Abstract
Emotion regulation plays a crucial role in adaptive functioning and mounting evidence suggests that some emotion regulation strategies are often more effective than others. However, little attention has been paid to the different ways emotions can be generated: from the ‘bottom-up’ (in response to inherently emotional perceptual properties of the stimulus) or ‘top-down’ (in response to cognitive evaluations). Based on a process priming principle, we hypothesized that mode of emotion generation would interact with subsequent emotion regulation. Specifically, we predicted that top-down emotions would be more successfully regulated by a top-down regulation strategy than bottom-up emotions. To test this hypothesis, we induced bottom-up and top-down emotions, and asked participants to decrease the negative impact of these emotions using cognitive reappraisal. We observed the predicted interaction between generation and regulation in two measures of emotional responding. As measured by self-reported affect, cognitive reappraisal was more successful on top-down generated emotions than bottom-up generated emotions. Neurally, reappraisal of bottom-up generated emotions resulted in a paradoxical increase of amygdala activity. This interaction between mode of emotion generation and subsequent regulation should be taken into account when comparing of the efficacy of different types of emotion regulation, as well as when reappraisal is used to treat different types of clinical disorders.
Keywords: emotion elicitation, emotion regulation, amygdale, reappraisal, face
Introduction
Even the most cursory analysis suggests wide variation in how different individuals respond to emotional challenges. One important determinant of this variation is thought to be how individuals attempt to regulate their emotions. To understand this variation, one popular approach has been to examine the possibility that some emotion regulation strategies are more effective than others. This approach has been a productive one, and there is now a substantial body of research which suggests that different forms of emotion regulation have different consequences for affective, cognitive and social functioning (Gross, 2007).
One important question that has not yet been addressed; however, is whether the way that an emotion is generated influences the impact of subsequent emotion regulatory efforts. In particular, we now know that emotions can be generated primarily from the ‘bottom up’ (in response to inherently emotional perceptual properties of a stimulus) or ‘top down’ (in response to cognitive appraisals of an event). To the extent that emotion generation engages psychological processes and neural systems that are also implicated in some types of emotion regulation, it seems possible that certain regulation strategies are more effective when performed upon emotions generated in similar ways. In the present study, we tested the hypothesis that the way an emotion is generated interacts with the success of subsequent emotion regulation by measuring emotional outcomes. Below, we review the literatures on emotion generation and emotion regulation. Then, we propose that the method of emotion generation may be important for examining the success of subsequent emotion regulation.
Emotion generation
Emotions can be elicited in a number of ways, ranging from unexpected encounters with threatening creatures, to conclusions drawn from interpretations of complex social interactions. Most typically, perhaps, everyday emotions involve a blending of some bottom-up processing of encounters with emotional stimuli along with some top-down conceptual knowledge, memories and linguistic representations. However, emotional encounters can be characterized by relatively stronger bottom-up or top-down generation. We argue that these two types of emotion generation instantiate emotions using separable psychological processes and neural systems (Teasdale et al., 1999; Phelps et al., 2001; Ochsner et al., 2009), which may make them differentially malleable by subsequent emotion regulation.
Bottom-up emotion generation
Refers to the elicitation of emotion by the presentation of a stimulus that is thought to have simple physical properties that are inherently emotional. For example, fear might be elicited from the bottom-up when someone glances over to discover a spider crawling across his shoulder. In everyday life, bottom-up generated emotions can be elicited by a range of stimuli, but in the lab, bottom-up emotion generation usually involves the presentation of visual stimuli that are thought to have conveyed emotional information over much of evolutionary history, or be biologically prepared (Seligman, 1971). Human faces expressing fear or anger, snakes, spiders, predatory animals and sharp objects are thought to be biologically prepared negative stimuli (Öhman and Mineka, 2001; Bar and Neta, 2007).
The predominant theory regarding the processing of biologically prepared stimuli is that the brain has evolved the ability to detect the simple perceptual features of emotional objects quickly and accurately (LeDoux, 2000; Luo et al., 2007). Bottom-up emotion generation is a stimulus-focused view of emotional processing, and individual variation in the emotional response is thought to be due to differences in perceptual acuity, or in the biologically determined sensitivity and strength of the emotional response system.
