtt have not been described in the literature.
2. Materials and methods
2.1. Reagents
The Superdex-200 10/300 GE chromatographic separation column was purchased from GE Healthcare (Piscataway, NJ, USA). Chromogenic substrates were purchased from Aniara (Mason, OH, USA). Molecular weight standards were purchased from Invitrogen, (Carlsbad, CA, USA). Purified substrates factor Xa, bovine alpha thrombin, plasmin, and human fibrinogen were purchased from American Diagnostica Inc. (Stamford, CT, USA). ADP was from Chronolog Corp, (Havertown, PA, USA). Thrombin standards were from the National Institute for Biological Standards and Control (NIBSC, UK). Aprotinin was purchased from Bayer (Leverkusen, Germany). Bovine alpha thrombin, benzamidine/HCl, ethylene glycol-bis(2-aminoethylether)-N, N, N′, N′-tetraacetic acid (EGTA), ethylenediaminetetraacetic acid (EDTA), iodoacetic acid and other reagents used in this study were from Sigma Chemical Co (St. Louis, MO, USA).
2.2. Venoms
Pools of venoms from Mtt and Mff (Texas and Florida coral snakes, respectively) were purchased from the National Natural Toxins Research Center, Texas A&M University-Kingsville, Texas, USA. The lyophilized pool included venom from approximately 10 specimens from snakes found in the south Texas (Mtt) and approximately 20 specimens found in Osceola Co., Florida (Mff). The venoms were stored at −80° C.
Micrurus venoms from snakes captured in diverse Venezuela geographical locations were represented by a pool from at least eight different specimens. Theses snakes include Micrurus isozonus from Calabozo (Guárico state), Caracas (Capital District), La Boyera (Miranda state) and Maracay (Aragua state), Venezuela. The snakes are currently housed in the Serpentarium of the Institute of Tropical Medicine of the Universidad Central de Venezuela. The venoms were obtained by extracting once from each animal into sterile Petri dishes, then lyophilized, divided into 5 mg samples and kept at −80 °C until use.
2.3. Animals
Female BALB/c mice weighting 18–22 g were obtained from the Animal House of the Instituto Venezolano de Investigaciones Científicas (IVIC), Caracas, Venezuela and maintained at 22–24 °C, with a relative humidity of 45–70%, and a 12-h light/dark cycle (lights on at 07.00 h). Mice were acclimated at least 1 week prior to each experiment and received water and food ad libitum. The Animal House authorities’ surveillance reports established that the mice were free of known pathogenic bacteria, viruses, mycoplasmas, and parasites.
2.4. Ethical statement
All the experimental procedures regarding the use of live animals were done by specialized personnel according to the Venezuelan pertinent regulations and institutional guidelines approved by the Animal Bioethics Commissions of the Instituto Venezolano de Investigaciones Cientificas and the Institute of Anatomy of the Universidad Central de Venezuela. The norms were obtained from the guidelines for the care and use of laboratory animals published by the United States National Institute of Health (NIH, 1985).
2.5. Protein concentration determination
The protein concentration of crude venoms was measured by the method of Lowry et al. (1951) and spectrophotometrically estimated by assuming that 1 unit of absorbance/cm of path length at 280 nm corresponds to 1 mg protein/ml (Stoscheck, 1990).
2.6. SDS-PAGE
Polyacrylamide gel electrophoresis of Mtt venom was carried out on a 10–20% Tricine gel using an XCell Sure-Lock® system (Invitrogen, Carlsbad, CA). A total of 22 µg of non-reduced and reduced crude venom were run at 125 V for 90 min. The gel was stained with Simply Blue Safe stain (Invitrogen).
2.7. Molecular exclusion chromatography
Micrurus t. tener venom was fractionated by molecular exclusion chromatography on a Superdex-200 10/300 column equilibrated with 50 mM ammonium acetate buffer, pH 6.9. The venom sample (5 mg/100 µl) was resuspended in equilibrium buffer and injected into the column. The elutionwas carried out with the same buffer at a flow rate of 0.4 ml/min and monitored at 280 nm using a Waters 2487 high performance liquid chromatography (HPLC) system.
2.8. Micrurus venoms lethality
Lethality of crude venom was determined by intravenous injections into mice and the LD50 value calculated according to Spearman-Karber method (WHO, 1981). The venom was diluted in phosphate-buffered saline solution (PBS). The endpoint of lethality of the mice was determined after 48 h. All solutions throughout the experiments were stored at 4 °C and warmed to 37 °C immediately prior to being injected into mice. The lethal toxicity was determined in five groups containing five mice. A total of 0.2 ml of venom (dosages from 0.03 to 1.9 mg/kg) was injected into the tail vein of 18–20 g female BALB/c mice. An equivalent volume of PBS was injected as a negative control group.
2.9. The effects of Micrurus venoms on platelet aggregation
Platelet aggregation was estimated by turbidimetry using a dual-channel Chronolog model 560 CA aggregometer. Platelet-rich plasma (PRP) was prepared by mixing fresh blood sample with trisodium citrate solution (3.8%, w/v) in a volume ratio of 9:1, followed by centrifugation at 190 × g, 24 °C for 20 min to sediment leukocytes and erythrocytes. The platelet count was adjusted to 3.0 × 105 platelets/µl with platelet-poor plasma. Four hundred ninety microliters of citrated PRP were pre-incubated at 37 °C with a stir bar in a silicone-treated glass cuvette. Then, 10 µl/10 µg of crude venom or fractions dissolved in PBS and PBS alone was added 4 min prior to the addition of the platelet aggregation inducer. Aggregation was induced by adding 5 µl of adenosine diphosphate (ADP) (final concentration of 10 µM), and the changes in light transmittance were continuously recorded for 8 min. The maximum aggregation response obtained after the addition of an inducer in the absence of crude venom or fractions was taken as 100% aggregation. The inhibition percentage was calculated by comparing light transmittance obtained in presence of venom against the control sample. The IC50 value was calculated from a dose-dependent curve that was achieved from at least five different venom concentrations using the software program Graph Pad Prism.
2.10. Coagulant activity of Micrurus venoms
Procoagulant activity in Micrurus Spp. venoms was determined by the method adapted from Salazar et al. (2007). In a borosilicate tube (10 × 75 mm), 0.1 ml of 0.05 M Tris–HCl buffer, pH 7.4 (coagulation buffer) containing 0.1 ml venom samples (1–50 µg, diluted in coagulation buffer) was incubated at 37 °C. Then 0.1 ml of fresh citrate human plasma or 0.3% of human fibrinogen solution in coagulation buffer was added. The solution was mixed thoroughly and the coagulation time recorded. All experiments and appropriate controls were repeated at least four times.
2.11. Amidolytic activity of Micrurus venoms
The Micrurus Spp. venoms were evaluated for amidolytic activity using a micro-method standardized in our laboratory (Guerrero and Arocha-Piñango, 1992). A mixture of 80 µl of the recommended buffer for each chromogenic substrate, 10 µl of the venom sample (2 mg/ml) and 10 µl of substrate (final concentrations of 0.60 mM S-2238, 1.20 mM S-2288, 0.16 mM S-2586, 0.80 mM S-2222, 0.80 mM S-2251, 0.44 mM S-2302 or 0.16 mM S-2444) were placed in each well of 96 well polystyrene plates. Bovine thrombin, bovine factor Xa, urokinase-PA and plasmin were used as positive controls. After incubation at 37 °C for 15 and 30 min, the absorbance at 405 nm was measured. One unit of amidolytic activity was expressed as ΔA 405 nm/min. Specific activity was calculated as mUA/min/µg.
2.12. The effects of Micrurus venoms on thrombin, factor Xa or plasmin activities
The effects of Micrurus Spp. venoms on thrombin, factor Xa or plasmin amidolytic activity was evaluated by the micro-method described above. Briefly, in polystyrene plates of 96 wells, 10 µl of Micrurus venom (20 µg) were mixed with 20 µL of the enzyme (0.25 nKcat plasmin, 0.1 IU thrombin or 0.05 IU factor Xa), and incubated for 30 min at 37 °C. Then 60 µl of the recommended buffer solution for each substrate and 10 µl of chromogenic substrate were added. After incubation at 37 °C for 10 min, the absorbance at 405 nm was measured. The enzymes incubated with buffer were used as controls, which measured 100% activity.
2.13. Effect of M. t. tener venom on factor Xa and thrombin coagulant activity
Anticoagulant activity of Mtt venom on factor Xa and thrombin was also evaluated using the Salazar et al. (2007) method. Factor Xa (0.05 IU/mL) or thrombin (2.5 IU/mL) was incubated at 37°C for 30 min with 0.1 ml of venom sample (1–25 µg). Then the residual coagulant activity was evaluated for factor Xa using fresh citrate human plasma in presence of 0.025 M CaCl2 and for thrombin using fresh citrate human plasma or human fibrinogen solution (0.3% in coagulation buffer). All experiments and appropriate controls were repeated at least four times.
