Abstract
Cocaine-induced neuroadaptation of stress-related circuitry and increased access to cocaine each putatively contribute to the transition from cocaine use to cocaine dependence. The present study tested the hypothesis that rats receiving extended versus brief daily access to cocaine would exhibit regional differences in levels of the stress-regulatory neuropeptide corticotropin-releasing factor (CRF). A secondary goal was to explore how CRF levels change in relation to the time since cocaine self-administration. Male Wistar rats acquired operant self-administration of cocaine and were assigned to receive daily long access (6 hours/day, LgA, n = 20) or short access (1 hour/day, ShA, n = 18) to intravenous cocaine self-administration (fixed ratio 1, ~0.50 mg/kg/infusion). After at least 3 weeks, tissue CRF immunoreactivity was measured at one of three timepoints: pre-session, post-session or 3 hours post-session. LgA, but not ShA, rats showed increased total session and first-hour cocaine intake. CRF immunoreactivity increased within the dorsal raphe (DR) and basolateral, but not central, nucleus of the amygdala (BLA, CeA) of ShA rats from pre-session to 3 hours post-session. In LgA rats, CRF immunoreactivity increased from pre-session to 3 hours post-session within the CeA and DR but tended to decrease in the BLA. LgA rats showed higher CRF levels than ShA rats in the DR and, pre-session, in the BLA. Thus, voluntary cocaine intake engages stress-regulatory CRF systems of the DR and amygdala. Increased availability of cocaine promotes greater tissue CRF levels in these extrahypothalamic brain regions, changes associated here with a model of cocaine dependence.
Keywords: Amygdala, cocaine addiction or dependence, CRF or CRH or corticotropin-releasing factor or corticotropin-releasing hormone, operant intravenous drug self-administration, stress, neuropeptide
INTRODUCTION
Cocaine dependence is a chronic disorder of compulsive use and drug seeking. After stopping cocaine intake, stress-like abstinence symptoms emerge (Gawin & Kleber 1986) that may drive cocaine use via negative reinforcement, predicting relapse (Kampman et al. 1998) and poor outcome (Kasarabada et al. 1998).
Corticotropin-releasing factor (CRF), the hypothalamic peptide that elicits pituitary adrenocorticotropic hormone release, is also an extrahypothalamic mediator of behavioral and autonomic stress responses (Zorrilla & Koob 2004) that has been implicated in cocaine’s actions. Central administration of CRF antisera or receptor antagonists blocked cocaine-induced anxiety-like behavior and locomotor activity (Sarnyai et al. 1992, 1995; Lu et al. 2003) and cocaine withdrawal–induced anxiety-like behavior (Sarnyai et al. 1995; Basso et al. 1999). Supporting the motivational relevance of CRF systems for cocaine dependence, intracranial CRF administration reinstated cocaine-seeking behavior (Erb et al. 2006b; Brown et al. 2009). Conversely, CRF1 antagonists reduced acquisition of cocaine-conditioned place preference (Lu et al. 2003), footshock-, cocaine priming– or drug cue–associated cocaine-seeking behavior (Shaham et al. 1998; Gurkovskaya & Goeders 2001; Przegalinski et al. 2005), stress-induced renewal of cocaine-conditioned place preference (Lu, Liu & Ceng 2001) and intravenous cocaine self-administration (Goeders & Guerin 2000; Specio et al. 2008).
Cocaine-induced adaptation in CRF circuitry may underlie both cocaine withdrawal symptoms and the transition from uncomplicated cocaine use to cocaine dependence (Koob 2008). Repeated cocaine administration cross-sensitizes locomotor and amygdala c-fos responses to intracerebroventricular CRF administration (Erb, Funk & Le 2003, 2005; Erb, Kayyali & Romero 2006a). Cocaine-induced facilitation of long-term potentiation in the central amygdala (CeA) is CRF-dependent (Pollandt et al. 2006; Fu et al. 2007). Functional evidence of cocaine-induced CRF system adaptation also is seen in rats receiving daily long access (6 hours/day, LgA) to cocaine self-administration, as compared with rats receiving short access (1 hours/day, ShA). LgA rats develop signs of cocaine dependence, including increased drug-taking and drug-seeking behavior, upward shifts in the cocaine dose–response function, impaired brain reward function upon drug withdrawal, resistance to extinction and facilitated reinstatement of drug-seeking/taking behavior (Ahmed & Koob 1998, 1999; Ahmed et al. 2002; Wee, Specio & Koob 2007). Stressors and CRF administration more effectively reinstate cocaine-seeking behavior in LgA rats than in ShA rats (Mantsch et al. 2008). Moreover, CRF1 antagonists reduce cocaine self-administration more effectively in LgA rats than in ShA rats (Specio et al. 2008).
