Abstract
Probiotic lactic acid bacteria (LAB) have been shown to alleviate inflammation, enhance the immunogenicity of rotavirus vaccines, or reduce the severity of rotavirus diarrhoea. Although the mechanisms are not clear, the differential Th1/Th2/Th3-driving capacities and modulating effects on cytokine production of different LAB strains may be the key. Our goal was to delineate the influence of combining two probiotic strains L. acidophilus and L. reuteri on the development of cytokine responses in neonatal gnotobiotic pigs infected with human rotavirus (HRV). We demonstrated that HRV alone, or HRV plus LAB, but not LAB alone, initiated serum cytokine responses, as indicated by significantly higher concentrations of IFN-α, IFN-γ, IL-12, and IL-10 at post-inoculation day (PID) 2 in the HRV only and LAB+HRV+ pigs compared to LAB only and LAB-HRV- pigs. Peak cytokine responses coincided with the peak of HRV replication. LAB further enhanced the Th1 and Th2 cytokine responses to HRV infection as indicated by significantly higher concentrations of IL-12, IFN-γ, IL-4 and IL-10 in the LAB+HRV+ pigs compared to the LAB-HRV+ pigs. The LAB+HRV+ pigs maintained relatively constant concentrations of TGF-β compared to the HRV only group which had a significant increase at PID 2 and decrease at PID 7, suggesting a regulatory role of LAB in maintaining gut homeostasis. At PID 28, cytokine secreting cell (CSC) responses, measured by ELISpot, showed increased Th1 (IL-12, IFN-γ) CSC numbers in the LAB+HRV+ and LAB-HRV+ groups compared to LAB only and LAB-HRV- pigs, with significantly increased IL-12 CSCs in spleen and PBMCs and IFN-γ CSCs in spleen of the LAB+HRV+ group. Thus, HRV infection alone, but not LAB alone was effective in inducing cytokine responses but LAB significantly enhanced both Th1 and Th2 cytokines in HRV-infected pigs. LAB may also help to maintain immunological homeostasis during HRV infection by regulating TGF-β production.
Keywords: Lacto acid bacteria, probiotics, cytokines, human rotavirus, gnotobiotic pigs
1. Introduction
Lactobacilli are natural inhabitants of the gastrointestinal microflora of most humans and also pigs. Many Lactobacillus spp have been tested for the prevention or treatment of a variety of pathological conditions. Metchnikoff was the first to propose in 1908 that lactic-acid-producing bacteria (LAB) could be used to prevent diarrhoea and intestinal diseases when introduced in the diet (Mercenier et al., 2003). LAB, mostly Lactobacillus spp., replicates within the human gastrointestinal tract and generates lactase (Marteau et al., 1990). This enzyme facilitates the digestion of lactose present in milk and decreases the symptoms of malabsorption, which usually accompany acute infectious diarrhoea. L. rhamnosus GG has been reported in many clinical studies to reduce the severity and duration of rotavirus (RV) diarrhoea in children (Guarino et al., 1997; Isolauri et al., 1991; Rosenfeldt et al., 2002). L. acidophilus NCFM mixed with L. reuteri and Bifidobacterium infantis BBI also significantly decreased the duration and incidence of rotavirus diarrhoea in children 12–32 months of age (Sanders and Klaenhammer, 2001). Possible mechanisms for the therapeutic effects of probiotics include the production of inhibitory substances, blocking of adhesion sites, competition for nutrients, and stimulation of the immune system (Rolfe, 2000). To date, no single probiotic strain possesses all of these qualities or properties, which is why mixed cultures of probiotics are frequently used in therapeutic treatments (Isolauri et al., 2002). Because of the complexity of the gastrointestinal ecosystem of commensals within which biotherapeutic agents interact and also because different probiotic strains mixtures may interact with each other, it is not always possible to predict the effect of an LAB mixture based on functional characteristics of individual LAB strains. We choose to study the effects of the mixture of L. acidophilus and L. reuteri because L. acidophilus is commercially available in fermented foods and in dietary supplements in the US since the mid-1970s (Sanders and Klaenhammer, 2001). L. acidophilus is known to stimulate innate and adaptive immune responses (Konstantinov et al., 2008; Liu et al., 2010; Zhang et al., 2008b). In vitro studies have shown that L. acidophilus is a strong inducer of Th1 cytokine (IL-12, IFN-γ) (Gackowska et al., 2006; Zeuthen et al., 2006) and it is involved in the up-regulation of dendritic cells surface markers such as HLA-DR, CD40, CD86 and CD83 (Zeuthen et al., 2006). L. reuteri has anti-inflammatory effects through increasing CD4+CD25+Foxp3+ T cells (Karimi et al., 2009). L. reuteri has also been shown to activate human DCs and promoted Th1 cytokine IL-12, IL-18 and IFN-γ production (Mohamadzadeh et al., 2005). Furthermore, both strains have been reported to reduce the risk, severity and duration of rotavirus diarrhea (Van Niel et al., 2002; Shornikova et al., 1997), although the mechanisms are undefined.