Bottom-up emotion generation reliably elicits activity from the amygdala, a neural structure that is thought to be important for emotional learning and the processing of emotional information more generally (Zald, 2003; Phelps and LeDoux, 2005). A considerable amount of research has identified certain perceptual features that elicit amygdala activation outside conscious awareness (e.g. before a backward mask, Vuilleumier et al., 2003; Whalen et al., 2004; Spezio et al., 2007). However, many of these studies have not measured emotion experience while eliciting emotion in these bottom-up ways (Whalen et al., 1998; Hariri et al., 2000; Lieberman et al., 2007). In studies that do measure subjective experience, the negative emotion elicited by emotional faces has been relatively weak (Hariri et al., 2002; Britton et al., 2006).
Top-down emotion generation
Refers to the elicitation of emotion by the activation of appraisals that a situation is relevant to an individual’s goals (Frijda, 1988; Scherer, 2001). For example, fear might be elicited from the top-down when someone interprets a curt email from a prospective employer as indicative of disinterest and a low likelihood of being hired. In the lab, top-down emotion generation involves the use of language, in the form of tailored autobiographical scripts, or narrations of events that might elicit an emotion-inducing appraisal (Teasdale et al., 1999; Phelps et al., 2001; Kim et al., 2004; Ochsner et al., 2009).
Functional accounts of top-down emotion generation emphasize the adaptive value of allowing the highly conserved emotional systems that respond to bottom-up emotional stimuli to also respond to top-down input. This gives the organism the power and flexibility to respond to internal, mental demands in addition to external, physical ones. Top-down emotion generation is a cognition-focused view of emotional processing, and variation in the emotional response is thought to be due to differences in individuals’ goal states or appraisal biases.
Studies of top-down emotion generation indicate that in some situations, top-down emotion generation elicits activity in the amygdala (Phelps et al., 2001; Kim et al., 2004; Ochsner et al., 2009) as well as dorsomedial prefrontal cortex (dMPFC) which is thought to represent the high-level self-relevant appraisals that lead to strong subjective experience of emotion (Teasdale et al., 1999; Ochsner et al., 2009; Waugh et al.). Top-down generated emotion is thought to elicit psychophysiological and amygdala activation primarily when accessible to conscious awareness (Olsson et al., 2007). Top-down generated emotions consistently elicit self-reported emotional experience (Britton et al., 2006).
Comparing bottom-up with top-down emotion generation
Bottom-up emotions are elicited largely by perceptions, which need not be accessible to conscious awareness and are often biologically prepared. Top-down emotions elicited largely by cognitions, which are not tied to any particular perceptual stimulus, but rather to linguistically represented appraisals that are usually accessible to conscious awareness. Bottom-up emotions help us respond quickly and accurately to emotion-relevant aspects of our environment, whereas top-down emotions help us achieve greater flexibility in producing these emotional responses. Bottom-up generated emotions, especially those elicited by emotional faces, elicit stronger amygdala activity—but weaker subjective reports of emotion—than top-down generated or mixed emotions (Hariri et al., 2002; Britton et al., 2006; Ochsner et al., 2009).
Emotion regulation
Emotion regulation refers to any process an individual uses to influence the onset, offset, magnitude, duration, intensity or quality of one or more aspects of an emotional response (Gross, 2007). One particularly important form of emotion regulation is cognitive reappraisal, which typically involves the re-consideration or re-framing of an emotional event in less emotional terms (Giuliani and Gross, 2009). Several studies have shown that reappraisal is relatively effective at reducing several aspects of negative emotional responding (Ochsner and Gross, 2008). Comparisons with other emotion regulation strategies, such as expressive suppression (Gross, 1998; Richards and Gross, 2000; Goldin et al., 2009) and distraction (Sheppes and Meiran, 2007; McRae et al., 2010) indicate that cognitive reappraisal is a reliable, effective way to reduce negative responding, as measured by self-reported negative affect as well as amygdala activation. In addition, these studies indicate that reappraisal engages linguistic and executive function processes involved in cognitive control (Ochsner and Gross, 2008). The down-regulation of negative emotions using reappraisal has remained a focus of research attention because it is effective, and it is closely related to widely used interventions for mood and anxiety disorders.