2.14. Fibrinolytic activity of M. t. tener venom
Fibrinolytic activity of Mtt venom was studied by the fibrin plate method as described by Marsh and Arocha-Pinango (1972). Fibrin plates were made using 3-cm diameter Petri dishes by allowing 1.5 ml of fibrinogen solution (10% plasminogen as contaminant, 0.1% in 5 mM imidazol saline buffer, pH 7.4) to clot by adding 75 µl of bovine thrombin (10 IU/ml, in 0.025 M CaCl2). The mixture was incubated at 22–25 °C for 30 min, and then 10 µl (25–50 µg) of sample was applied over the fibrin. After 24 h incubation at 37 °C, the fibrin hydrolysis diameter (lysed area) was measured. The activity was then recorded as the lysis area consisting of the maximum and smallest size diameter, which could be measured at right angles, and this was expressed in mm2. Specific fibrinolytic activity (mm2/µg) was calculated dividing the lysed area (mm2) by the given protein dose (µg). Human plasmin (0.25 nKcat) was used as positive control.
2.15. The effects of M. t. tener venom on plasmin fibrinolytic activity
The effects of Mtt venom and chromatographic fractions on plasmin fibrinolytic activity were tested by a modification of the method described by Marsh and Arocha-Pinango (1972). Fibrin films were formed in wells of a 96 well polystyrene plate: 200 µl of fibrinogen (0.1%) was allowed to clot with 12 µl of bovine thrombin (10 IU/ml, in 0.025 M CaCl2). The mixture was incubated at 22–25 °C for 60 min. The venom’s effect on plasmin fibrinolytic activity (0.25 nKcat) was tested after pre-incubating plasmin with 10 µl of crude venom (20 µg) at 37 °C for 30 min and then applying it to the fibrin for 6 h at 37 °C. The assay was also carried out with crude venom pre-treated with serine protease (10 mM benzamidine and 100 IU/ml aprotinin), metalloprotease (10 mM EDTA), or cysteine protease (iodoacetic acid 10 mM) inhibitors (CPI). As controls, the fibrinolytic activity with plasmin (0.25 nKcat), Mtt venom (20 µg), and chromatographic fractions (20 µg) was tested on the fibrin film. After the 6 h incubation period, the absorbance was measured at 405 nm. The effect of Mtt venom on plasmin fibrinolytic activity was expressed as the change in absorbance of the fibrin film.
2.16. Statistical analysis
All experiments were repeated three times. Results were expressed as the mean ± standard deviation and analyzed using the two-tailed Student’s t-test for samples with equal variances. Differences were statistically significant if p was less than 0.05.
3. Results
3.1. SDS-PAGE analysis of M. t. tener venom
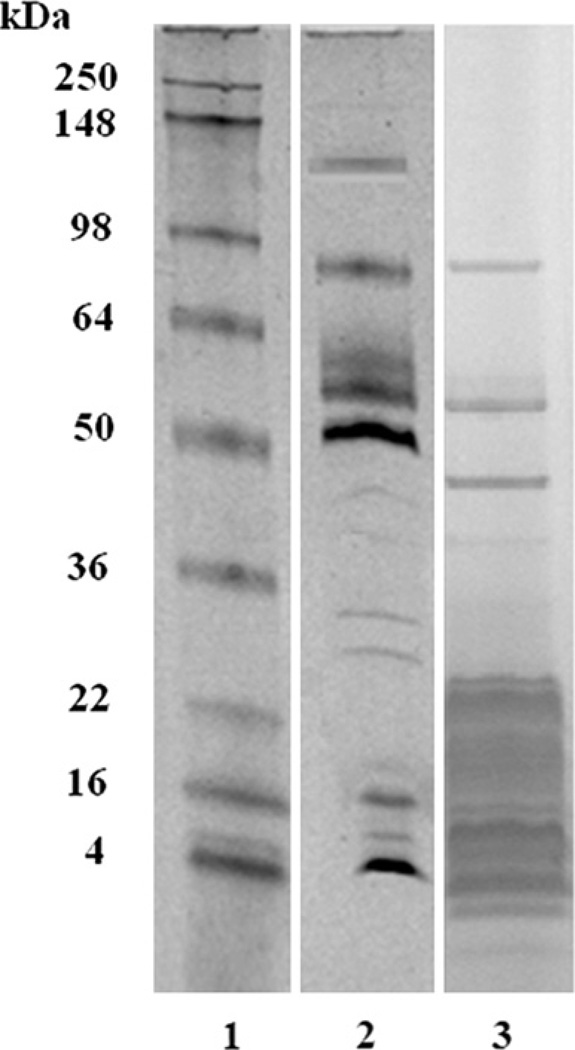
The electrophoretic profile of crude Mtt venom was analyzed by SDS-PAGE in a gradient of 10–20% (Fig. 1). Under non-reduced conditions, 17 well-defined venom protein bands were observed. Two were observed between 45 and 55 kDa, one between 34 and 45 kDa, 12 bands between 18 and 7 kDa, and two between 4 and 7 kDa.
Fig. 1.
An 10–20% SDS-PAGE of M. t. tener (Mtt) crude venom. Lanes: 1) Molecular weight markers; 2) Mtt (50 µg) under non-reducing conditions; 3) Mtt (50 µg) under reducing conditions. The gel was stained with Simply blue.
3.2. Chromatographic profile of M. t. tener venom
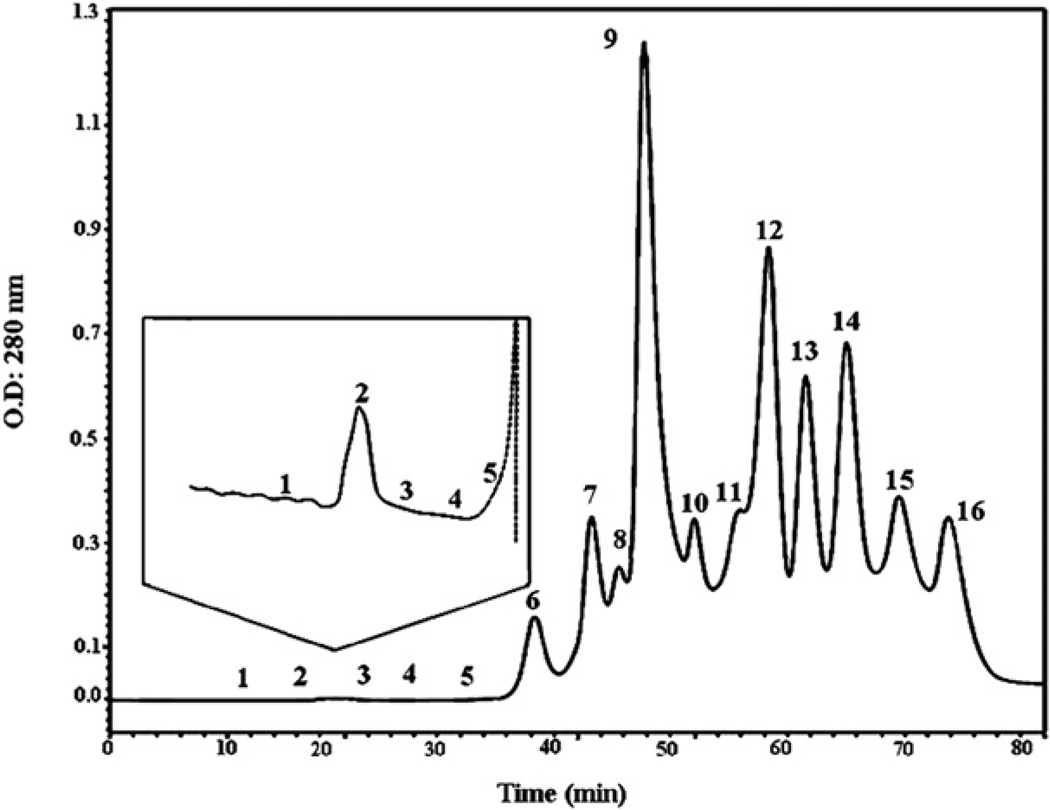
Sixteen Mtt venom fractions were collected (F1 to F16) from a Superdex-200 10/300 molecular exclusion column (Fig. 2). Fractions 1 to 5, having very low concentrations and elution times between 12 and 34 min, are shown in the insert of Fig. 2. Fractions F7 to F14 had the highest protein concentrations. The molecular weights for the proteins in F6 to F10 ranged from 75 to 5 kDa, and F2, which contained a very low protein concentration, had proteins at 150 kDa.
Fig. 2.
Molecular exclusion chromatographic profile of M. t. tener venom. Crude venom (5 mg) was reconstituted in 100 µl of 50 mM ammonium acetate buffer, pH 6.9 and fractionated through a Superdex-200 column (10 × 300 mm) previously equilibrated with the same buffer. The venom was run for 80 min at 0.4 ml/min, and the proteins were detected at 280 nm. The insert shows the fractions F1–F5, which showed the fractions with a high anti ADP-inducer-platelet aggregation and anti-plasmin activities.