Both lactobacilli strains were previously evaluated for their colonization pattern in gnotobiotic pigs and similar results were reported (Glass, 2003). Because studies using combination of probiotics predict synergism or additive effects on a mixture (Timmerman et al., 2007), we hypothesized a mixture of L. acidophilus and L. reuteri would achieve better immune stimulating effects than monoassociated probiotics. Thus, we used a mixture of L. reuteri and L. acidophilus (LA+LR) in our earlier study as we attempted to achieve optimal immunomodulating effects, i.e. to reduce RV diarrhoea and at the same time to enhance immune responses to RV infection. However, the mixture of LA+LR did not confer protection against RV diarrhoea in gnotobiotic pigs although immunostimulatory and regulatory effects on total B cell, dendritic cell (DC) and macrophage, γδ T cell and toll-like receptor responses in the RV-infected pigs were evident (Wen et al., 2009; Wen et al., 2011; Zhang et al., 2008a; Zhang et al., 2008c). The goal of the current study was to evaluate the immunomodulating effects of the mixture of LA+LR on cytokine responses in RV-infected gnotobiotic pigs, because cytokines play important roles in RV pathogenesis and immunity. In vitro studies have shown that different LAB strains induce different cytokine responses in DCs (Christensen et al., 2002; Zeuthen et al., 2006) and epithelial cells (Liu et al., 2010). Different cytokines, in turn, contribute to distinct functions in the induction of antiviral cytotoxic T cells (by Th1 cytokines), isotype-specific B cell responses and mucosal IgA (by Th2 cytokines), and regulatory T helper (Treg) cells (by Treg cytokines). Th2 cytokines promote the maturation and activation of B cells; transforming growth factor (TGF)-β acts synergistically with Th2 cytokines to stimulate B cells to switch from IgM to IgA antibody secreting cells (Simecka, 1998). TGF-β is secreted by Treg cells and has multiple suppressive actions on T and B cells, macrophages, and other cell types and can directly suppress Th1, Th2 and Th17 immune responses (Hong et al., 2011; Weiner, 2001). We compared cytokine profiles, kinetics, and duration of proinflammatory, Th1, Th2 and Treg cytokine responses in serum, intestinal contents and intestinal and systemic lymphoid tissues of gnotobiotic pigs after infection with a virulent human RV (VirHRV) (mimic natural infection) in the presence or absence of LA+LR colonisation. There are few in vivo studies of cytokines in either humans or animals in association with probiotic LAB or following HRV exposure, and most studies focused on cytokines in blood and not at the local site of colonisation or infection, the gut.
2. Material and Methods
Virus
The virulent Wa HRV from pooled intestinal contents of gnotobiotic pigs was used to infect the pigs at a dose of 105 50% infectious dose (ID50). The ID50 of the virulent Wa HRV inoculum for gnotobiotic pigs was previously determined to be at least 1 focus forming unit (FFU) (Ward et al., 1996).
Lactobacillus
The Lactobacillus reuteri strain ATCC 23272 and Lactobacillus acidophilus strain NCFM™ (ATCC, Manassas, VA, USA) were used to colonise gnotobiotic pigs with a mixture of 1:1 every other day from 3 to 11 days of age. LAB inoculums were prepared and titrated as previously described (Zhang et al., 2008a). The LAB mixture was fed to pigs at 3, 5, 7 and 9 days of age at doses of 103, 104, 105 and 106 colony forming unit (CFU), respectively. The feeding protocol was based on results from monoassociate colonisation of gnotobiotic pigs (Glass, 2003) and a pilot study using the mixture of L. reuteri and L acidophilus to ensure colonisation without causing side effects in newborn gnotobiotic pigs.
Inoculation and challenge of gnotobiotic pigs
Near-term pigs were derived by surgery and maintained in gnotobiotic isolator units as described (Meyer et al., 1964). Pigs were assigned to one of four groups and inoculated as follows: (1) Colonised with LAB and inoculated with 1 oral dose of VirHRV at 6 days of age (LAB+HRV+); (2) Inoculated with 1 oral dose of VirHRV only (LAB-HRV+); (3) Colonised with LAB only (LAB+HRV-); or (4) inoculated with 1 oral dose of minimum essential media (LAB-HRV-). Pigs were bled at post-inoculation days (PIDs) 0, 2, 5, 7, 10, 21 and euthanized at PID 28. All procedures were conducted in accordance with protocols reviewed and approved by The Ohio State University’s Institutional Laboratory Animal Care and Use Committee.