Studies of emotion regulation have focused upon comparing and contrasting the processes engaged during regulation, but have largely ignored the processes engaged during the generation of the emotion to be regulated. The results from studies employing a large variety of generation methods have been combined and considered together to describe the success of different emotion regulation processes. For example, a large number of studies on cognitive reappraisal have used the same picture set (Lang et al., 2001; Kalisch, 2009) which combines elements of bottom-up and top-down emotion generation. However, the down-regulation of the emotion elicited by these pictures is commonly compared with and contrasted with studies that use only bottom-up stimuli, such as studies manipulating attention to and verbal processing of emotional faces (Hariri et al., 2003; Etkin et al., 2006; Lieberman et al., 2007) and the extinction of fear conditioning with electrical shock (Delgado et al., 2008).
Does mode of emotion generation influence emotion regulation?
Bottom-up and top-down emotions differ in their psychological and neural mechanisms. It is possible that these differences have important consequences for subsequent emotion regulation attempts, making emotion regulation more or less successful depending on the method of generation.
The idea that the method of emotion generation might interact with subsequent regulation is reminiscent of other examples in which the activation of one cognitive process facilitates a subsequent similar or identical process. Most directly, a generation-regulation interaction may be akin to a property of memory known as transfer appropriate processing (TAP; Roediger et al., 1989). TAP specifies that the greater the overlap between the processes engaged during encoding and the processes engaged during retrieval, the more successful is retrieval.
More broadly, process priming has been observed when seemingly unrelated cognitive processes are supported by a common neural substrate. For example, the induction of approach-related emotions enhances performance on verbal working memory, and the induction of withdrawal-related emotions improves performance on spatial working memory (Gray, 2001). The proposed mechanism is shared hemispheric processing in the brain: both approach tendencies and verbal processes are thought to be supported by the left hemisphere, whereas both withdrawal and spatial processes are thought to be supported by the right hemisphere.
In the case of emotion regulation, which processes might overlap between emotion generation and regulation? One process that is engaged during top-down generation and reappraisal is the degree to which the affective meaning or appraisal is brought into the forefront of attention. Reappraisal requires that this appraisal is re-considered, changed and that altered reappraisal is maintained in working memory. Because top-down generation activates the appraisal, and reappraisal manipulates it, it is possible that reappraisal is more effective when performed upon top-down generated emotions. To date, however, no one has investigated the possibility of an interaction between the method of generation (top-down or bottom-up) and the success of subsequent emotion regulation.
The present study
The goal of the present study was to test for an interaction between emotion generation and emotion regulation. One challenge is that the methods most commonly used to generate emotions from the top-down and bottom up differ on many low-level characteristics that impact amygdala activation, such as the extent of linguistic vs visual processing required, luminance, size of stimulus on visual field and the amount of time required to recognize the stimulus as emotional. Therefore, the strongest test of emotion generation—regulation interactions is to compare bottom-up and top-down generation as history effects, when these low-level differences are no longer present. Therefore, we adapted a design that has been used earlier to demonstrate the use of top-down generated emotion to modulate the neural response to a neutral stimulus (Kim et al., 2004). In this paradigm, emotional information pervades an otherwise neutral context, imbuing a previously innocuous stimulus with an emotional character.
We expanded this design, so that the present study is the first attempt to use bottom-up emotion generation to bias the response to a neutral stimulus, and the first to collect self-reported negative affect as a measure of this bias. In addition, we added a top-down emotion regulation instruction, in which individuals used reappraisal to consider the bottom-up or top-down emotion that was generated earlier in less negative terms. This allowed us to compare the success of using reappraisal to decrease negative emotion on emotions that had a history of being generated in these two ways, without the low-level differences that usually are confounded with bottom-up and top-down emotion generation. We predicted an interaction between emotion generation and regulation, such that top-down generated emotions would be more successfully decreased by reappraisal than bottom-up generated emotions, as evidenced by self-reported negative affect and amygdala activation.