3.3. Micrurus venoms lethality
The LD50 values, determined by probit analysis at 95% confidence, showed differences among the Micrurus venoms tested. The most potent venom (Table 1) was Mff from Florida, USA (0.32 mg/kg), and Mtt from Texas, USA was the least active (0.78 mg/kg). The M. isozonus venoms showed LD50s ranging from 0.52 to 0.61 mg/kg. A pool of these M. isozonus venoms presented a LD50 of 0.57 mg/kg.
Table 1.
LD50s of Venezuelan and United States coral snake venoms.
| Species | Pool | LD50a ± SD (mg/kg) |
|---|---|---|
| M. isozonus | La Boyera, Miranda state (Venezuela) | 0.56 ± 0.072 |
| M. isozonus | Caracas, Capital District (Venezuela) | 0.52 ± 0.090 |
| M. isozonus | Calabozo, Guárico state (Venezuela) | 0.61 ± 0.086 |
| M. isozonus | Maracay, Aragua state (Venezuela) | 0.58 ± 0.080 |
| M. tener tener | Kingsville, Texas state (United States) | 0.78 ± 0.140 |
| M. fulvius fulvius | Tampa, Florida state (United States) | 0.32 ± 0.120 (p < 0.05) |
The LD50 is the concentration of venom required to kill 50% of a mouse population (n = 40/group) after 48 h. Results are expressed in mg venom/kg body weight.
3.4. The effect of Micrurus venoms on platelet aggregation
The effects of Micrurus venoms on ADP-induced platelet aggregation are displayed in Table 2. Micrurus venoms inhibited 50–95% of ADP-induced platelet aggregation. Micrurus isozonus venoms from different Venezuelan geographical regions (10 µg) inhibited platelet aggregation from 50 to 68.2%, with the Capital District venom having the highest effect (68.2%). Micrurus t. tener was the most active with a 95.2% inhibitory effect. The results showed that this crude venom inhibited ADP-induced platelet aggregation with an IC50 of 12.79 µg/ml.
Table 2.
The effects of coral snake venoms (10 µg) on ADP-induced platelet aggregation.
| Venoms | % Inhibitiona |
|---|---|
| M. isozonus, La Boyera, Miranda state (Venezuela) | 54.8 ± 5.2 |
| M. isozonus, Calabozo, Guárico state (Venezuela) | 50.1 ± 6.4 |
| M. isozonus, Maracay, Aragua state (Venezuela) | 55.9 ± 8.0 |
| M. isozonus, Caracas, Capital District, (Venezuela) | 68.2 ± 10.2 |
| M. tener tener, Kingsville, Texas state (United States) | 95.2 ± 13.3 *(p < 0.05) |
| M. fulvius fulvius, Tampa, Florida state (United States) | 60.3 ± 8.9 |
p < 0.05 in comparison with other venoms.
The activity is expressed in percentage inhibition. The maximum aggregation response obtained after addition of inducer in presence of PBS was taken as 100% aggregation.
Chromatography fractions of Mtt venom (10 µg) were also evaluated. Fractions F1, F2, F6 to F8 and F11 to F16 inhibited platelet aggregation by more than 60% (Table 3). However, the highest inhibitory activity (>80%) was observed in fractions F2, F11 and F13 to F15.
Table 3.
The effects of Micrurus t. tener molecular exclusion fractions on hemostatic functions.
| Fractions or CV | Mr kDaa | Activity | ||||
|---|---|---|---|---|---|---|
| % Anti- Platelet aggregation (ADP) (10 µg) |
α Fibrinogenaseb | α/β Fibrinogenase | Fibrinolytice,f | % Anti-Plasmin (S-2251) (20 µg) |
||
| CV | 95.2 | + | − | + | 71.3 | |
| F1 | 86.3 | + | +c | + | − | |
| F2 | − | + | − | + | 66.2 | |
| F3 | − | + | − | + | − | |
| F4 | − | − | − | + | − | |
| F5 | >158.000 | 61.1 | − | − | − | 47.9 |
| F6 | 73.147 | 70.2 | + | +d | − | 74.8 |
| F7 | 26.678 | 60.0 | + | − | + | 41.4 |
| F8 | 15.814 | − | + | − | + | 81.1 |
| F9 | 12.095 | − | − | − | − | 41.6 |
| F10 | <5.000 | 87.2 | − | − | − | − |
| F11 | 51.9 | − | − | − | − | |
| F12 | 80.3 | − | − | − | − | |
| F13 | 84.6 | − | − | − | 37.3 | |
| F14 | 81.7 | − | − | − | 81.9 | |
| F15 | 61.2 | − | − | − | 62.4 | |
| F16 | ||||||
+: Active; −: Inactive.
Relative molecular mass determinate by molecular exclusion chromatography.
Activity abolished by metalloprotease inhibitor (MPI).
Activity abolished by MPI inhibitor.
Activity abolished partially inhibited by EDTA, serine protease inhibitor (SPI) and/or iodoacetic acid.
Activity abolished by MPI plus serine protease inhibitors.
Activity determinate by fibrin plate using micro-plate method.
3.5. Coagulant activity of Micrurus venoms
Micrurus Spp. venoms obtained from diverse geographical Venezuelan and USA locations did not have significant procoagulant activity on plasma or on fibrinogen doses between 20 and 50 µg. However, Mtt venom at doses between 1 and 10 µg induced the formation of an instable fibrin gel at 25–30 s incubation time.
3.6. Amidolytic activity of Micrurus venoms
All Micrurus crude venoms did not have significant thrombin, factor Xa, or plasmin-like activities using amidolytic methods at doses up to 20 µg. Additionally, when fresh citrated human plasma was incubated 10 min with the venoms, no effect was observed when kallikrein, factor Xa or thrombin chromogenic substrates were used. These results indicated that the venoms, under such conditions, did not exhibit pre-kallikrein, factor X or prothrombin activators, as well as thrombin-like amidolytic activity.
3.7. The effects of Micrurus venoms on factor Xa, thrombin and plasmin amidolytic activities
The effects of Micrurus crude venoms (20 µg) on plasmin (0.25 nKcat), thrombin (0.1 IU) and factor Xa (0.05 IU) amidolytic activities are shown in Table 4. All the crude venoms tested inhibited plasmin amidolytic activity on S-2251 chromogenic substrate between 40 and 99%. All the M. isozonus venoms produced diverse effects. For instance, M. isozonus of the Capital District inhibited plasmin (98.5%) by almost twice as much as the venoms of La Boyera, Guárico, and Aragua states (~50%). Micrurus t. tener venom was more active than Mff venom having 71 and 40% inhibitory activities, respectively.
Table 4.
The effects of coral snake venom on plasmin, factor Xa and thrombin amidolytic activities.
| Venoms (20 ug) | % Inhibition | ||
|---|---|---|---|
| Plasmin (0.25 nKcat) S-2251 |
Factor Xa (0.05 IU) S-2222 |
Thrombin (0.1 IU) S-2238 |
|
| M. isozonus La Boyera, Miranda state (Venezuela) | 56.8 ± 7.2 | 16.5 ± 2.7 | 6.5 ± 0.72 |
| M. isozonus Calabozo, Guárico state (Venezuela) | 52.5 ± 5.5 | 21.6 ± 2.8 | 8.3 ± 1.0 |
| M. isozonus Maracay, Aragua state (Venezuela) | 49.2 ± 5.1 | 17.4 ± 2.0 | 7.8 ± 0.5 |
| M. isozonus Caracas, Capital District, (Venezuela) | 98.5 ± 10.2 (p < 0.05) | 21.0 ± 2.6 | 10.1 ± 1.6 |
| M. tener tener Kingsville, Texas state (United States) | 71.3 ± 6.9 | 0 | 0 |
| M. fulvius fulvius Tampa, Florida state (United States) | 39.8 ± 4.0 | 0 | 1.4 ± 0.7 |
In reference to factor Xa amidolytic activity, M. isozonus venoms produced minimal inhibition values ranging from 16.5 to 21.6%. Micrurus t. tener and Mff venoms did not inhibit factor Xa amidolytic activity. Regarding thrombin amidolytic activity, all M. isozonus venoms showed limited inhibitory effects. Micrurus t. tener and Mff venoms did not inhibit thrombin amidolytic activity.
Studies with the Mtt chromatographic fractions (20 µg) demonstrated that F2, F5 to F9 and F13 to F15 induced a significant inhibition of plasmin amidolytic activity, with the most active fractions being F6, F8 and F14 with inhibitory activities from 75 to 82% (Table 3).