Isolation of MNC for ELISPOT-Cytokine assay
For the isolation of mononuclear cells, the small intestines (ileum), spleen, and blood were collected from euthanized pigs and processed as previously described (VanCott et al., 1993; Yuan et al., 1996). An ELISPOT-Cytokine assay for interferon (IFN)-γ, interleukin (IL)-10, IL-12, IL-4, IL-6, tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β was conducted as follows: Multiscreen™-IP sterile 96-well plates (Millipore, Bedford, MA) were prepared according to the manufacture and coated with anti-porcine IFN-γ (5μg/ml), anti-porcine IL-10 (5μg/ml), anti-porcine TGF-β (Biosource, Camarillo, CA), anti-porcine IL-12 (1μg/ml), anti-porcine IL-4 (1μg/ml), anti-porcine IL-6 (0.8μg/ml) (R&D System, Minneapolis, MN) and anti-porcine TNF-α (4μg/ml) (Endogen) overnight at room temperature (RT). The anti-porcine TGF-β was diluted following the manufacturer recommended dilution. Prior to use, the plates were blocked with Roswell Park Memorial Institute (RPMI)-1640 media containing 10% foetal bovine serum (FBS) for 2 hrs at RT, then cell suspensions from each tissue were added in concentrations of 5×105 and 5×104 cells per well. Cells were stimulated with 50μg/ml of semi-purified (ultracentrifugation through 35% sucrose cushions) attenuated (Att) HRV, 10μg of phytohemagglutinin (PHA) or RPMI. After 48 hrs of incubation at 37°C and 5% CO2, the cells were removed by washing and the following reagents were added: biotinylated monoclonal antibodies to porcine IFN-γ (1μg/ml), IL-10 (1μg/ml), TGF-β (Biosource), IL-12 (0.2μg/ml) or IL-4 (1μg/ml), IL-6 (0.1μg/ml) (R&D Systems), TNF-α (0.7μg/ml) (Endogen) and incubated overnight at 4°C. Horseradish peroxidase-conjugated streptavidin (Biosource) was added at a concentration of 0.3μg/ml and incubated for 2 hrs at RT. The spots were developed with H2O2 and 3-amino-9-ethylcarbazole (AEC) (Sigma-Aldrich, St. Louis, MO) for 1 hr. The numbers of cytokine secreting cells (CSC) were counted using an ImmunoSpot® series 3A Analyzer (Cellular Technology Ltd, Cleveland, OH) and presented as the number of spots per 5×105 MNC.
Detection of cytokine levels by ELISA in serum and intestinal contents
Pigs were bled at PID 0, 2, 5, 7, 10, 21 and 28, and small intestinal contents were collected at euthanasia (PID 28). Serum samples were processed and stored at -20°C until tested. Intestinal contents (IC) were diluted 1:2 in MEM. Protease inhibitor cocktail was added to the IC samples to prevent cytokine degradation (Azevedo et al., 2004) and the samples were stored at −20°C until tested.
The ELISA test was conducted as follows: Nunc Maxisorp 96-well plates were coated with anti-porcine IFN-γ (1.5μg/ml), anti-porcine IL-10 (2μg/ml), anti-porcine TGF-β (1.5μg/ml) (Biosource), anti-porcine IL-12 (0.75μg/ml), anti-porcine IL-4 (1.5μg/ml), anti-porcine IL-6 (0.8μg/ml), anti-porcine IFN-α (0.3μg/ml) (R&D Systems) or anti-porcine TNF-α (2μg/ml) (Endogen) overnight at room temperature (RT). Prior to use, the plates were blocked with phosphate buffer saline (PBS)/tween (T) 0.1%/bovine serum albumin (BSA) 0.5% for 2 hrs at RT. Samples were added to the wells in a volume of 50μl plus 50μl of PBS/BSA 1%. Control samples were diluted in PBS/BSA 1%. Plates were incubated for 2 hrs at RT, then were washed 5 times with PBS/T 0.1% and the following reagents were added: biotinylated monoclonal antibodies to porcine IFN-γ (0.5μg/ml), IL-10 (0.5μg/ml), TGF-β (0.75μg/ml) (Biosource), IL-12 (0.2μg/ml), IL-4 (0.25μg/ml), IL-6 (0.1μg/ml), TNF-α (0.2μg/ml) or unconjugated mouse anti-porcine IFN-α (0.5μg/ml)(R&D Systems) and incubated for 2 hrs at RT. Horseradish peroxidase-conjugated streptavidin (Biosource) or Horseradish peroxidase goat anti-mouse (KPL) were added at concentrations of 0.1μg/ml and 0.2μg/ml respectively, and incubated for 1 hr at RT. Standard curves were generated using recombinant porcine IFN-γ, IL-10 (Biosource), IL-12, IL-4, IL-6, TNF-α and IFN-α (R&D Systems), and human recombinant TGF-β (Biosource). The standard curves were calculated using a computer generated 4 parameters curve-fit for each cytokine. Sensitivities for these ELISAs were 7.5pg/ml for IFNγ, IL-10, IL-4 and IL-12, 15pg/ml for IL-6, TNF-α and TGF-β, and 75pg/ml for IFN-α.
Statistical analysis
The CSC numbers and concentrations of cytokines in serum at PID 0, 2, 7, 10, 21 and 28 and intestinal contents at PID 28 were compared among and within the groups using the Kruskal-Wallis rank sum test (nonparametric). Statistical significance was assessed at p<0.05.
3. Results
Colonization with a mixture of lactobacilli and human rotavirus infection in gnotobiotic pigs
After gnotobiotic pigs were inoculated with VirHRV and fed LA+LR, bacterial colonisation, virus shedding, and diarrhoea were monitored and the results have been reported previously (Zhang et al., 2008a). LA+LR effectively colonised the gnotobiotic pigs; however all the VirHRV-inoculated pigs with or without LA+LR feeding developed diarrhoea and shed virus.