METHOD
Participants
Participants were recruited via online advertising from the San Francisco Bay Area community. Participants were screened via email to exclude those with: past or current mood/anxiety disorders, current use of psychoactive medications, or fMRI rule-outs (e.g. pregnancy, metal in body, tattoos on head or neck). We recruited only women to reduce the heterogeneity of emotional reactivity and regulation observed earlier between men and women (Kring and Gordon, 1998; McRae et al., 2008). Twenty-six women, ages 18–35 completed the entire experimental procedure (mean age = 24.88, s.d. = 5.58, 15 Caucasian, 5 Asian-American, 2 Hispanic, 4 other or multiple ethnicities). Participants provided written informed consent and were compensated for their participation. This project was approved by the institutional review board at Stanford University.
Task
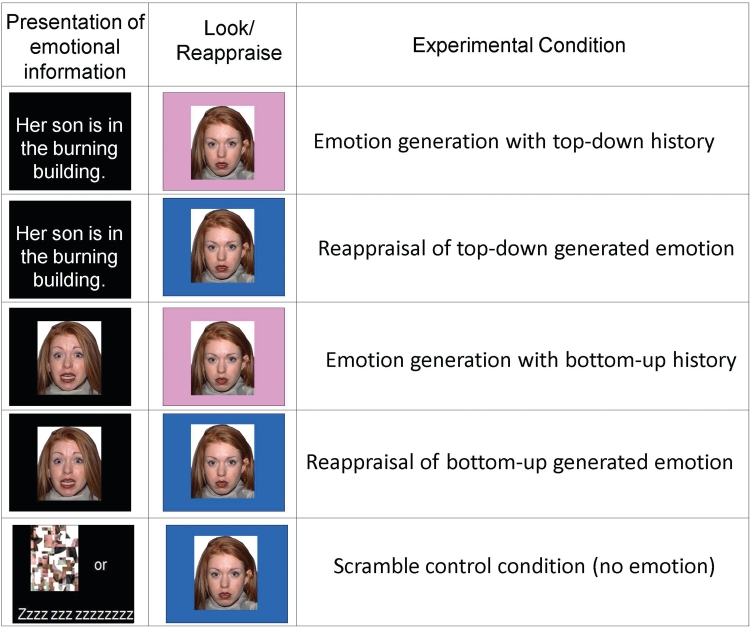
For each trial, participants saw a piece of background information (top-down negative sentences, bottom-up fearful faces, or scrambled faces or sentences) for 4 s. Participants then saw a fixation cross for a variable duration between 0 and 4 s, averaging 2 s (Figure 1). A neutral face (Tottenham et al., 2009) was then presented for 6 s (with a matching identity to the background fearful face in the bottom-up condition). A colored frame bordered the neutral face, and participants were trained to look and respond naturally when one frame color appeared, and use reappraisal to decrease their emotional response when the other color appeared. The assignment of background information and neutral faces to the look or reappraise condition was counterbalanced across participants. Following the neutral face with frame, participants responded to the question ‘How negative do you feel?’ on a 5-point rating scale (labeled, ‘not all negative’ to ‘very negative’). Finally, a fixation cross appeared for a variable duration between 2 and 6 s, averaging 4 s, between trials.
Fig. 1.
Emotion generation and regulation task. Participants were first presented with either fearful faces (bottom-up) followed by the instruction to look or reappraise, or negative sentences (top-down) followed by the instruction to look or reappraise. Emotion generation refers to the look instruction, or the presentation of the neutral face with the instruction to look and respond naturally, considering the relevant background information. Emotion regulation refers to the reappraisal instruction, or the presentation of the neutral face with the instruction to decrease negative affect using reappraisal, considering the relevant background information. Also presented were scrambled pictures or words presented before the instruction to look, combined and used as a control condition.
Task training
Participants were trained on the experimental task before entering the scanner. The experimenter explained that during the task, the participant would see a series of faces. Before each face, the participant would be provided a piece of background information (a negative sentence, face or a neutral scrambled face or sentence), which would inform the participant as to what was going on for that person at that point in time. Participants were encouraged to consider the background information while viewing the neutral face that followed it. Participants were instructed to think of the scrambled faces and sentences as not containing significant background information, and these were combined to form a common control condition that contained elements of both types of emotional background information.