The present study therefore tested the hypothesis that LgA rats exhibit regional differences in CRF tissue content relative to ShA rats in the amygdala and dorsal raphe (DR), CRF-rich structures that subserve mood and arousal (Cummings et al. 1983; Palkovits, Brownstein & Vale 1985). Passive cocaine administration is known to increase CeA CRF release, depleting CRF tissue content (Richter et al. 1995; Maj et al. 2003), but the effects of self-administered cocaine are unknown. Therefore, cocaine self-administration was hypothesized here to alter tissue CRF content in the CeA, with greater changes expected in LgA rats. Withdrawal from cocaine also increases CeA CRF release (Richter & Weiss 1999) concurrent with compensatory CRF mRNA synthesis (Zhou et al. 2003; Erb et al. 2004). Thus, the present study also explored the timing of changes in CRF tissue content relative to drug self-administration.
MATERIALS AND METHODS
Subjects
Male Wistar rats (n = 38, 250–300 g) were obtained from Charles River (Kingston, NY and Raleigh, NC) and group-housed (2–3/cage) in wire-topped, plastic cages (19 × 10.5 × 8 inches) in a 12-hour/12-hour reverse light cycle (08:00 h lights off), humidity- (60%) and temperature-controlled (22°C) vivarium. All behavioral testing occurred during the dark cycle. Food and water were freely available unless otherwise specified. Experimental procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication number 85-23, revised 1996) and the ‘Principles of laboratory animal care’ and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Cocaine hydrochloride (National Institute on Drug Abuse, Rockville, MD, USA) was dissolved in sterile physiological saline to ~0.5 mg/kg/infusion (~0.2–0.25 mg depending on body weight infused in a volume of 0.1 ml over 4 seconds).
Apparatus
Behavioral training was conducted in operant-conditioning chambers (Coulbourn Instruments, Allen-town, PA, USA) housed in sound-attenuating cubicles. Chambers were equipped with two retractable levers, a food pellet trough and a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT, USA) delivering 0.1 ml of cocaine solution over 4 seconds via Tygon tubing attached to liquid swivels (Model 375, Instech Laboratories, Plymouth Meeting, VA, USA). A time-out period (20 seconds) followed each infusion, during which a cue light above the active lever was illuminated. At the start of a session, two levers were presented. Responding on the active lever resulted in reinforcement, whereas responding at the inactive lever had no scheduled consequences but was recorded. Sessions were controlled by a personal computer with a custom interface and software.
Self-administration procedure
To establish operant behavior, rats were initially food-restricted (15 g/rat/day) and allowed to press a lever to obtain food pellets (45 mg Formula A/I, Research Diets, New Brunswick, NJ, USA) under a fixed-ratio 1 schedule in 30-minute sessions. Food training sessions were performed twice daily for 5 days, across which the post-reinforcement time-out duration was gradually increased (1, 5, 10 and 20 seconds). After animals reached the 20-second time-out duration, food was made available ad libitum for the remainder of the study. The rats were then implanted with an indwelling catheter into the right jugular vein under 1–3% isoflurane anesthesia as described previously (Caine et al. 1993). Catheters were flushed daily with 0.2 ml of sterile antibiotic solution containing Timentin (100 mg/ml; SmithKline Beecham Pharmaceuticals, Philadelphia, PA, USA) and heparin (30 USP units/ml). Catheter patency was checked by briefly aspirating blood from the catheter or using an ultra short-acting barbiturate, Brevital® (JPH Pharmaceutical, Rochester, MI, USA) (methohexital sodium, 10 mg/ml, 2 mg/rat).
After recovery from surgery, rats self-administered 0.50 mg/kg per infusion of cocaine in daily 1-hour sessions under a fixed-ratio 1 schedule for a maximum of 11 days. Following these baseline sessions, animals were separated into two groups balanced for body weight and cocaine intake. The session length was kept at 1 hour for one group (short access, ShA, n = 18) and was increased to 6 hours for the other group (long access, LgA, n = 20). Rats received 21–27 sessions under the different access conditions and then were randomly assigned to be sacri-ficed immediately at one of three timepoints: (1) the time at which the next scheduled self-administration session would otherwise occur (‘pre-session’); (2) immediately following the self-administration session (‘post-session’); or (3) 3 hours after completion of the self-administration session (‘3 hours post-session’). In the 3 hours post-session condition, rats remained in the self-administration chambers, with the levers retracted until the time of sacrifice. Sessions were scheduled so that all rats at a given timepoint were sacrificed within 1 hour of one another, to reduce time-of-day effects.