Serum cytokines
LA+LR colonisation significantly increased Th1 and maintained Th3 cytokine levels in serum during HRV infection of gnotobiotic pigs
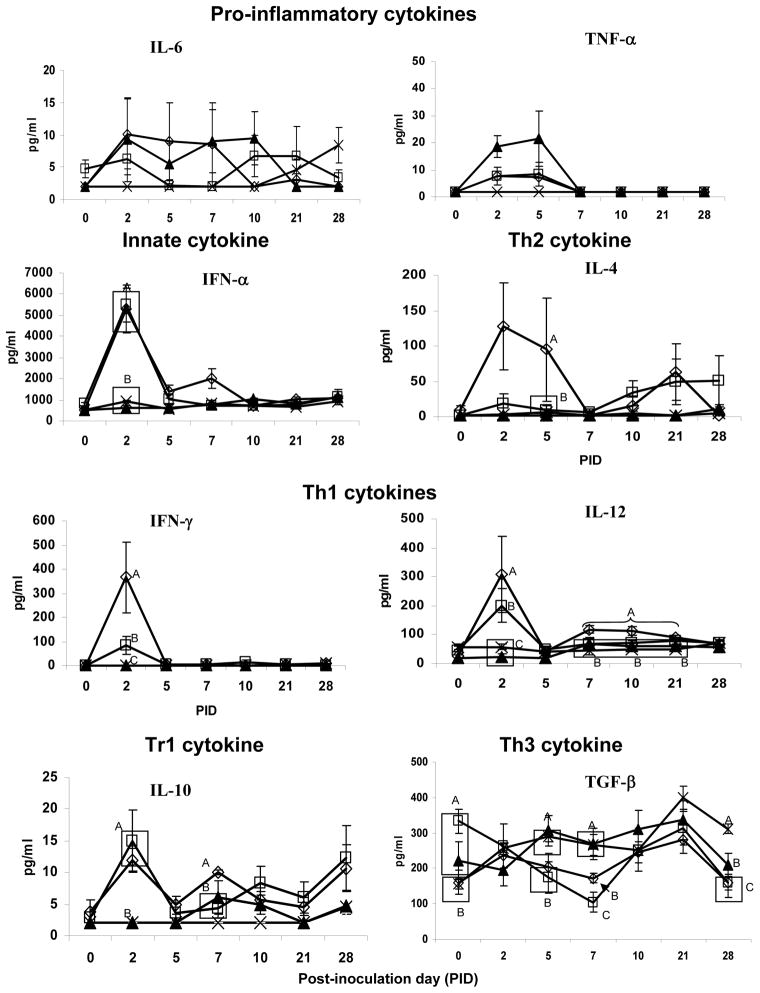
Concentrations of Th1 (IL-12, IFN-γ), Th2 (IL-4), Th3 (IL-10 and TGF-β) and proinflammatory cytokines (IL-6, TNF-α and IFN-α) in serum were compared among pigs colonised with or without LA+LR and infected with HRV or mock-inoculated (Figure 1). HRV infection or LA+LR colonisation slightly elevated proinflammatory cytokine IL-6 and TNF-α levels compared to the mock-inoculated control pigs; however LA+LR colonisation did not alter serum IL-6 and TNF-α levels in HRV infected pigs. Levels of IFN-α and IL-12 cytokine concentrations in HRV alone and LAB+HRV+ pigs were significantly elevated at PID 2 compared to the LAB alone and mock control pigs. The IL-12 concentrations in the LAB+HRV+ pigs remained significantly higher until PID 21 compared to all the other groups. The IFN-γ concentrations in the LAB+HRV+ and HRV alone pigs were significantly higher than those in the LAB alone and control pigs, indicating that HRV infection effectively activated Th1 type responses in neonatal gnotobiotic pigs with or without bacterial gut colonisation. However, LA+LR enhanced the Th1 cytokine responses to HRV infection, as indicated by significantly higher IFN-γ (5-fold) and higher IL-12 (1.5-fold) concentrations at PID 2 in the serum of LAB+HRV+ pigs than those of the LAB-HRV+ pigs. Interesting to note, pigs in all four groups had similar mean levels of TGF-β at 2 days of age (ranging from 155–194 pg/ml, data not shown). After two doses of LA+LR feeding (3 and 5 days of age), pigs in the LAB alone and HRV alone groups had significantly elevated concentrations of TGF-β compared to the pigs in the LAB+HRV+ and LAB-HRV- groups that remained the same (157–161 pg/ml) on PID 0 (6 days of age). After HRV infection at PID 7, the HRV alone pigs had the lowest mean TGF-β concentration. Although TGF-β concentrations in pigs of both HRV+ groups decreased significantly at PID 7 compared to pigs in the LAB alone and the control groups that had similar TGF-β levels at PID 0 and PID 7, TGF-β concentrations in the LAB+HRV+ pigs were significantly higher than the HRV alone pigs. Thus, LA+LR colonized and HRV infected pigs maintained relatively higher TGF-β levels during HRV infection, suggesting a regulatory role for LA+LR in the Th3 responses to HRV infection. At PID 10 and 21, the TGF-β levels in both HRV+ groups recovered to similar levels as the LAB alone and control groups (Figure 1). However, at PID 28, the TGF-β concentrations in both HRV+ groups declined and became again significantly lower than the LAB alone and control pigs, coinciding with the establishment of the Th1 type response in the two groups of HRV infected pigs.