Participants were then told that when they viewed these neutral faces they would also be asked to either (i) look and have their natural response to the person and their situation (look) or (ii) try to think about the person and their situation in a way that made it less negative (reappraise). Several examples of reappraisals were provided, and the participant was required to generate at least two appropriate reappraisals during this training. Examples of appropriate reappraisals were: the situation is not as bad as it first seemed, this person has special skills to get him/her out of the situation, or he/she is feeling better now.
Scan parameters
Twenty-four axial slices (4.4 mm thick) were collected at a 3T (GE Signa LX Horizon Echospeed) scanner with a T2* sensitive gradient echo spiral-in-out pulse sequence (TR = 2.00, TE = 40 ms, 80° flip angle, 24-cm field of view, 64 × 64 data acquisition matrix) which has been shown to effectively reduce signal dropout at high field strengths (Preston et al., 2004). Two hundred and thirty whole-brain images were taken in each of four 7-min, 40-s runs. High-resolution SPGR scans were acquired for anatomical normalization and localization of the amygdala.
Data analysis
Ratings of negative affect from each trial were averaged by condition (scramble look, top-down look, bottom-up look, top-down reappraise, and bottom-up reappraise) for each participant. Mean ratings were entered into a repeated measures GLM in SPSS with generation method (top-down vs bottom-up) and instruction (look or reappraise) as within-subject factors.
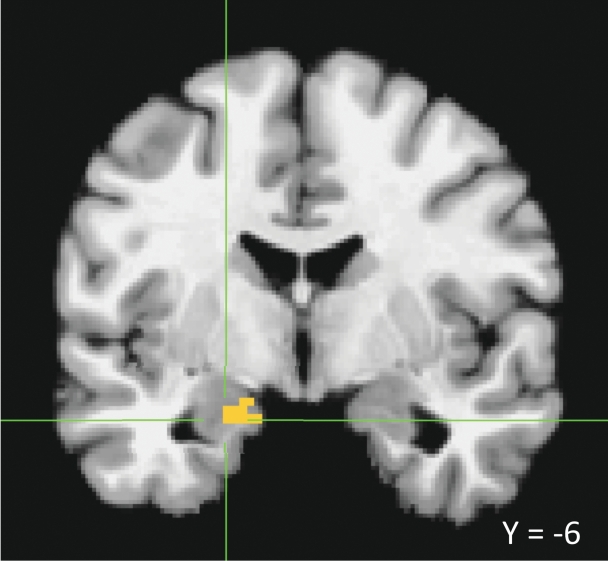
Standard pre-processing steps were completed in AFNI. Functional images were corrected for motion across scans using an empirically determined baseline scan and then manually co-registered to each subject’s high resolution anatomical. Anatomical images were then normalized to a structural template image, and normalization parameters were applied to the functional images. Finally, images were re-sliced to a resolution of 2 mm × 2 mm × 2 mm and smoothed spatially with a 4 mm filter. We then used a GLM (3dDeconvolve) in AFNI to model two different trial parts: the emotion presentation period when top-down, bottom-up or scrambled information was presented, and the emotion generation/regulation period, when individuals were either looking and responding naturally or using cognitive reappraisal to try to decrease their negative affect toward a neutral face. This resulted in 10 conditions: two trial parts during five conditions (Figure 1). Linear contrasts were then computed to test for the hypothesis of interest (an interaction between emotion generation and emotion regulation) for both trial parts. Because the amygdala was our primary a priori structure of interest, we used an a priori ROI approach. Voxels demonstrating the predicted interaction [(top-down look > top-down reappraise > bottom-up look > bottom-up reappraise)] were identified using joint voxel and extent thresholds determined by the AlphaSim program [the voxel threshold was t = 2.74 (corresponding with a P < 0.01) and the extent threshold was 10, resulting in an overall threshold of P < 0.05). Significant clusters were then masked with a pre-defined amygdala ROI at the group level, and parameter estimates for supra-threshold voxels inside the amygdala (figure 2) were then extracted and averaged for each condition for display.
Fig. 2.
Left amygdala ROI identified in hypothesized interaction [(top-down look—top-down reappraise) > (bottom-up look—bottom-up reappraise)] at a voxel threshold of t = 2.74 and extent threshold of 10, for an overall P of <0.05, then masked with an amygdala ROI defined at the group level.