3.8. Fibrinogenolytic activity of Micrurus t. tener venom
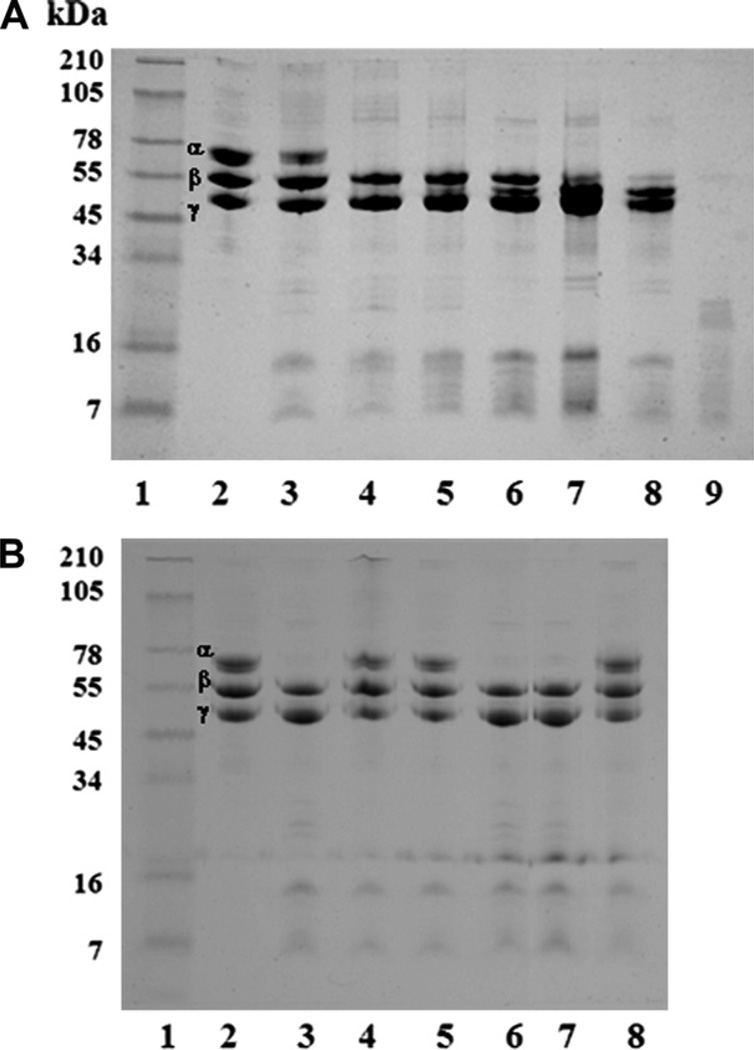
The study of Mtt crude venom on fibrinogen demonstrated that the fibrinogen:venom ratios of 100:1, 100:5 and 100:10, incubated for 4 h, induced fibrinogen Aα chain degradation, generating fragments with diverse molecular masses (data no shown). The results using a 100:1 ratio at different times demonstrated that the proteases present in Mtt venom induced a rapid degradation of fibrinogen Aα chains (1 h) and a slower degradation of Bβ chains (18 h). It was also observed that the Aα chains degradation started at 5 min, with a maximum at 1 h, and degradation of Bβ chains was from 18 to 24 h, and full degradation was observed at 24 h. The γ chains were unaffected (Fig. 3A). This fibrinogenolytic activity was completely inhibited by metalloprotease, and cysteine protease inhibitors (iodoacetic acid), while the serine protease inhibitors did not modify the degradation pattern (Fig. 3B).
Fig. 3.
Fibrinogenolytic activity of M. t. tener (Mtt) crude venom. A) A ratio of 100 µg of fibrinogen (Fg):1 µg venom was incubated at 37 °C at different times. Lanes: 1) Molecular weight markers; 2) Fg control (25 µg); 3 – 8) Fg + Mtt at 5 min, 1, 2, 4, 18 and 24 h, respectively; 9) crude venom (15 µg). B) A ratio of 100 µg Fibrinogen (Fg):1 µg venom was incubated in presence of protease inhibitors, for 2 h at 37 °C. Lanes: 1) Molecular weight markers; 2) 25 µg Fg control; 3) Fg + Mtt; 4) Fg + (Mtt + EDTA); 5) Fg + (Mtt + EGTA); 6) Fg + (Mtt + Benzamidine); 7) Fg + (Mtt + Aprotinin); 8) Fg + (Mtt + Iodoacetic acid). An 8% SDS-PAGE under reduced conditions was used and stained with Coomassie blue.
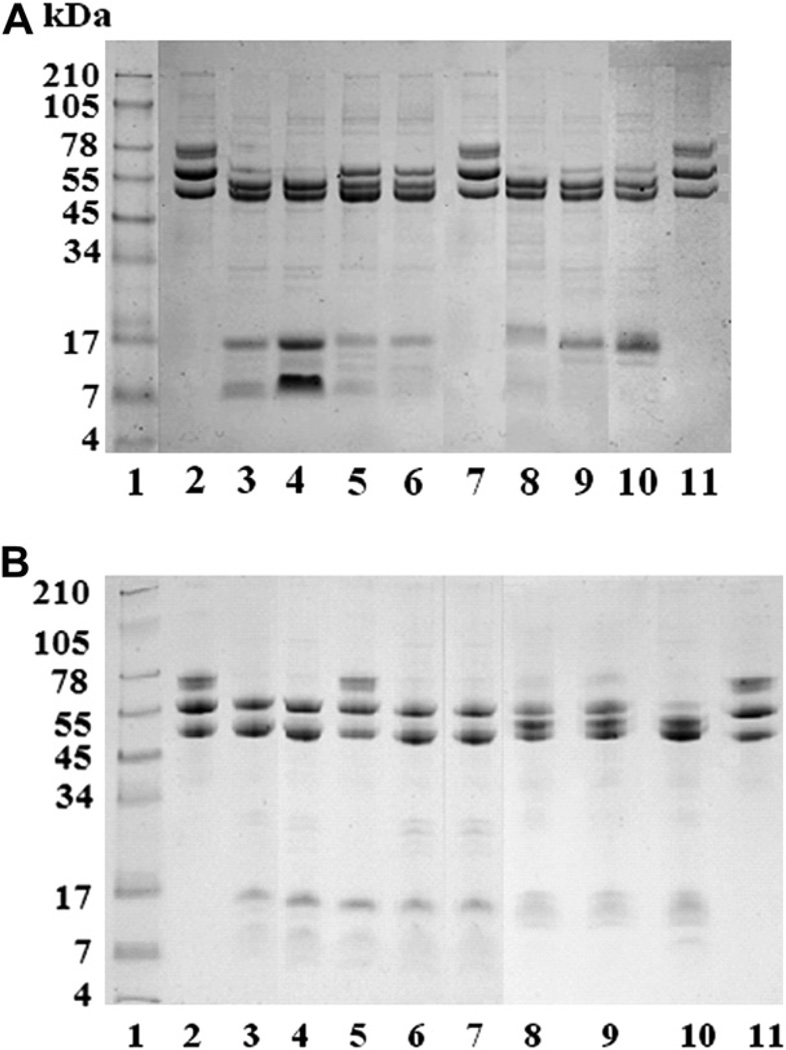
Fig. 4A shows the fibrinogenolytic activity of the Mtt venom fractions at a 100:1 (Fg:venom) ratio and 24 h incubation. Fractions F1–F4 and F6–F8 degraded the Aα chains with different intensities. Fractions F2 and F7 completely degraded both fibrinogen Aα and Bβ chains. The γ chains were not affected by the fractions.
Fig. 4.
Fibrinogenolytic activity of M. t. tener chromatographic venom fractions. A) A ratio of 100 µgfibrinogen (Fg):1 µg fraction was incubated for 24 h at 37 °C. A. Lanes: 1) Molecular weight markers; 2 – 11) Fg control at 24 h; 3–11) Fg + fractions F1, F2, F3, F4, F5, F7, F8, and F9, respectively. B) Fibrinolytic activity of fractions in presence of protease inhibitors. Lanes: 1) Molecular weight markers; 2) Fg control at 24 h; 3) Fg + F3; 4) Fg + (F3 + SPI); 5) Fg + (F3 + MPI); 6) Fg + (F3 + CPI); 7) Fg + F7; 8) Fg + (F7 + SPI); 9) Fg+(F7 + MPI); 10) Fg+(F7 + CPI); 11) Fg+(F7+SPI+MPI). An 8% SDS-PAGE was used under reduced conditions and stained with Coomassie blue.
The fibrinogenolytic activities of the fractions were also evaluated in presence of protease inhibitors. The results showed that the proteolytic activity of fractions F1, F3, F4, F6 and F8 was completely inhibited only by MPI, while the activity of fractions F2 and F7 was partially inhibited by EDTA, SPI and iodoacetic acid. The fibrinolytic activity of F2 and F7 fractions was completely inhibited by MPI and SPI. Fig. 4B shows the results obtained with F3 and F7 fractions in presence or absence of protease inhibitors.