Figure 1. Cytokine mean concentration in serum of pigs with or without LAB and inoculated with virulent Wa HRV. Numbers with different letters differ significantly at the same time for the same cytokine among groups (Kruskal Wallis Test, p<0.05, n=4–10).
◇ - LAB+HRV+; □ - LAB-HRV+; ▲ - LAB+HRV−;
 - LAB-HRV−
- LAB-HRV−
LA+LR colonisation enhanced early Th2 cytokines in serum during HRV infection and induced a delayed enhancement of IL-10
Concentrations of IL-4 were significantly higher in the LAB+HRV+ group than those in the LAB-HRV+ group at PID5, and remained higher in both HRV+ groups at PID 10 and PID 21. HRV and HRV+LAB induced an IL-10 response at PID 2 that was significantly higher than the LAB only and control groups. LAB significantly enhanced the concentrations of IL-10 in the LAB+HRV+ pigs at PID 7 (Figure 1).
Intestinal cytokines
LA+LR colonisation did not significantly influence cytokine profiles in intestinal contents of HRV infected pigs
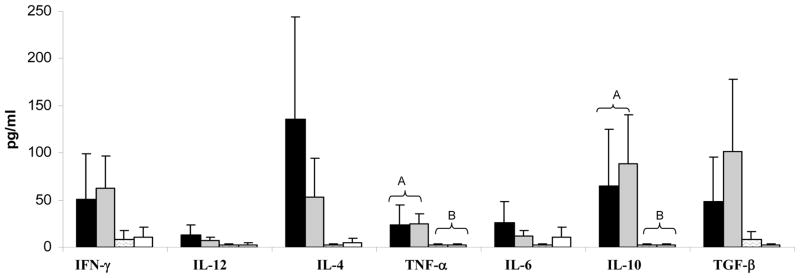
Cytokine concentrations in the small intestinal contents were measured when pigs were euthanized at PID 28. LAB alone and mock-inoculated pigs had very low or no detectable cytokines in the small intestinal contents, indicating that LAB alone was immune tolerated at PID28. HRV infection with or without LAB induced overall higher levels of IFN-γ, IL-12, IL-4, TNF-α, IL-6, IL-10 and TGF-β, however, only TNF-α and IL-10 concentrations were significantly higher in the LAB+HRV+ and LAB-HRV+ pigs compared to the LAB alone and control groups (Figure 2). There were no significant differences in the cytokine concentrations between the LAB+HRV+ and LAB-HRV+ pigs. Because most of the cytokines are not stable in the gastrointestinal lumen due to pH and enzymatic activity as we reported previously (Azevedo et al., 2006), variability of cytokine concentrations in intestinal contents is high and it may not be a good indicator of immune status in the gut. Therefore we also studied cytokine secreting cell responses in the small intestinal site (ileum) and the systemic sites (spleen and blood) at PID 28, to better understand the influence of LA+LR colonisation and HRV infection on the cytokine milieu in the gut.
Figure 2. Cytokine mean concentration in intestinal contents of pigs with or without LAB and inoculated with virulent Wa HRV. Numbers with different capital letters on top differ significantly at the same time for the same cytokine among groups; numbers with shared letters or without letters do not differ significantly (Kruskal Wallis Test, p<0.05, n=4–10).
■ - LAB+HRV+;
 - LAB-HRV+;
- LAB-HRV+;
 - LAB+HRV−; □ - LAB-HRV−
- LAB+HRV−; □ - LAB-HRV−
Cytokine secreting cell responses after human rotavirus infection and colonisation with a mixture of lactobacilli
LA+LR colonisation enhanced the Th1 (IL-12 and IFN-γ) CSC responses in systemic sites after HRV infection whereas it decreased the pro-inflammatory TNF-α CSC response
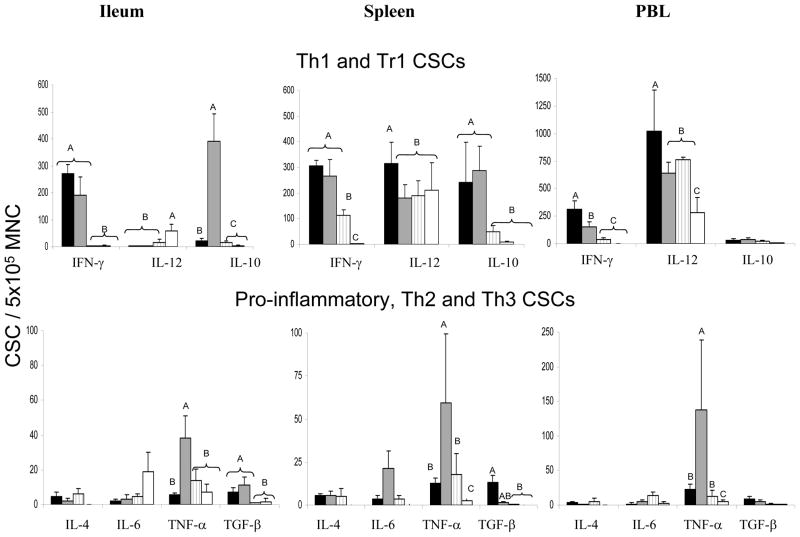
HRV infection with or without LAB induced significantly higher IFN-γ CSC numbers in ileum, spleen and PBL compared to the LAB alone and control groups at PID 28. LA+LR colonisation significantly enhanced IL-12 CSC numbers in spleen and PBL and IFN-γ CSC numbers in PBL of LAB+HRV+ pigs compared to LAB-HRV+ pigs, indicating an immunostimulatory effect of the LA+LR on the Th1 type response to HRV in systemic sites. LA+LR fed pigs showed a significant reduction in TNF-α CSC numbers in ileum, spleen and PBL of LAB+HRV+ group compared to LAB-HRV+ group (Figure 3), indicating an anti-inflammatory effect of the LA+LR on CSC.