RESULTS
Manipulation check
During the presentation of the emotional stimulus (background information), we observed greater amygdala activity in response to bottom-up generated emotion (mean = 0.154, s.e.m. = 0.036) than top-down generated emotion (mean = 0.030, s.e.m. = 0.051) or the scramble control condition (mean = −0.031, s.e.m. = 0.039). In a repeated measures GLM with emotion generation type and regulation factors, there was a main effect of type of generation type [F(1, 25) = 5.20, P < 0.04] but no interaction with emotion regulation instruction during this period [as participants were not yet instructed to regulate or not; F(1, 25) = 0.11, P = 0.75].1
Emotion generation
Self-reported negative affect
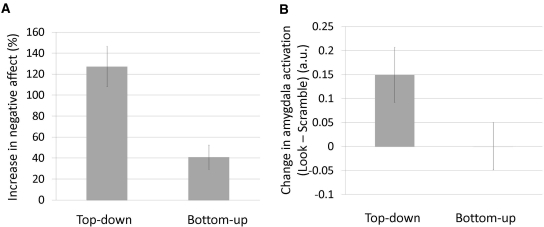
Ratings of self-reported negative affect from the non-regulation condition (look) indicated that individuals felt significantly more negative during the negative conditions, compared with the scramble control condition. This was true when emotions were generated from the top-down [t(1, 25) = 9.37, P < 0.001] and the bottom-up [t(1, 25) = 3.90, P < 0.002]. Top-down generated emotions elicited greater reports of negative affect than bottom-up generated emotions [t(1, 25) = 8.39, P < 0.001; Figure 3 and Table 1].
Fig. 3.
Emotion generation, or unregulated responding to a neutral face that was previously preceded by the presentation of top-down or bottom-up negative information. (A) Percentage increase in self-reported negative affect reflecting top-down and bottom-up emotion generation compared to a scramble control. (B) Parameter estimates from the left amygdala ROI (Figure 2) during the presentation of a neutral face that has a top-down or bottom-up emotional history compared to a scramble control.
Table 1.
Mean parameter estimates from amygdala ROI and mean self-reported negative affect
| Measure | Instruction Condition | Emotion generation condition |
||
|---|---|---|---|---|
| Top-down | Bottom-up | Scramble | ||
| Amygdala parameter estimates | Generation | 0.096 (0.05) | −0.053 (0.04) | −0.054 (0.06) |
| Regulation | 0.014 (0.08) | 0.046 (0.07) | – | |
| Self-reported negative affect | Generation | 3.696 (0.17) | 2.402 (0.16) | 1.842 (0.14) |
| Regulation | 2.311 (0.12) | 1.726 (0.12) | – | |
Amygdala activation
During the look/reappraise portion of the trial (when neutral faces were presented, endowed with top-down or bottom-up emotional history), we examined the amygdala activation under the look instruction to examine unregulated emotion generation with top-down and bottom-up histories. These responses indicate that during the presentation of emotional information, the amygdala showed greater activation for top-down generated emotions than bottom-up generated emotions [t(1, 25) = 2.90, P < 0.009; Figure 3 and Table 1].2
Interactions between emotion generation and regulation
Self-reported negative affect
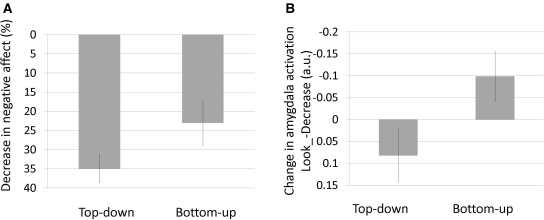
We observed a significant interaction between type of generation and regulation instruction [F(1, 25) = 21.02, P < 0.001]. This interaction was such that top-down generated emotions were down-regulated [mean look-reappraise difference = 1.38, t(25) = 9.07, P < 0.001] to a greater extent than bottom-up generated emotions [mean look-reappraise difference = 0.68, t(25) = 3.91, P < 0.002; Figure 4 and Table 1]. To address the potential concern that this interaction reflects the amount of emotion induced rather than the amount regulated, we also expressed reappraisal as the percentage of the induced emotion that was reduced by reappraisal for each participant. According to this metric, top-down reappraisal was also significantly more effective than bottom-up reappraisal [top-down = 34.9% decrease, bottom-up 23.0% decrease; t(25) = 2.33, P < 0.029].