Peptide acid extraction and CRF radioimmunoassay
Rats were decapitated; the brains were quickly removed and sliced coronally (1–2 mm sections) in a brain matrix. Punches containing the CeA, basolateral amygdala (BLA) and DR were collected on an ice-cold stage. Brain regions were ultrasonicated in 20 vol ice-cold 1 N HCl. A 20 μl aliquot was removed for determination of protein content using a modification of the Bradford method (Bio-Rad, Hercules, CA, USA). The remaining homogenate was boiled for 10 minutes and immediately centrifuged (20 minutes, 7500 rpm, 4°C). The supernatant was removed, lyophilized, reconstituted with 10 μl of 1 N NaOH and diluted to a final volume of 1 ml in gelatin assay buffer (0.15 M K2HPO4, 0.2 mM ascorbic acid, 0.1% gelatin, pH 7.5). Tissue CRF-like immunoreactivity content was quantified with a sensitive and specific solid-phase radioimmunoassay (Zorrilla, Valdez & Weiss 2001). Immulon-4 96-well plates (Dynatech, Chantilly, VA, USA) were coated with protein A/G (1 μg/100 μl 1 M NaHCO3/well, pH 9.0; Calbiochem, La Jolla, CA) over-night. Plates were rinsed with wash buffer (0.15 M K2HPO4 supplemented with 0.2 mM ascorbic acid and 0.1% Tween-20, pH 7.5) to dislodge loose protein A/G. Wells were incubated for 48 hours at 4°C with 50 μl anti-CRF serum (rC68, generously provided by W. Vale, The Salk Institute, La Jolla, CA, USA) at a titer of 1:400 000 in gelatin assay buffer. After three rinses to dislodge loose antibody, 50 μl of dilute sample (in duplicate) or standard (0.03–100 ng/ml, in quadruplicate) were incubated overnight at 4°C. Following incubation, 50 μl of [125I-Tyr°]r/hCRF (~10 000 cpm/50 μl; New England Nuclear, Boston, MA, USA) was added to each well and incubated for an additional 24 hours at 4°C. Wells were rinsed, blotted dry and separated. Residual radioactivity was counted by a gamma counter. Sensitivity of the assay was approximately 0.3 fmol/well, and inter- and intra-assay coefficients of variation at the ED50 dose ranged from 5% to 10%. A four-parameter logistic curve fit model was used for interpolation of the standard curves (Sigma-Plot 9.0, Systat Software, Point Richmond, CA, USA).
Data analysis
Data were expressed as first hour and total session cocaine intake (mg/kg). Subjects and tissue samples were run in two independent cohorts, balanced for access condition and timepoint of sacrifice. To control for main effects of cohort (e.g. cohort-to-cohort or assay-to-assay variability), cohort was a covariate in all analyses. To confirm the expected access-related changes in cocaine intake during the escalation period, a repeated-measures two-way analysis of covariance (ANCOVA) was used (access × session), with cohort as the covariate, access a between-subjects factor and session a within-subject factor. The effects of cocaine self-administration access on regional CRF peptide immunoreactivity were evaluated using two-way factorial ANCOVA, with cohort as a covariate and access and timepoint as between-subject factors. Following significant omnibus tests (with the α-level of significance set at 0.05), post hoc comparisons were conducted using Fisher’s protected least-significant difference (LSD) tests (Levin, Serlin & Seaman 1994). No significant interactions of cohort with access, session or timepoint were observed, indicating that effects of these variables did not differ reliably between cohorts. The statistical package used was Systat 12.0 (Systat Software Inc., Chicago, IL, USA).