Figure 3.
Cytokine secreting cells (CSC) in intestinal and systemic lymphoid tissues of pigs with or without LAB and infected Wa HRV or mock-infected at PID 28. Data are presented as CSC/ 5×105 MNC. Numbers with different letters differ significantly among groups within the same tissue for the same cytokine; numbers with shared letters or without letters do not differ significantly. (Kruskal-Wallis Test, p<0.05, n=4–10).
Legend: ■- LAB+HRV+;
 - LAB-HRV+;
- LAB-HRV+;
 - LAB+HRV−; □ - LAB-HRV−
- LAB+HRV−; □ - LAB-HRV−
LA+LR colonisation significantly decreased IL-10 CSC numbers in the ileum
IL-10 CSC numbers in ileum and spleen of the two HRV+ groups were significantly higher than the LAB alone and control group at PID 28. However, IL-10 CSC numbers were significantly lower in ileum of the LAB+HRV+ pigs (17-fold) compared to the LAB-HRV+ pigs, suggesting a regulatory effect of LA+LR on the Tr1/Th2 cytokine IL-10 response to HRV in the ileum. TGF-β CSC numbers were significantly higher in ileum of the two HRV+ groups and in spleen of the LAB+HRV+ group compared to the LAB alone and control group. LA+LR colonisation did not significantly alter IL-6 CSC numbers in any tissue among all groups. IL-4 CSC numbers in all tissues and IL-10 and TGF-β in PBL were low and did not differ significantly among groups at PID 28.
4. Discussion and conclusions
In this study, we delineated the influence of LAB colonization on the development of pro-inflammatory, Th1, Th2 and Treg cytokine responses in neonatal gnotobiotic pigs colonised with a mixture of L. acidophilus and L. reuteri (LA+LR) and infected with HRV. We demonstrated that LA+LR colonisation significantly increased Th1 and maintained Th3 cytokine levels, enhanced early Th2 cytokines and induced a delayed enhancement of IL-10 in serum during HRV infection. LA+LR colonisation enhanced the Th1 (IL-12 and IFN-γ) CSC responses and decreased the pro-inflammatory TNF-α CSC response in systemic sites, and significantly decreased IL-10 CSC numbers in the ileum at 28 days after HRV infection. Our findings in this study clearly suggest that LAB modulate cytokine responses during a viral infection.
LAB alone did not induce significant cytokine responses in serum or intestinal contents of the neonatal gnotobiotic pigs (Figures 1 and 2), indicative of immune tolerance of the intestinal immune system to probiotic bacterial antigens. HRV infection alone initiated serum cytokine responses, as observed by significantly higher concentrations of IFN-α, IFN-γ, IL-12, and IL-10 in the HRV only (and LAB+HRV+) pigs compared to LAB+HRV- pigs, demonstrating that a gastrointestinal virus can induce maturation of the gut immune system in a germ-free animal in the absence of other microorganisms. Our results also confirmed that the intestinal mucosal immune system of neonatal gnotobiotic pigs is able to discriminate and mount immune responses to enteric pathogens (rotaviruses), but concurrently develop immune tolerance to commensal/probiotic bacterial antigens in the intestinal environment. LA+LR colonisation significantly enhanced early Th1 (IFN-γ), Th2 (IL-4) and Th3 (TGF-β on PID 7) serum cytokine responses to HRV infection compared to HRV only pigs. Therefore, LA+LR further promoted maturation of the neonatal immune system of gnotobiotic pigs and augmented a balanced Th1/Th2/Th3 immune response to HRV infection.