Fig. 4.
Emotion regulation, or response to a neutral face that was previously preceded by the presentation of top-down or bottom-up negative information under the instruction to cognitively reappraise to decrease negative emotion. (A) Percentage decrease in self-reported negative affect reflecting regulation success for top-down and bottom-up generated emotions (downward bars signal more successful down-regulation). (B) Parameter estimates from the left amygdala ROI (Figure 2) regulation success for top-down and bottom-up generated emotions for the decrease condition subtracted from the look condition (downward bars signal more successful down-regulation).
Amygdala activation
As noted above, there were no effects of regulation instruction on amygdala activity during the presentation of top-down or bottom-up background information, as individuals were not yet instructed to look or reappraise. However, during the look/reappraise phase of the trial, we observed an interaction between type of generation and regulation instruction [F(1, 25) = 11.74, P < 0.003; Figure 4 and Table 1]. This interaction revealed opposite effects of reappraisal on emotions generated from the top-down and bottom-up. For top-down generated emotions, reappraisal resulted in a relative (though non-significant) decrease in amygdala activity during reappraisal compared with look [t(25) = 1.35, P = 0.19, 85% decrease]. For bottom-up generated emotions, we observed a trend for greater amygdala activity during reappraisal than look [t(25) = 1.76, P = 0.09, 93% increase].3 This interaction can also be described as greater amygdala activation during top-down generated emotion than bottom-up generated emotion on look trials [t(25) = 3.61, P < 0.003], but no significant difference on reappraise trials [t(25) = 0.51, P = 0.617].4
DISCUSSION
Does the way emotions are generated influence subsequent regulation-related success? In the present study, we observed significantly greater decreases in self-reported negative affect when emotions were generated from the top-down than from the bottom-up. In addition, we observed an interaction in the amygdala such that using reappraisal to decrease negative emotion resulted in paradoxically increased amygdala activation for bottom-up generated emotions. Taking both measures together, these findings suggest that top-down generated emotions are more successfully down-regulated by reappraisal than bottom-up emotions, and using reappraisal to decrease bottom-up generated emotions may even be counterproductive.
Emotion generation interacts with emotion regulation
We observed an interaction in the amygdala between the type of emotion generation and the degree to which that emotion is down-regulated by cognitive reappraisal. It is important to keep in mind that this interaction could have several sources. We have focused upon the change that occurs during regulation, as we were most interested in the effects of using reappraisal to decrease negative emotion when emotions were generated in these two different ways. It is also informative to focus upon the difference in amygdala activation between the top-down look and bottom-up look conditions.
Although bottom-up emotion generation elicited substantial amygdala activation during the presentation of the fearful face, this response was greatly diminished when the neutral face followed it. Therefore, the low amygdala activation in the bottom-up look condition does not reflect a floor effect in terms of emotion generation, but rather a failure for the emotional information processing in the amygdala to persist beyond the physical presentation of the fearful face. By contrast, the top-down emotion generation was hardly diminished between presentation and the subsequent neutral face, indicating that the emotional information processed by the amygdala is longer-lasting, or perhaps more transferrable, when generated from the top-down. In this case, the trend for a paradoxical increase in amygdala activation is still interpretable, because it reflects a tendency for the attempt to use reappraisal to decrease negative emotion to interrupt the decrement that would naturally occur when the bottom-up stimulus is no longer physically present.
Underlying mechanisms
We propose that the processes engaged during top-down emotion generation facilitated the processes engaged when participants used reappraisal to decrease negative emotion. Although conceptualizing emotion generation–emotion regulation interactions in this way is novel, the idea that one process may prime or facilitate another is present in previous studies of memory, language and cognitive control (Inhoff et al., 1984; Roediger et al., 1989; Gray et al., 2002). What psychological processes might overlap between top-down emotion generation and subsequent top-down regulation? One similarity between top-down emotion generation and cognitive reappraisal is the extent to which the emotional appraisal is available to conscious attention.