RESULTS
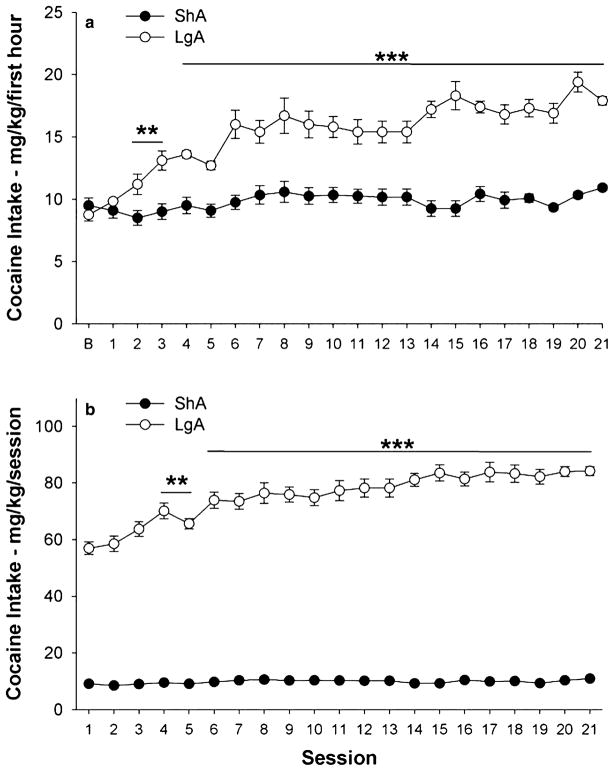
Figure 1 illustrates cocaine intake (mg/kg) for ShA and LgA groups during the first hour and entire session. As expected, a significant Access × × Session interaction [F(21,735) = 5.48, P < 0.0001] confirmed that LgA rats, but not ShA rats, showed significant escalation of first hour cocaine self-administration beginning with session 2 (Fig. 1a). As a result, LgA rats showed greater first hour cocaine self-administration than ShA rats by the second session (P < 0.01), a difference that progressively increased through the end of the escalation period (P < 0.001).
Figure 1.
Self-administration of cocaine by rats under a fixed-ratio schedule during the escalation period. Data from the first hour of sessions (a) and from the entire session (b). The data represent least squares mean (±SEM) cocaine intake adjusted for body weight (mg/kg). Filled symbols represent data for rats in 1-hour short-access sessions (ShA, n = 18). Open symbols represent data for rats in 6-hour long-access sessions (LgA, n = 20). Note that error bars in panel b for ShA rats are smaller than the symbol. *P < 0.05, **P < 0.01, ***P < 0.001 versus ShA group and versus baseline (‘B’) first hour intake (a) or session 1 intake (b) (Fisher’s protected LSD tests)
Total session cocaine self-administration of LgA (but not ShA) rats also increased relative to their initial intake, evident beginning with session 4 [Access × Session interaction: F(20,700) = 6.78, P < 0.0001]. Accordingly, mean cocaine intake of LgA rats, but not ShA rats, during week 3 significantly exceeded their mean week 1 intake (P < 0.05, see Fig. 1b).
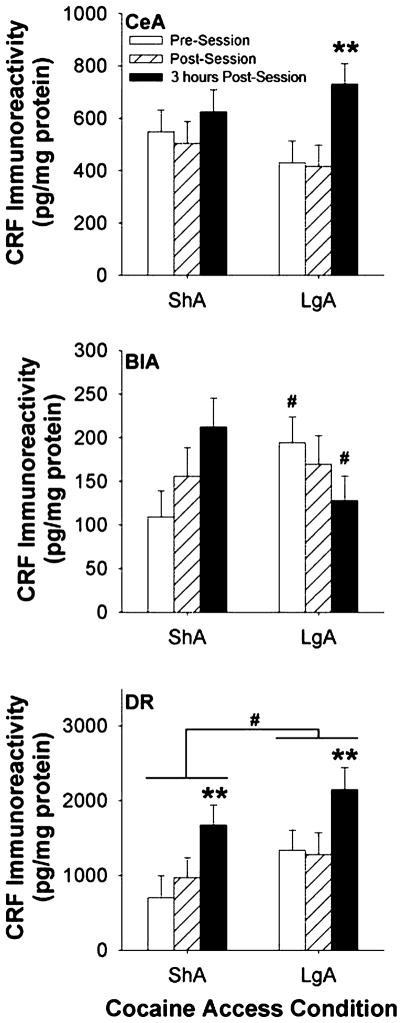
As shown in Fig. 2 (top panel), CRF immunoreactivity in the CeA significantly changed in relation to the time from the cocaine self-administration session [Time: F(2,30) = 4.27, P = 0.02]. A significant main effect of Access was not observed [F(1,30) = 0.25, P = 0.62], indicating that CeA CRF levels did not consistently differ between ShA and LgA groups. Pairwise comparisons showed that CRF tissue content in the CeA was significantly greater 3 hours post-session than it was immediately prior to or following the cocaine self-administration session. The post-session increase tended to be more pronounced in LgA rats, which exhibited a significant Time effect when considered alone [F(2,15) = 4.59, P = 0.03; P = 0.01 versus post- and pre-session time points], unlike ShA rats [F(2,14) = 0.84, P = 0.45]. However, the Access × Time point interaction did not reach significance [F(2,30) = 1.10, P = 0.34].
Figure 2.