3.9. Fibrinolytic activity of Micrurus t. tener venom
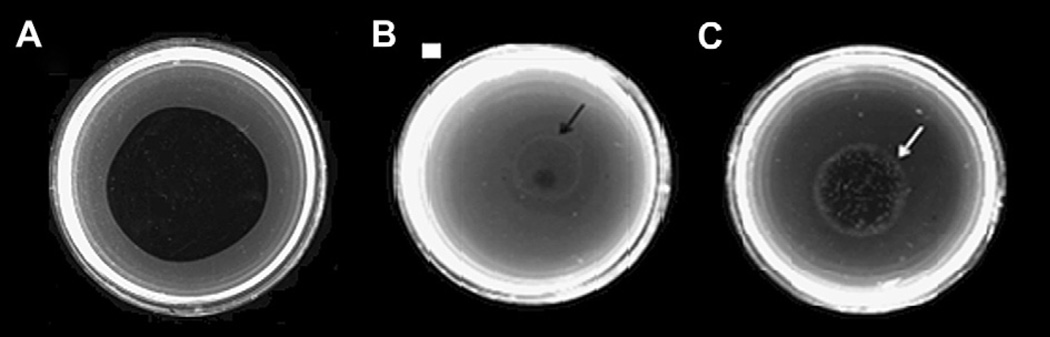
The fibrinolytic activity of Mtt venom at different doses was evaluated on plasminogen-rich fibrin plates. Plasmin (0.25 nKcat), used as a positive control, produced a fibrin lysis area of 418 mm2 (Fig. 5A). Twenty-five micrograms of Mtt showed a fibrin lysis area of 16 mm2 with a central white halo, which could indicate the presence of a plasmin inhibitor(s) present in the crude venom (Fig. 5B). At 50 µg, Mtt venom had a fibrin lysis area of 100 mm2 and an increase in the diameter of the white halo (Fig. 5C).
Fig. 5.
Fibrinolytic activity on fibrin plates (in presence of plasminogen) observed with M. t. tener (Mtt) crude venom. A) 0.125 nKcat plasmin; B) Mtt (25 µg); C) Mtt (50 µg). The arrows point to the plasmin inhibition halo.
The fibrinolytic activity of the chromatographic fractions (20 µg) obtained from Mtt venom was evaluated on fibrin film using a micro-method. The results revealed that F1 to F4 and F7 and F8 contained fibrinolytic activity (Table 3).
3.10. The effect of M. t. tener venom on plasmin fibrinolytic activity
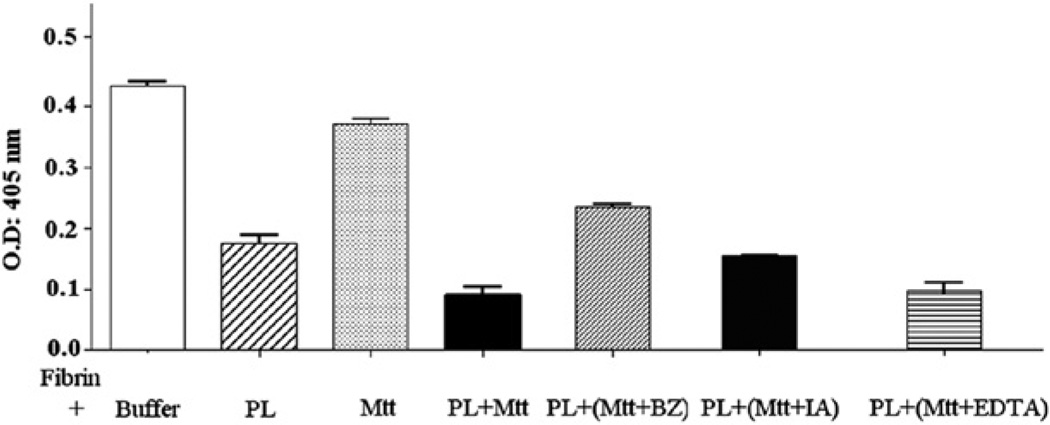
The fibrin film in presence of a buffer control showed an absorbance of 0.4348 ± 0.0195 at 405 nm. The fibrin incubated with plasmin (0.25 nKcat) during 6 h presented an absorbance of 0.1753 ± 0.0335 (p < 0.001), which denoted a 59.58% decrease in optical density (OD), representing 100% lysis. The fibrin incubated with Mtt crude venom (20 µg) presented an absorbance of 0.3716 ± 0.0237 (p < 0.01), which represented a 15.15% decrease in OD and a lysis of 24.3%. The fibrin incubated with plasmin (0.25 nKcat) pre-treated with Mtt crude venom (20 µg) presented an absorbance of 0.0961 ± 0.0331 (p < 0.001), which indicated a 77.9% decrease in OD and a 130% lysis, representing complementary fibrinolytic activity with both plasmin and venom (Fig. 6).
Fig. 6.
The effects of M. t. tener crude venom on plasmin fibrinolytic activity evaluated in micro-plates. (PL: plasmin; Mtt: M. t. tener venom; BZ: benzamidine; IA: Iodoacetic acid; EDTA: ethylenediaminetetraacetic acid).
The pre-treatment of the Mtt crude venom with proteases inhibitors showed that only benzamidine significantly inhibited fibrin lysis induced by plasmin and venom (Fig. 6).
3.11. The effect of M. t. tener venom on factor Xa and thrombin coagulant activity
Table 5 shows that Mtt venom inhibits factor Xa coagulant activity in a dose-dependent manner, an effect evidenced by the increase in coagulation time of this enzyme pre-treated with crude venom using as substrate citrated human plasma in presence of calcium. In contrast, this venom increases, in a dose-dependent manner, the thrombin coagulant activity, which was evidenced by the decrease in coagulation time of this enzyme pre-treated with crude venom.
Table 5.
The effects of M. t. tener crude venom on factor Xa and thrombin coagulant activity.
| Venom (µg) |
Thrombin (2.5 IU/mL) | Factor Xa (0.05 IU/mL) | |
|---|---|---|---|
| Substrate | |||
| Plasma | Fibrinogen | Human Plasma (in presence of CaCl2) | |
| Coagulation | Time (s) | ||
| 0 | 39.9 ± 0.14 | 31.1 ± 0.14 | 20.8 ± 0.55 |
| 1 | 35.3 ± 0.14 | 18.8 ± 1.13 | 27.9 ± 0.14 |
| 5 | 29.4 ± 0.14 | 15.1 ± 0.14 | 58.5 ± 0.71 |
| 25 | 20.4 ± 0.56 | 13.7 ± 0.14 | 251.0 ± 1.41 |
4. Discussion
Elapidae venoms are described as complex mixtures containing protein components with neurotoxic functions that can cause alterations in the nervous system, and many also contain hemorrhagic components, which induce alterations of the capillaries (Rosso et al., 1996; Markland, 1998; Wijeyewickrema et al., 2007). Several Elapidae snake venoms with neurotoxic activity, such as the cobras (Elapidae), also contain proteins that activate or inhibit the hemostatic system (Utkin and Osipov, 2007; Osipov et al., 2010). For instance, a metalloprotease enzyme capable of activating factor V was described in the venom of Naja naja oxiana (Gerads et al., 1992). Other elapids, Australians for example, showed that their hemostatic activities were limited to prothrombin activation and contain no thrombin-like enzymes (Chester and Crawford, 1982; Fry, 1999; Braud et al., 2000; Matsui et al., 2000; Rao et al., 2003). St Pierre et al. (2005) described from the cDNA gland of Australian elapids, two prothrombin activators, factor X and factor V- like.
The isolation and characterization of coral snake venom components have not been well documented to date. In this study we have evaluated the biochemical and biological characteristics, in regards to coagulation, fibrinolysis and platelet functions, of several venoms of the Micrurus genus, with particular reference to Mtt venom.
Micrurus snakes belonging to the Elapidae family are the most representative genus in respect to abundance and diversity, with a great number of species found in South America and the Southern United States. Coral snake envenomations are comparatively infrequent because of their subfossorial behavior and the high incidence of dry bites; nevertheless, the mortality attributed to muscle respiratory paralysis is high (Rengifo and Rodríguez-Acosta, 2004). In spite of being considered amongst the most toxic snakes in America (Roze, 1996), their venom hemostatic activities have been scarcely described since disorders of blood coagulation are not common in human envenomations (Barros et al.,1994; Francis et al.,1997; Urdaneta et al., 2004; Cecchini et al., 2005; Dokmetjian et al., 2009).
Several biological activities have been demonstrated in comparative studies among venoms from several Micrurus taxa (Gutiérrez et al., 1983, 1992; Aird and da Silva, 1991; Alape-Girón et al., 1994; Barros et al., 1994). Lethality studies in mice are utilized as a direct evaluation of the pathological action of general venom toxicity. In the current work, the LD50 values of the three Micrurus venoms were shown to be different. The most lethal was Mff venom (0.32 mg/kg) followed by M. isozonus (averaging 0.57 mg/kg), and then Mtt (0.78 mg/kg). These results are in accordance with those reported by other authors (Arce et al., 2003; Sánchez et al., 2008) who studied the lethal toxicities of Mff (LD50 = 0.32 mg/kg) and Mtt (LD50 = 0.78 mg/kg).