Infection with HRV significantly decreased early serum Th3 (TGF-β); however, TGF-β levels were significantly higher in LA+LR colonised than in noncolonised pigs, suggesting a role for probiotics in maintaining regulatory Th3 responses during infection. The role of cytokines in RV disease and protection is currently under investigation. Our previous studies of serum cytokine profiles of neonatal gnotobiotic pigs showed that HRV induced high Th1 (IL-12 and IFN-γ) cytokine responses acutely after HRV infection and proinflammatory cytokines (TNF-α and IL-6) that correlated with the peak of diarrhoea and viremia (Azevedo et al., 2006). Likewise, TNF-α has been associated with the severity of RV diarrhoea in children (Jiang et al., 2003), possibly due to its ability to induce increased Cl− secretion by intestinal epithelial cells (Kandil et al., 1994). Stimulation of adult human blood MNC by HRV induced IL-2 and IFN-γ secretion which augmented NK cell activity and other forms of non-specific CTL activity (Yasukawa et al., 1990). Because pro-inflammatory and Th1 cytokines coincide with the peak of HRV diarrhoea in non-colonised pigs, and colonised pigs had lower pro-inflammatory cytokines (TNF-α, IL-6) than non-colonised HRV infected pigs (based on CSC, Figure 3), upregulation and induction of anti-inflammatory Treg cytokines may be essential to curtail or moderate HRV diarrhoea. LAB may help to maintain the immunological homeostasis during HRV infection by promoting production of regulatory cytokines. Furthermore, in the absence of LAB colonisation, IL-10 CSC numbers were significantly increased in the gut of LAB-HRV+ compared to LAB+HRV+, which may be an alternate mechanism to maintain gut homeostasis.
However, the mixture of L. acidophilus and L. reuteri provided no enhanced protection upon early HRV challenge (Zhang et al., 2008a). Failure of the LA+LR mixture to reduce HRV diarrhoea in gnotobiotic pigs may be due to the following. The interval between colonisation and HRV infection (2 days) was too short to induce gut immune maturation and to restore homeostasis; the LAB strains chosen were ineffective in stimulating gut repair (Boirivant and Strober, 2007); and the regions colonised or concentrations were not optimal to influence HRV infection and diarrhoea. It is also likely that the different immune modulating effect of L. acidophilus (immunostimulatory) antagonized the anti-inflammatory effect of L. reuteri when used together. In vitro studies showed that combining IFN-γ-inducing and non-inducing LAB completely abrogates DC-mediated IFN-γ production by NK cells (Fink et al., 2007). Combining a weakly IL-12 and TNF-α inducing LAB (L. reuteri DSM12246) with a strongly IL-12 and TNF-α inducing LAB (L. acidophilus X37) inhibited the IL-12 and TNF-α-inducing capacity of L. acidophilus X37 in DCs (Zeuthen et al., 2006). Therefore mixing LAB with opposite immune modulating effects may have weakened the effect of each individual LAB strain. These findings in vitro and in vivo have important implications in selecting LAB strains to be used in a mixture of probiotics for therapeutic or immunostimulating purposes.
Nonetheless, LA+LR modulated CSC profiles in the HRV-infected pigs by reducing numbers of pro-inflammatory cytokines (TNF-α) and IL-10 (Treg) CSC in the gut, and significantly enhancing systemic Th1 CSC responses. It is possible that the magnitude of this modulation is below the threshold necessary to have an impact on the disease and that a single LAB strain (i.e. Lactobacillus rhamnosus GG) capable of inducing a stronger Th3 response is necessary to impact rotavirus diarrhoea. Our observation that pigs infected with HRV and colonised with LA+LR had significantly enhanced systemic Th1 CSC responses coincided with the significantly enhanced systemic IFN-γ+CD4+ T cell frequency in the same animals (Yuan et al., 2008). LAB+HRV+ significantly enhanced Th1 and Th2 cytokines in serum and systemic Th1 CSC, suggesting an additive role for LA+LR colonisation with rotavirus infection in enhancement of immune responses to HRV.
The beneficial effects of different probiotic bacteria include improving immune responses against pathogens and correcting adverse immune responses in allergy and autoimmune diseases in humans and animals which bear a normal flora. The neonatal gnotobiotic pig model was used in this study because its gnotobiotic status avoids confounding factors from extraneous bacteria and viruses, making it possible to dissect and clarify the role of lactobacillus in modulating immune responses to human rotavirus (HRV). Because of these characteristics, this model has been used extensively in studies of rotavirus pathogenesis, immunity and vaccines (Yuan and Saif, 2002).
In summary, our findings indicate that the intestinal immune system is largely immunotolerant of LAB colonisation alone; however, LAB can significantly modulate cytokine immune responses induced by HRV infection. Such modulating effects of LAB are potentially beneficial in the development of immunity against rotavirus reinfection as the Th1, Th2 and Treg cytokine responses all were enhanced. Further HRV challenge studies are needed to confirm the immunological significance of the LAB-enhanced cytokine responses in gnotobiotic pigs.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R21AT002524 LY and R01AI033561 to LJS) and Ohio Agricultural Research and Development Center, The Ohio State University (OHOA1208 to LY). We thank Dr. Juliette Hanson and Mr. Rich McCormmick for animal care and veterinary clinical assistance, respectively. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University.