Regional brain corticotropin-releasing factor (CRF) levels in rats receiving daily short-access (1 hour/day, ShA, n = 18) or long-access (6 hours/day, LgA, n = 20) to intravenous cocaine self-administration. Panels show CRF concentrations in the (top) central nucleus of the amygdala (CeA), (middle) basolateral nucleus of the amygdala (BlA) or (bottom) dorsal raphe (DR). The data represent least squares mean (±SEM) CRF immunoreactivity normalized for total protein content (pg/mg) across the following 3 timepoints of observation (n = 6–7 per timepoint): the time of day the self-administration would otherwise occur (pre-session), immediately following the completion of the self-administration session (post-session), or 3 hours following the completion of the self-administration session (3 hours post). Note scale differences across regions. **P < 0.01 versus other timepoints, # P < 0.05 versus respective ShA group (Fisher’s protected LSD tests)
As shown in Fig. 2 (middle panel), CRF immunoreactivity in the BLA differentially changed across timepoints as a function of access condition [Access × Time point: F(2,27) = 3.90, P = 0.03]. Pairwise comparisons showed that CRF levels in the BLA were higher in LgA rats than ShA rats at the onset of self-administration sessions but lower in LgA rats than in ShA rats 3 hours post-session (P = 0.05). The latter difference partly reflected that ShA rats, but not LgA rats, showed a significant increase in CRF immunoreactivity from pre-session to 3 hours post-session (P < 0.05). Main effects of Time and Access were not significant [F(2,27) = 0.18, P = 0.83 and F(1,27) = 0.03, P = 0.86, respectively].
As shown in Fig. 2 (bottom panel), CRF immunoreactivity in the DR differed across time points [F(2,26) = 6.11, P = 0.007] as well as across access conditions [F(1,26) = 4.07, P = 0.05]. Post hoc comparisons showed that CRF immunoreactivity was higher 3 hours post-session in rats of both access conditions than at pre-session or immediately post-session (P < 0.01), and that LgA rats exhibited higher CRF immunoreactivity than ShA rats (P = 0.05). No significant Access × Time point interaction was observed [F(2,26) = 0.16, P = 0.85].
DISCUSSION
The results demonstrate that self-administration of cocaine alters levels of the stress-regulatory peptide CRF in the amygdala and DR of rats differentially in relation to cocaine availability. In rats with daily short access to cocaine (ShA), CRF immunoreactivity increased from pre-session to 3 hours post-session within the DR and BLA, but not CeA. In rats with daily extended access to cocaine (LgA), CRF immunoreactivity increased from pre-session to 3 hours post-session within the CeA and DR, but tended to decrease in the BLA. Thus, relative to ShA rats, LgA rats showed larger increases in CRF content in the CeA and a trend for decreasing, rather than increasing, CRF content in the BLA after cocaine intake. Moreover, LgA rats, a putative model of compulsive cocaine self-administration, showed higher CRF content in the DR at all timepoints and increased pre-session CRF levels in the BLA as compared with ShA rats.
In humans, an increase in cocaine availability can precipitate a transition to increased cocaine use and ‘binge-like’ patterns of intake, with development of dependence and increased withdrawal symptom severity (Gawin & Ellinwood 1989). Toward identifying neuro-adaptations related to cocaine addiction, as opposed to uncomplicated, limited access drug use, the present results show the presence of differential activity of CRF systems in the LgA group receiving extended access to cocaine. Here, tissue CRF peptide content was higher in the DR and also, pre-session, in the BLA in LgA rats as compared with ShA rats. Higher CRF tissue content, which represents both intracellular and extracellular CRF, may represent an increased releasable pool of peptide, perhaps because of increased peptide synthesis. Consistent with this possibility, stressors, which elicit CRF release, reinstate cocaine-seeking behavior more effectively in LgA rats than ShA rats, and CRF1 antagonists more effectively reduce cocaine self-administration in LgA rats than in ShA rats (Mantsch et al. 2008; Specio et al. 2008). Future work comparing the mRNA synthesis and dynamic secretion of CRF peptide (e.g. via in vivo microdialysis) between cocaine access groups may better clarify the molecular basis for and significance of differences in peptide tissue content.