Some snake venom components are recognized as modulators of platelet receptors and their ligands. The platelets play a crucial function in hemostasis, but in addition are liable for the pathogenic thrombosis triggering severe clinical manifestations of vascular atherothrombotic disease (Varon et al., 2009; Fabre and Gurney, 2010). Several authors have proposed that snake venoms of the family Elapidae are capable of inhibiting platelets aggregation through the action of l-amine oxidases or phospholipases A2 (Rosso et al., 1996; Alape-Girón et al., 1999; Sakurai et al., 2001; Satish et al., 2004). Moreover, Oyama and Takahashi (2007) evaluated the effects of venom from 11 species of the Elapidae family on platelet aggregation, specifically inhibiting platelet aggregation induced by ADP. In the present study, it was demonstrated that three M. isozonus venoms (10 µg) from snakes captured in diverse Venezuelan localities as well as venoms from Mtt and Mff, both from the USA (10 µg), exhibited an inhibition of ADP-induced platelet aggregation, with M. t. tener venom displaying the highest activity (95.2% inhibitory effect). This effect could be related to disintegrin-like components, or to the proteolytic effects against ADP receptors or to the presence of ADPases. In future studies the possible mechanism of action with isolated fractions of these toxins will be evaluated.
Although no significant coagulopathy has been reported in Elapidae venoms, the cobra (N. naja) induces blood clots in vitro by activating prothrombin, as demonstrated by thrombin-specific chromogenic substrates (Sundell et al., 2003). In our study, none of the Micrurus venoms presented a significant factor Xa, thrombin or chymotrypsin-like amidolytic activities. These results suggested the absence of a significant procoagulant activity related to prothrombin or factor X activators. In contrast, Mtt at very low doses (between 1 and 20 µg) pre-incubated with purified fibrinogen induced an instable fibrin gel formation, which evidence a thrombin-like coagulant activity in Mtt venom. The fibrinogenolytic activity present in this venom probably masked this thrombin-like coagulant activity, which should not activate factor XIII, given that the formed fibrin gel is unstable.
Several biochemical and hemostatic characteristics from Mtt venom were analyzed. Electrophoretic analysis evidenced venom components with molecular masses of 130 kDa. Several venom components in Mtt venom were between 64 and 50 kDa, which is uncommon since neurotoxins are mostly of low molecular weights (Tanaka et al., 2010). After gel electrophoresis analysis, M. t. tener venom was fractionated by molecular exclusion chromatography in order to isolated fibrinogenases and fibrinolytic enzymes as well as anti-plasmin inhibitors. Micrurus t. tener venom chromatographic profiles showed 16 fractions (Fig. 2). The first fractions showed very low absorbencies at 280 nm (F1 to F4) corresponding to proteins of high molecular masses (>75 kDa), which contained fibrinogenolytic activity as well as inhibitory effects on the plasmin amidolytic activity and on ADP-induced platelet aggregation, which can all be related to metalloproteases activities. Additionally there is a group of proteins with molecular masses between 75 and 12 kDa (F6 to F9) where these activities are also present. The molecular masses around 26 kDa are in the same range of metalloproteases such as those described in various venoms (Bjarnason and Fox, 1994; Markland, 1998). This is also the molecular weight ranges (26–13 kDa) in which serine proteases with hemostatic activities such as Bothrombin (Nishida et al., 1994) and Bothrojaracin (Zingali et al., 1993) have been described.
The Micrurus venoms have also been characterized as possessing low or non-proteolytic activity. The presence of hyaluronidase activity was demonstrated in several Micrurus species from different geographic Brazilian locations and one Northern American species (Mff) (Tanaka et al., 2010). Fibrinogenolytic activity tested with Mtt crude venom showed that the fibrinogen Aα chains was hydrolyzed much earlier than the Bβ-chains, while the γ-chain remained resistant to proteolysis even after 24 h. The α-fibrinogenases enzymes specifically degraded the Aα chains and are metalloproteases, they also cleave the Bβ-chains but at a slower rate (Markland, 1998; Swenson and Markland, 2005). Alpha-fibrinogenases have been isolated from the venoms of Bothrops (Maruyama et al., 1992), Agkistrodon (Moran and Geren, 1981), and Cerastes (Daoud and Tu, 1986) among others.
The F1, F3, F4, F6 and F8 fractions induced degradation of fibrinogen Aα chains, which was inhibited with metal chelating agents signifying that these toxins were mainly metalloproteases. In contrast, F2 and F7 fractions induce degradation of both fibrinogen Aα and Bβ-chains, which were partially inhibited by metallo- and serine proteases inhibitors, which demonstrated that these fractions contained both serine and metalloproteases activities. These results were in accordance with Jagadeesha et al. (2002) who isolated and characterized a non-toxic anticoagulant metalloprotease, (NN-PF3; 67.81 kDa), from N. naja naja venom with strong anticoagulant and fibrinogenolytic activities. Gowda et al. (2006) also demonstrated that the fibrinogenolytic activity of N. naja venom was due to metalloproteases.
Venoms may cause fibrin degradation due to some serine proteases which directly degrade fibrin in a plasmin-like manner (Zhang et al., 1995; Kamiguti et al., 1996; Cho et al., 2004; Swenson and Markland, 2005). Furthermore, the fibrinolytic activity of Mtt venom at different doses was evaluated on plasminogen-rich fibrin plates, and showed a fibrin lysis area as well as a white halo, which could be indicating the presence of plasmin inhibitor(s) or plasminogen activator inhibitor(s) in the crude venom. Furthermore, the plasmin activity (enzyme that physiologically degrades fibrin) evaluated by a fibrin lysis micro-method was significantly increased in presence of Mtt crude venom (Fig. 6), which confirmed the fibrinolytic activity presence in this venom.
To corroborate the presence of plasmin inhibitor(s) in Mtt venom, the plasmin amidolytic activity was evaluated. The results demonstrated that the plasmin amidolytic activity (S-2251) was significantly inhibited (between 40 and 99%) in the presence of all Micrurus sp venoms tested. M. isozonus venom (Caracas)was the most active (99%), followed by M. t. tener (71%) (Table 4). These results revealed that Micrurus venoms also contain plasmin inhibitors.
Additionally, Mtt fractions were used to evaluate plasmin inhibitors. The results showed that F2, F5 to F9 and F13 to F15 fractions induced an inhibition of the plasmin amidolytic activity. Fractions F6, F8 and F14 were the most active, showing inhibitory effects as high as 75%. The inhibition effect on plasmin amidolytic activity can be associated with inhibitory molecules or with proteolytic activity against the plasmin molecule. These results stimulate future studies with the isolation and characterization of plasmin inhibitor(s); for instance, plasmin inhibitors can be used as potential anti-fibrinolytic components in hemophilia and/or local surgical procedures such as dental extractions. Snake venoms from N. naja naja and Pseudonaja textilis textilis (Textilinins) have been accounted to contain plasmin inhibitory toxins. Textilinins, a Kunitz-type serine protease inhibitor with a high specificity for plasmin, showed homology with aprotinin and was capable of inhibiting hemorrhages in experimental mice (Takahashi et al., 1974; Ritonja et al., 1983; Masci et al., 2000; Millers et al., 2009).
Few anticoagulant components have been described in Elapids (Kini and Evans, 1991; Sundell et al., 2003; White, 2005; Gowda et al., 2006; Kumar et al., 2010). A protein complex, the hemextin AB, was isolated from the Hemachatus haemachatus (African Ringhals cobra) venom, which is an inhibitor of factor VIIa (Banerjee et al., 2005). Recently, the venom of Naja pallida was also shown to contain anticoagulant activity (Suntravat et al., 2010). A basic phospholipase A2 (CM-IV) was isolated from Naja nigricollis venom which inhibited the prothrombinase complex by a novel non-enzymatic mechanism (Stefansson et al., 1990). Kerns et al. (1999) evidenced that the phospholipase A2 CM-IV in presence of factor Va inhibits thrombin formation by factor Xa. In order to evaluate the presence of other Mtt crude venom inhibitors, the activity of this venom was evaluated on thrombin and factor Xa amidolytic and coagulant activities. The results evidenced that Mtt venom (Tables 4 and 5) was capable of induce inhibition of the amidolytic activity and the coagulant activity of factor Xa in human plasma in presence of calcium. Thus factor Xa is the target protein of this anticoagulant present in Mtt venom. It was possible to identify a significant factor Xa inhibitory activity in Mtt venom; and therefore, studies must be continued at this level.
Osipov et al. (2010), isolated and characterized a phospholipase A2 from Naja haje cobra venom, identified as TI-NH, which was the first direct thrombin inhibitor found in the venom of the Elapidae family and the first phospholipase with that function. Clinically, the specific inhibition of factor Xa or thrombin would reduce the risk of hemorrhagic syndromes.
This work constitutes, to our knowledge, the first report on fibrino(geno)lytic and inhibitors of plasmin and factor Xa activities in Micrurus venoms. These molecules, once purified and characterized, may be useful as therapeutic tools for thrombosis, strokes and other hemorrhagic diseases.