References
- Azevedo MS, Yuan L, Iosef C, Chang KO, Kim Y, Nguyen TV, Saif LJ. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clinical & Diagnostic Laboratory Immunology. 2004;11:12–20. doi: 10.1128/CDLI.11.1.12-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. Journal of Virology. 2006;80:372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M, Strober W. The mechanism of action of probiotics. Current Opinion in Gastroenterology. 2007;23:679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- Christensen HR, Frokiar H, Pestka JJ. Lactobacilli Differentially Modulate Expression of Cytokines and Maturation Surface Markers in Murine Dendritic Cells. Journal of Immunology. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- Fink LN, Zeuthen LH, Christensen HR, Morandi B, Frokiaer H, Ferlazzo G. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. International Immunology. 2007;19:1319–1327. doi: 10.1093/intimm/dxm103. [DOI] [PubMed] [Google Scholar]
- Gackowska L, Michalkiewicz J, Krotkiewski M, Helmin-Basa A, Kubiszewska I, Dzierzanowska D. Combined effect of different lactic acid bacteria strains on the mode of cytokines pattern expression in human peripheral blood mononuclear cells. J Physiol Pharmacol. 2006;57(Suppl 9):13–21. [PubMed] [Google Scholar]
- Glass M. Master Thesis Thesis. The Ohio State University; Wooster: 2003. Effects of Lacobacillus acidophilus and Lactobacillus reuteri on bovine Cryptosporidium parvum and C. hominis in vitro and in vivo. [Google Scholar]
- Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. Journal of Pediatric Gastroenterology & Nutrition. 1997;25:516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Hong T, Xing J, Li L, Tyson JJ. A mathematical model for the reciprocal differentiation of T helper 17 cells and induced regulatory T cells. PLoS Computational Biology. 2011;7:e1002122. doi: 10.1371/journal.pcbi.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50(Suppl 3):III54–59. doi: 10.1136/gut.50.suppl_3.iii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Snipes-Magaldi L, Dennehy P, Keyserling H, Holman RC, Bresee J, Gentsch J, Glass RI. Cytokines as mediators for or effectors against rotavirus disease in children. Clinical & Diagnostic Laboratory Immunology. 2003;10:995–1001. doi: 10.1128/CDLI.10.6.995-1001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil HM, Berschneider HM, Argenzio RA. Tumour necrosis factor alpha changes porcine intestinal ion transport through a paracrine mechanism involving prostaglandins. Gut. 1994;35:934–940. doi: 10.1136/gut.35.7.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. American Journal of Respiratory and Critical Care Medicine. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li G, Wen K, Bui T, Cao D, Zhang Y, Yuan L. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunology. 2010;23:135–149. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau P, Flourie B, Pochart P, Chastang C, Desjeux JF, Rambaud JC. Effect of the microbial lactase (EC 3.2.1.23) activity in yoghurt on the intestinal absorption of lactose: an in vivo study in lactase-deficient humans. British Journal of Nutrition. 1990;64:71–79. doi: 10.1079/bjn19900010. [DOI] [PubMed] [Google Scholar]
- Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Current Pharmaceutical Design. 2003;9:175–191. doi: 10.2174/1381612033392224. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Bohl EH, Kohler EM. Procurement and Maintenance of Germ-Free Seine for Microbiological Investigations. Applied Microbiology. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. Journal of Nutrition. 2000;130:396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Pedersen P, Tvede M, Weyrehter H, Valerius NH, Paerregaard A. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatric Infectious Disease Journal. 2002;21:411–416. doi: 10.1097/00006454-200205000-00012. [DOI] [PubMed] [Google Scholar]
- Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. Journal of Dairy Science. 2001;84:319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari T. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatrica. 1997;86:460–465. doi: 10.1111/j.1651-2227.1997.tb08913.x. [DOI] [PubMed] [Google Scholar]
- Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Advanced Drug Delivery Reviews. 1998;34:235. doi: 10.1016/s0169-409x(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Timmerman HM, Niers LE, Ridwan BU, Koning CJ, Mulder L, Akkermans LM, Rombouts FM, Rijkers GT. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr. 2007;26:450–459. doi: 10.1016/j.clnu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- VanCott JL, Brim TA, Simkins RA, Saif LJ. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. Journal of Immunology. 1993;150:3990–4000. [PubMed] [Google Scholar]
- Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. Journal of General Virology. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes & Infection. 2001;3:947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- Wen K, Azevedo MS, Gonzalez A, Zhang W, Saif LJ, Li G, Yousef A, Yuan L. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Veterinary Immunology & Immunopathology. 2009;127:304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen K, Li G, Zhang W, Azevedo MS, Saif LJ, Liu F, Bui T, Yousef A, Yuan L. Development of gammadelta T cell subset responses in gnotobiotic pigs infected with human rotaviruses and colonized with probiotic lactobacilli. Veterinary Immunology & Immunopathology. 2011;141:267–275. doi: 10.1016/j.vetimm.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa M, Nakagomi O, Kobayashi Y. Rotavirus induces proliferative response and augments non-specific cytotoxic activity of lymphocytes in humans. Clinical & Experimental Immunology. 1990;80:49–55. doi: 10.1111/j.1365-2249.1990.tb06440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Veterinary Immunology & Immunopathology. 2002;87:147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. Journal of Virology. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Wen K, Azevedo MSP, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-[gamma] producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26:3322–3331. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen LH, Christensen HR, Frokiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clinical & Vaccine Immunology: CVI. 2006;13:365–375. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Veterinary Immunology & Immunopathology. 2008a;122:175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MSP, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008b;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Veterinary Immunology & Immunopathology. 2008c;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]