The DR is a serotonin-rich nucleus that widely innervates the forebrain, and increased DR serotonin transmission has traditionally been associated with increased anxiety-like behavior (Gingrich & Hen 2001). The higher CRF concentrations seen in LgA rats as compared with ShA rats in the DR could represent greater local synthesis or distribution in CRF-positive afferents in LgA rats, because CRF is present in both cell bodies and terminals in the raphe (Cummings et al. 1983; Merchenthaler 1984; Palkovits et al. 1985; Chappell et al. 1986; Austin, Rhodes & Lewis 1997; Valentino, Liouterman & Van Bockstaele 2001). In vitro, CRF increases firing rates of a ventral subpopulation of DR neurons that project primarily to basal forebrain corticolimbic structures. Activation of this CRF-responsive subpopulation is sensitized after prior exposure to stress (Lowry et al. 2000). In vivo, intraraphe CRF produces biphasic effects on neuronal firing, with inhibition at low doses and excitation at high doses (Price et al. 1998; Kirby, Rice & Valentino 2000; Price & Lucki 2001). Both CRF receptor subtypes are expressed in the DR (Chalmers, Lovenberg & De Souza 1995; Van et al. 2000; Waselus et al. 2009), and it has been proposed that the biphasic effects of CRF represent a differential recruitment of CRF1 versus CRF2 receptors in relation to CRF concentration (Waselus et al. 2009). Thus, chronic cocaine self-administration, by yielding a greater releasable pool of CRF in the DR, may yield more serotonergic signaling and, perhaps thereby, more stress sensitivity and susceptibility to relapse in LgA rats as compared with ShA rats.
The BLA, which subserves both unconditioned and conditioned stress-related emotional behavior, is rich in CRF1 receptors but exhibits very little CRF synthesis under basal conditions. Rather, BLA CRF1 receptors are putatively activated by CeA-derived CRF via volume transmission, as occurs following stress for example (Roozendaal et al. 2002). Microinfusion of CRF receptor agonists into the BLA elicits anxiogenic-like behavior in the social interaction test via CRF receptors (Sajdyk et al. 1999; Sajdyk & Gehlert 2000). Stress-induced consolidation of aversively motivated learning also is mediated by BLA CRF1 receptors (Roozendaal et al. 2002). Moreover, repeated microinfusion of subthreshold doses of CRF and related peptides sensitizes panic-like responses to otherwise innocuous stimuli (Sajdyk et al. 1999; Sajdyk & Gehlert 2000). Conversely, chronic administration of mood stabilizers (Gilmor et al. 2003), antidepressants (Aubry, Pozzoli & Vale 1999) or anxiolytics (Skelton et al. 2000) downregulates BLA CRF1 expression in parallel with the therapeutic time course of these agents. In view of these observations, it is notable that repeated passive cocaine treatment transiently decreases the number of BLA CRF1-like binding sites in rat (Ambrosio, Sharpe & Pilotte 1997), an effect that may reflect compensatory downregulation in response to the increased amygdalar CRF release associated with repeated cocaine exposure (Richter et al. 1995). Future studies that examine the effects of voluntary cocaine self-administration on CRF receptor levels would clearly complement the present results. The present finding of increased pre-session CRF immunoreactivity in the BLA of LgA rats as compared with ShA rats lends support to the hypothesis that chronic cocaine exposure facilitates BLA CRF1–mediated neurotransmission, with possible long-term neuro-adaptive consequences for emotional behavior and learning. Insofar as levels in LgA rats were greater than those in ShA rats at baseline and not following cocaine intake, the results in LgA rats are potentially consistent with a withdrawal-induced elevation in BLA CRF tissue content. An alternative possibility is that differential classical conditioning may underlie differences between the access groups. That is, a single experimenter-administered dose of cocaine is known to increase amygdala CRF content (Sarnyai et al. 1993). The greater history of cocaine self-administration in the LgA group, as compared with ShA group, may thereby yield greater conditioned responses of amygdala CRF systems at the scheduled self-administration time (‘pre-session’).
A limitation of the present study is that a cocaine-naive comparison group was not included. Thus, it cannot be ruled out for some ‘null’ findings that both ShA and LgA rats may exhibit altered CRF levels as compared with drug-naive subjects. Also, where group or time differences are present, it cannot be stipulated which concentrations deviate from a cocaine-naive group in a normative sense. A comparison group was not included in part because most previous behavioral comparisons of LgA versus ShA groups have not included cocaine-naive subjects and because it is a complex question of what constitutes an appropriate cocaine-naive comparison group (e.g. a cocaine-naive group without self-administration history versus one with ShA to a non-cocaine reinforcer versus one with LgA to a non-cocaine reinforcer versus some combination thereof). Also, the focus of the present study was to identify regions and timepoints at which LgA and ShA groups differed from one another. Such group differences, irrespective of their comparison to cocaine-naive subjects, might be key to the motivational differences seen between groups to self-administer cocaine. For example, the LgA group might have CRF tissue content that is higher than (e.g. greater withdrawal/exposure-induced CRF synthesis), lower than (e.g. greater withdrawal/exposure-induced CRF release and depletion) or equivalent to a cocaine-naive group (e.g. loss of cocaine’s aversive stimulus properties). Yet each scenario might be germane to the motivational difference between LgA versus ShA groups if the LgA group differs from the ShA group.