Acknowledgments
This research was supported by FONACIT grant (G-2005000400), Caracas, Venezuela, and funds from Texas A&M University-Kingsville, Kingsville, Texas, U.S.A.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Aguilar I, Girón ME, Rodríguez-Acosta A. Purification and characterization of a hemorrhagic fraction from the venom of the Uracoan rattlesnake Crotalus vegrandis. Biochim. Byophys. Acta. 2001;1548:57–65. doi: 10.1016/s0167-4838(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Aird SD, da Silva NJ., Jr Comparative enzymatic composition of Brazilian coral snake (Micrurus) venoms. Comp. Biochem. Physiol. B. 1991;99:287–294. doi: 10.1016/0305-0491(91)90043-d. [DOI] [PubMed] [Google Scholar]
- Alape-Girón A, Lomonte B, Gustafsson B, Da Silva NJ, Thelestam M. Electrophoretic and immunochemical studies of Micrurus snake venoms. Toxicon. 1994;32:713–723. doi: 10.1016/0041-0101(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Alape-Girón A, Persson B, Cederlund E, Flores-Díaz M, Gutiérrez JM, Thelestam M, Bergman T, Jörnvall H. Elapid venom toxins: multiple recruitments of ancient scaffolds. Eur. J. Biochem. 1999:225–234. doi: 10.1046/j.1432-1327.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- Arce V, Rojas E, Ownby CL, Rojas G, Gutiérrez JM. Preclinical assessment of the ability of polyvalent (Crotalinae) and anticoral (Elapidae) antivenoms produced in Costa Rica to neutralize the venoms of North American snakes. Toxicon. 2003;41:851–860. doi: 10.1016/s0041-0101(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Banerjee Y, Mizuguchi J, Iwanaga S, Kini M. Hemextin AB complex - A snake venom anticoagulant protein complex that inhibits factor VIIa activity. Pathophysiol. Haemost. Thromb. 2005;34:184–187. doi: 10.1159/000092420. [DOI] [PubMed] [Google Scholar]
- Barros ACS, Fernandes DP, Ferreira LCL, Santos MC. Local effects induced by venoms from five species of genus Micrurus sp. (coral snakes) Toxicon. 1994;32:445–452. doi: 10.1016/0041-0101(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Bjarnason JB, Fox JW. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994;62:325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Braud S, Bon C, Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82:851–859. doi: 10.1016/s0300-9084(00)01178-0. [DOI] [PubMed] [Google Scholar]
- Cecchini AL, Marcussi S, Silveira LB, Borja-Oliveira CR, Rodrigues-Simioni L, Amara S, Stabeli RG, Giglio JR, Arantes EC, Soares AM. Biological and enzymatic activities of Micrurus sp. (Coral) snake venoms. Comp. Biochem. Physiol. Part A. 2005;140:125–134. doi: 10.1016/j.cbpb.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Chester A, Crawford GP. In vitro coagulant properties of venoms from Australian snakes. Toxicon. 1982;20:501–504. doi: 10.1016/0041-0101(82)90014-9. [DOI] [PubMed] [Google Scholar]
- Cho IH, Choi ES, Lim HG, Lee HH. Purification and characterization of six fibrinolytic serine-proteases from earthworm Lumbricus rubellus. J. Biochem. Mol. Biol. 2004;37:199–205. doi: 10.5483/bmbrep.2004.37.2.199. [DOI] [PubMed] [Google Scholar]
- Daoud E, Tu AT, el-Asmar MF. Isolation and characterization of an anticoagulant proteinase, cerastase F-4, from Cerastes cerastes (Egyptian sand viper) venom. Thromb. Res. 1986;42:55–62. doi: 10.1016/0049-3848(86)90196-9. [DOI] [PubMed] [Google Scholar]
- de Roodt AR, Paniagua-Solis JF, Dolab JA, Estévez-Ramiréz J, Ramos-Cerrillo B, Litwin S, Dokmetjian JC, Alagón A. Effectiveness of two common antivenoms for North, central, and south American Micrurus envenomations. J. Toxicol. Clin. Toxicol. 2004;42:171–178. doi: 10.1081/clt-120030943. [DOI] [PubMed] [Google Scholar]
- Dokmetjian JC, Del Canto S, Vinzón S, de Jiménez-Bonino MB. Biochemical characterization of the Micrurus pyrrhocryptus venom. Toxicon. 2009;53:375–382. doi: 10.1016/j.toxicon.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Fabre JE, Gurney ME. Limitations of current therapies to prevent thrombosis: a need for novel strategies. Mol. Biosyst. 2010;6:305–315. doi: 10.1039/b914375k. [DOI] [PubMed] [Google Scholar]
- Francis BR, da Silva NJ, Júnior, Seebart C, Casais E, Silva LL, Schmidt JJ, Kaiser II. Toxins isolated from the venom of the Brazilian coral snake (Micrurus frontalis frontalis) include hemorrhagic type phospholipases A2 and postsynaptic neurotoxins. Toxicon. 1997;35:1193–1203. doi: 10.1016/s0041-0101(97)00031-7. [DOI] [PubMed] [Google Scholar]
- Fry BG. Structure-function properties of venom components from Australian elapids. Toxicon. 1999;37:11–32. doi: 10.1016/s0041-0101(98)00125-1. [DOI] [PubMed] [Google Scholar]
- Gerads I, Tans G, Yukelson LYa, Zwaal RF, Rosing J. Activation of bovine factor V by an activator purified from the venom of Naja naja oxiana. Toxicon. 1992;30:1065–1079. doi: 10.1016/0041-0101(92)90052-7. [DOI] [PubMed] [Google Scholar]
- Gowda CD, Nataraju A, Rajesh R, Dhananjaya BL, Sharath BK, Vishwanath BS. Differential action of proteases from Trimeresurus malabaricus, Naja naja and Daboia russellii venoms on hemostasis. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2006;143:295–302. doi: 10.1016/j.cbpc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B, Portilla E, Cerdas L, Rojas E. Local effects induced by coral snake venoms: evidence of myonecrosis after experimental inoculations of venoms from five species. Toxicon. 1983;21:777–783. doi: 10.1016/0041-0101(83)90066-1. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Rojas G, Pérez A, Argüello I, Lomonte B. Neutralization of coral snake Micrurus nigrocinctus venom by a monovalent antivenom. Braz. J. Med. Biol. Res. 1991;24:701–710. [PubMed] [Google Scholar]
- Gutiérrez JM, Rojas G, da Silva Júnior NJ, Núñez J. Experimental myonecrosis induced by the venoms of South American Micrurus (coral snakes) Toxicon. 1992;30:1299–1302. doi: 10.1016/0041-0101(92)90446-c. [DOI] [PubMed] [Google Scholar]
- Guerrero B, Arocha-Piñango CL. Activation of human prothrombin by the venom of Lonomia achelous (Cramer) caterpillars. Thromb. Res. 1992;66:169–177. doi: 10.1016/0049-3848(92)90187-f. [DOI] [PubMed] [Google Scholar]
- Jagadeesha DK, Shashidhara-Murthy R, Girish KS, Kemparaju K. A non-toxic anticoagulant metalloprotease: purification and characterization from Indian cobra (Naja naja naja) venom. Toxicon. 2002;40:667–675. doi: 10.1016/s0041-0101(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Kamiguti AS, Hay CRM, Theakston RDG, Zuzel M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon. 1996;34:627–642. doi: 10.1016/0041-0101(96)00017-7. [DOI] [PubMed] [Google Scholar]
- Kerns RT, Kini RM, Stefansson S, Evans HJ. Targeting of venom phospholipases: the strongly anticoagulant phospholipase A2 from Naja nigricollis venom binds to coagulation factor Xa to inhibit the prothrombinase complex. Arch. Biochem. Biophys. 1999;369:107–113. doi: 10.1006/abbi.1999.1345. [DOI] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. Inhibition of platelet aggregation by a fibrinogenases from Naja nigricollis venom is independent of fibrinogen degradation. Biochim. Biophys. Acta. 1991;1095:117–121. doi: 10.1016/0167-4889(91)90073-7. [DOI] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. Structural domains in venom proteins: evidence that metalloproteinases and non-enzymatic platelet aggregation inhibitors (Disintegrins) from the snake venoms are derived by proteolysis from a common precursor. Toxicon. 1992;30:265–293. doi: 10.1016/0041-0101(92)90869-7. [DOI] [PubMed] [Google Scholar]
- Kini RM. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol. Haemost. Thromb. 2005;34:200–204. doi: 10.1159/000092424. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Devaraj VR, Vishwanath BS, Kemparaju K. Anticoagulant activity of a metalloprotease: further characterization from the Indian cobra (Naja naja) venom. J Thromb. Thrombolysis. 2010;29:340–348. doi: 10.1007/s11239-009-0379-2. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosembrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;1193:265–275. [PubMed] [Google Scholar]
- Markland FS. Snake Venoms and the Hemostatic System. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- Marsh NA, Arocha-Piñango CL. Evaluation of the fibrin plate method for estimating plasminogen activators. Thromb. Diath. Haemorrh. 1972;28:75–88. [PubMed] [Google Scholar]
- Maruyama M, Suzuki M, Yoshida E, Mihara H, Nakajima N. Purification and characterization of two fibrinolytic enzymes from Bothrops jararaca (Jararaca) venom. Toxicon. 1992;30:853–864. doi: 10.1016/0041-0101(92)90383-g. [DOI] [PubMed] [Google Scholar]
- Masci PP, Whitaker AN, Sparrow LG, de Jersey J, Winzor DJ, Watters DJ, Lavin MF, Gaffney PJ. Textilinins from Pseudonaja textilis textilis. Characterization of two plasmin inhibitors that reduce bleeding in an animal model. Blood. Coagul. Fibrinolysis. 2000:385–393. doi: 10.1097/00001721-200006000-00011. [DOI] [PubMed] [Google Scholar]
- Matsui T, Fujimura Y, Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta. 2000;1477:146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- Millers EK, Trabi M, Masci PP, Lavin MF, de Jersey J, Guddat LW. Crystal structure of textilinin-1, a Kunitz-type serine protease inhibitor from the venom of the Australian common brown snake (Pseudonaja textilis) FEBS. J. 2009;276:3163–3175. doi: 10.1111/j.1742-4658.2009.07034.x. [DOI] [PubMed] [Google Scholar]
- Moran JB, Geren CR. Characterization of a fibrinogenase from northern copperhead (Agkistrodon contortrix mokasen) venom. Biochim. Biophys. Acta. 1981;659:161–168. doi: 10.1016/0005-2744(81)90280-1. [DOI] [PubMed] [Google Scholar]
- NIH. Principles of Laboratory Animal Care. vol. 85. USA: National Institute of Health; 1985. pp. 1–112. [Google Scholar]
- Nishida S, Fujimura Y, Miura S, Ozaki Y, Usami Y, Suzuki S, Titán K, Yoshida E, Sugimoto M, Yoshioka A, Fukui H. Purification and characterization of bothrombin, a fibrinogen-clotting serine protease from the venom of Bothrops jararaca. Biochemistry. 1994;33:1843–1849. doi: 10.1021/bi00173a030. [DOI] [PubMed] [Google Scholar]
- Osipov AV, Filkin SY, Makarova YV, Tsetlin VI, Utkin YN. A new type of thrombin inhibitor, noncytotoxic phospholipase A2, from the Naja naje cobra venom. Toxicon. 2010:186–194. doi: 10.1016/j.toxicon.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Oyama E, Takahashi H. Distribution of low molecular weight platelet aggregation inhibitors from snake venoms. Toxicon. 2007;49:293–298. doi: 10.1016/j.toxicon.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Rao VS, Swarup S, Kini RM. The non-enzymatic subunit of pseutarin C, a prothrombin activator from eastern brown snake (Pseudonaja textilis) venom, shows structural similarity to mammalian coagulation factor V. Blood. 2003;102:1347–1354. doi: 10.1182/blood-2002-12-3839. [DOI] [PubMed] [Google Scholar]
- Rengifo C, Rodríguez-Acosta A. Serpientes, venenos y su tratamiento en Venezuela. 1er ed. Caracas: Fondo de Publicaciones de la Facultad de Medicina de la Universidad Central de Venezuela; 2004. pp. 1–86. [Google Scholar]
- Ritonja A, Turk V, Gubensek F. Serine proteinase inhibitors from Vipera ammodytes venom. Isolation and kinetic studies. Eur. J. Biochem. 1983;133:427–432. doi: 10.1111/j.1432-1033.1983.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Rosso JP, Vargas-Rosso O, Gutiérrez JM, Rochat H, Bougis PE. Characterization of alpha-neurotoxin and phospholipase A2 activities from Micrurus venoms. Determination of the amino acid sequence and receptor-binding ability of the major alpha-neurotoxin from Micrurus nigrocinctus nigrocinctus. Eur. J. Biochem. 1996;238:231–239. doi: 10.1111/j.1432-1033.1996.0231q.x. [DOI] [PubMed] [Google Scholar]
- Roze JA. Coral Snakes of the Americas: Biology, Identification, and Venoms. Malabar: Krieger Publishing Company; 1996. pp. 1–340. [Google Scholar]
- Sakurai Y, Takatsuka H, Yoshioka A, Matsui T, Suzuki M, Titani K, Fujimura Y. Inhibition of human platelet aggregation by L-amino acid oxidase purified from Naja naja kaouthia venom. Toxicon. 2001;39:1827–1833. doi: 10.1016/s0041-0101(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Rodríguez-Acosta A, Girón ME, Aguilar I, Guerrero B. A comparative analysis of the clotting and fibrinolytic activities of the mapanare (Bothrops atrox) snake venom from different geographical areas in Venezuela. Thromb. Res. 2007;120:95–104. doi: 10.1016/j.thromres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Lopez-Johnston JC, Rodríguez-Acosta A, Pérez JC. Neutralization of two North American coral snake venoms with United States and Mexican antivenoms. Toxicon. 2008;51:297–303. doi: 10.1016/j.toxicon.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish S, Tejaswini J, Krishnakantha TP, Gowda TV. Purification of a Class B1 platelet aggregation inhibitor phospholipase A2 from Indian cobra (Naja Naja) venom. Biochimie. 2004;86:203–210. doi: 10.1016/j.biochi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Stefansson S, Kini RM, Evans HJ. The basic phospholipase A2 from Naja nigricollis venom inhibits the prothrombinase complex by a novel nonenzymatic mechanism. Biochemistry. 1990;29:7742–7746. doi: 10.1021/bi00485a024. [DOI] [PubMed] [Google Scholar]
- St Pierre L, Masci PP, Filippovich I, Sorokina N, Marsh N, Miller DJ, Lavin MF. Comparative analysis of prothrombin activators from the venom of Australian elapids. Mol. Biol. Evol. 2005;22:1853–1864. doi: 10.1093/molbev/msi181. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Sundell IB, Rånby M, Zuzel M, Robinson KA, Theakston RD. In vitro procoagulant and anticoagulant properties of Naja naja naja venom. Toxicon. 2003;42:239–247. doi: 10.1016/s0041-0101(03)00137-5. [DOI] [PubMed] [Google Scholar]
- Suntravat M, Nuchprayoon I, Pérez JC. Comparative study of anticoagulant and procoagulant properties.f 28 snake venoms from families Elapidae, Viperidae, and purified Russell’s viper venom-factor X activator (RVV-X) Toxicon. 2010;56:544–553. doi: 10.1016/j.toxicon.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson S, Markland FS. Snake venom fibrin(ogen)olytic enzymes. Toxicon. 2005;45:1021–1039. doi: 10.1016/j.toxicon.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Iwanaga S, Kitagawa T, Hokama Y, Suzki T. Snake venom proteinase inhibitors. Chemical structure of inhibitor II isolated from the venom of Russell’s viper (Viper russelli) J. Biochem. 1974;76:721–733. [PubMed] [Google Scholar]
- Tanaka GD, Furtado MF, Portaro FC, Sant’Anna OA, Tambourgi DV. Diversity of Micrurus snake species related to their venom toxic effects and the prospective of antivenom neutralization. PLoS Negl Trop. Dis. 2010;4:e622. doi: 10.1371/journal.pntd.0000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdaneta AH, Bolaños F, Gutiérrez JM. Feeding behavior and venom toxicity of coral snake Micrurus nigrocinctus (Serpentes: Elapidae) on its natural prey in captivity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;138:485–492. doi: 10.1016/j.cca.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Utkin YN, Osipov AV. Non-Lethal Polypeptide components in cobra venom. Current. Pharmaceutical. Design. 2007;13:2906–2915. doi: 10.2174/138161207782023757. [DOI] [PubMed] [Google Scholar]
- Varon D, Shai E, Spectre G. Frontiers in platelet inhibition. Discov. Med. 2009;8:242–246. [PubMed] [Google Scholar]
- White J. Snake venoms and coagulopathy. Toxicon. 2005;45:951–967. doi: 10.1016/j.toxicon.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Wijeyewickrema LC, Gardiner EE, Shen Y, Berndt MC, Andrews RK. Fractionation of snake venom metalloproteinases by metal ion affinity: a purified cobra metalloproteinase Nk, from Naja kaouthia binds Ni2+agarose. Toxicon. 2007;50:1064–1072. doi: 10.1016/j.toxicon.2007.07.006. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Vol. 58. Geneva: WHO Offset Publication; 1981. Progress in the Characterization of Venoms and Standardization of Antivenoms. [PubMed] [Google Scholar]
- Zaganelli GL, Zaganelli MGM, Magalhaes A, Diniz CR, de Lima ME. Purification and characterization of fibrinogenase from the venom of Jararacucu (Bothrops jararacussu) Toxicon. 1996;34:807–819. doi: 10.1016/0041-0101(96)00006-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wisner A, Xiong Y, Bon C. A novel plasminogen activator from snake venom. Purification, characterization, and molecular cloning. J. Biol. Chem. 1995;270:10246–10255. doi: 10.1074/jbc.270.17.10246. [DOI] [PubMed] [Google Scholar]
- Zingali RB, Jandrot-Perrus M, Guillén MC, Bon C. Bothrojaracin, a new thrombin inhibitor isolated from Bothrops jararaca venom: characterization and mechanism of thrombin inhibition. Biochemistry. 1993;32:10794–10802. doi: 10.1021/bi00091a034. [DOI] [PubMed] [Google Scholar]