Increased immunoreactivity was not observed in the CeA of LgA rats as compared with ShA rats at any time-point. This negative result is consistent with studies that observed changes in CeA CRF peptide or mRNA levels only after acute, and not repeated, cocaine exposure (Maj et al. 2003; Zhou et al. 2003). Increased extracellular CeA CRF immunoreactivity was observed previously using in vivo microdialaysis ~12 hours following ‘binge-like’ (12-hour session) cocaine access (Richter & Weiss 1999), but that study reflected interstitial (rather than whole tissue) CRF levels, and did not involve a repeated history of LgA cocaine exposure. We also previously observed reduced CRF tissue content in whole amygdala ~24 hours following two ‘binge’ cocaine session (Zorrilla et al. 2001), but that finding again did not follow a repeated history of LgA cocaine exposure and reflected concentrations in whole amygdala rather than CeA only. A final key caveat is that it cannot be ruled out that ShA and LgA rats may both exhibit altered CRF levels in the CeA as compared with drug-naive subjects, since each of the above studies involved comparison to cocaine-naive subjects rather than LgA versus ShA comparisons.
A final result was that CRF levels in the CeA and DR were higher 3 hours after the cocaine self-administration session than immediately before or after the session. These results extend upon studies that showed that passive exposure to cocaine acutely activates amygdalar CRF systems (Sarnyai et al. 1993; Zhou et al. 1996; Maj et al. 2003) by finding that voluntary cocaine self-administration also results in CRF system activation, and that the DR CRF system is involved as well. One interpretation of these data is that the delayed rise in CRF concentrations reflects the time needed to complete cocaine exposure-induced CRF synthesis (Zhou et al. 1996; Maj et al. 2003) or, alternatively, a rapid rise in CRF synthesis secondary to withdrawal from cocaine access (Zhou et al. 2003; Erb et al. 2004; Rudoy, Reyes & Van Bockstaele 2009). An alternative interpretation, however, is that the results reflect depleted levels of CRF both pre- and post-session—with levels normalizing by 3 hours post-session. Under the latter interpretation, the lower levels observed pre-session may reflect depletion secondary to withdrawal from the previous day’s cocaine self-administration session. Indeed, a microdialysis study found that after completion of binge-like cocaine self-administration, extracellular CRF levels did not significantly increase until 5 hours post-session and did not peak until at least 11–12 hours following the completion of cocaine self-administration (Richter & Weiss 1999). In that study, cocaine self-administration led to decreased CRF release as compared with cocaine-naive controls (Richter & Weiss 1999), an effect that might account for restoration of CRF tissue content by 3 hours post-session in the present study. Future comparison of CRF tissue content in drug-taking subjects to cocaine-naive controls would help determine whether the changing CRF levels seen here represent absolute elevations (e.g. 3 hours post-session) or depletions (e.g. pre- and post-session).
In summary, the present results confirm that voluntary cocaine self-administration, and not only passive cocaine exposure, dynamically engages stress-regulatory CRF systems of the amygdala. Even more novel, the results demonstrate recruitment of DR CRF systems by cocaine intake. Finally, it is well established that compared with rats receiving daily, short access (ShA) to cocaine, rats self-administering cocaine under LgA conditions show persistently increased cocaine intake, increased progressive-ratio responding, an upward shift in the dose–response function for the reinforcing effects of self-administered cocaine, withdrawal-like deficits in brain reward function that are relieved by renewed access to cocaine, resistance to extinction of cocaine-seeking behavior, and increased vulnerability to relapse to cocaine-seeking and use (Ahmed & Koob 1998, 1999; Ahmed et al. 2002; Paterson & Markou 2003; Wee et al. 2007). Here, cocaine dependence, as modeled in the comparison of LgA rats to ShA rats, was associated with higher levels of CRF in the amygdala in time-dependent fashion and in the DR at all timepoints. The results support the hypothesis that extrahypothalamic CRF systems participate in the transition from uncomplicated drug use to cocaine addiction.
Acknowledgments
We thank Mike Arends for editorial assistance, Mary Gichuhi for administrative assistance, and Jeanette Helfers for technical assistance. The authors thank Dr Wylie Vale (The Salk Institute) for generously providing the CRF antiserum. This publication was supported by grants from the National Institute on Drug Abuse (DA08467, DA04398) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK26741), and the Pearson Center for Alcoholism and Addiction Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Sharpe LG, Pilotte NS. Regional binding to corticotropin releasing factor receptors in brain of rats exposed to chronic cocaine and cocaine withdrawal. Synapse. 1997;25:272–276. doi: 10.1002/(SICI)1098-2396(199703)25:3<272::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Aubry JM, Pozzoli G, Vale WW. Chronic treatment with the antidepressant amitriptyline decreases CRF-R1 receptor mRNA levels in the rat amygdala. Neurosci Lett. 1999;266:197–200. doi: 10.1016/s0304-3940(99)00295-5. [DOI] [PubMed] [Google Scholar]
- Austin MC, Rhodes JL, Lewis DA. Differential distribution of corticotropin-releasing hormone immunoreactive axons in monoaminergic nuclei of the human brainstem. Neuropsychopharmacology. 1997;17:326–341. doi: 10.1016/S0893-133X(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioural Neuroscience: A Practical Approach. Vol. 2. Oxford: IRL Press; 1993. pp. 117–143. [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Funk D, Borkowski S, Watson SJ, Akil H. Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroscience. 2004;123:1003–1009. doi: 10.1016/j.neuroscience.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Prior, repeated exposure to cocaine potentiates locomotor responsivity to central injections of corticotropin-releasing factor (CRF) in rats. Psychopharmacology (Berl) 2003;170:383–389. doi: 10.1007/s00213-003-1556-1. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Cocaine pre-exposure enhances CRF-induced expression of c-fos mRNA in the central nucleus of the amygdala: an effect that parallels the effects of cocaine pre-exposure on CRF-induced locomotor activity. Neurosci Lett. 2005;383:209–214. doi: 10.1016/j.neulet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Erb S, Kayyali H, Romero K. A study of the lasting effects of cocaine pre-exposure on anxiety-like behaviors under baseline conditions and in response to central injections of corticotropin-releasing factor. Pharmacol Biochem Behav. 2006a;85:206–213. doi: 10.1016/j.pbb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 2006b;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, Gallagher JP, Shinnick-Gallagher P. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J Neurophysiol. 2007;97:937–941. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine dependence. Annu Rev Med. 1989;40:149–161. doi: 10.1146/annurev.me.40.020189.001053. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Skelton KH, Nemeroff CB, Owens MJ. The effects of chronic treatment with the mood stabilizers valproic acid and lithium on corticotropin-releasing factor neuronal systems. J Pharmacol Exp Ther. 2003;305:434–439. doi: 10.1124/jpet.102.045419. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Gurkovskaya O, Goeders NE. Effects of CP-154,526 on responding during extinction from cocaine self-administration in rats. Eur J Pharmacol. 2001;432:53–56. doi: 10.1016/s0014-2999(01)01465-0. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kasarabada ND, Anglin MD, Khalsa-Denison E, Paredes A. Variations in psychosocial functioning associated with patterns of progression in cocaine-dependent men. Addict Behav. 1998;23:179–189. doi: 10.1016/s0306-4603(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JR, Serlin RC, Seaman MA. A controlled, powerful multiple-comparison strategy for several situations. Psychol Bull. 1994;115:153–159. [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol. 2001;415:203–208. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Maj M, Turchan J, Smialowska M, Przewlocka B. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003;37:105–110. doi: 10.1016/s0143-4179(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5 (Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Vale W. Distribution of corticotropin-releasing factor in rat brain. Fed Proc. 1985;44:215–219. [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegalinski E, Filip M, Frankowska M, Zaniewska M, Papla I. Effects of CP 154,526, a CRF1 receptor antagonist, on behavioral responses to cocaine in rats. Neuropeptides. 2005;39:525–533. doi: 10.1016/j.npep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Richter RM, Pich EM, Koob GF, Weiss F. Sensitization of cocaine-stimulated increase in extracellular levels of corticotropin-releasing factor from the rat amygdala after repeated administration as determined by intracranial microdialysis. Neurosci Lett. 1995;187:169–172. doi: 10.1016/0304-3940(95)11365-4. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy CA, Reyes AR, Van Bockstaele EJ. Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal. Neuropsychopharmacology. 2009;34:1135–1148. doi: 10.1038/npp.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Gehlert DR. Astressin, a corticotropin releasing factor antagonist, reverses the anxiogenic effects of urocortin when administered into the basolateral amygdala. Brain Res. 2000;877:226–234. doi: 10.1016/s0006-8993(00)02638-x. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res. 1993;616:315–319. doi: 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Hohn J, Szabo G, Penke B. Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci. 1992;51:2019–2024. doi: 10.1016/0024-3205(92)90151-e. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000;20:1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- Van PK, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res. 2003;114:73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279:351–358. [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticoster-one during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]