When emotions are generated from the top-down, relevant aspects of the emotional appraisal may be brought to the forefront of attention. These appraisals are represented linguistically, are relatively specific, and activate semantic networks that represent the situational and cognitive antecedents of the elicited emotion. These representations are not dependent on physical properties of the stimulus, and may be relatively easily maintained in working memory. This activated representation may make these appraisals more accessible and amenable to efforts to manipulate or change them (as is done during cognitive reappraisal).
In the case of bottom-up emotion generation, emotional information is transmitted by physical properties of the stimulus, and appraisals are not necessarily brought into focal attention. In the bottom-up case, the emotion fades when the perceptual inputs are no longer present. Therefore, in order to manipulate the appraisal, the emotional meaning of the bottom-up stimulus must be transformed into a linguistic representation, and the appraisal must become active and available to conscious attention for the first time. Our results indicate that activating these appraisals while using reappraisal to decrease bottom-up generated emotion results in increased amygdala activation. It is possible that using reappraisal to decrease negative emotion maintains what would naturally fade with the removal of the stimulus.
The paradoxical amygdala effect while participants were using reappraisal to decrease bottom-up emotion is in some ways counter to the established finding that providing a verbal label for a stimulus results in relatively decreased amygdala activation (Lieberman et al., 2007). We believe that there are several differences in these experimental tasks that could account for this. First, during labeling studies, individuals are looking at emotional faces while providing a label. In the present study, individuals were viewing a neutral face that had been imbued with negative affect by a preceding emotional face. Secondly, verbal labeling is quite different than using cognitive reappraisal to decrease negative emotion. Providing a verbal label involves selecting one of a limited number of options, and the process of labeling ends when a response has been selected. Reappraisal, on the other hand, is a multi-step process that involves bringing to mind many details regarding the causes and significance of the emotional situation, enumerating several alternative appraisals, selecting among them, maintaining the selected appraisal and monitoring the success of the desired change in affect. Finally, studies of verbal labeling do not typically ask for subjective experience reports, and so it is difficult to say whether the multi-method characterization of bottom-up regulation reported here has the same or different effects as verbal labeling.
The present study demonstrated an interaction between emotion generation and emotion regulation in the case of self-reported negative affect and amygdala activation. It should be noted, however, that these two measures of negative emotion did not show identical interactions. In the case of self-reported negative affect, using reappraisal to decrease negative emotion was successful for both top-down and bottom-up generated emotions, but significantly more so for top-down generated emotions. For amygdala activation, the interaction was due to the tendency for heightened activation to bottom-up generated emotions when using reappraisal to decrease negative emotions.
Heightened amygdala activation, in this case, could represent a part of the negative emotional response that is not captured by self-reported negative affect. This would lead to the interpretation that attempting to use reappraisal to decrease negative emotion to a bottom-up stimulus `backfired’ in some way and produced more negative responding than not reappraising. However, future work should investigate the possibility that that this activation is due to the engagement of one of several other processes known to involve the amygdala, such as arousal, positive affect, novelty or effort (Hamann and Mao, 2002; Anderson et al., 2003; Wright et al., 2003) while individuals are using reappraisal to decrease negative emotion.
Methodological and clinical implications
These findings indicate that the success of emotion regulation may vary depending on whether an emotion is generated from the top-down or the bottom-up. Therefore, if one type of emotion regulation is found to be more successful than others, this finding may only apply to emotions generated in a particular way. In addition, it may be problematic to collapse across earlier studies of regulatory processes to compare the success of verbal labeling, extinction, reappraisal, expressive suppression and distraction if they were performed upon emotions generated using different methods (Hariri et al., 2003; Etkin et al., 2006; Lieberman et al., 2007; Delgado et al., 2008; Ochsner and Gross, 2008; Goldin et al., 2009; McRae et al., 2010). Researchers interested in comparing and contrasting different types of emotion regulation should be mindful that the type of emotion generation may impact the success of those processes.
The interaction between emotion generation processes and emotion regulation processes may be relevant to the treatment of clinical disorders. Although many disorders are quite mixed, it is possible that different clinical disorders are characterized by negative emotions generated primarily from encounters with specific stimuli (e.g. specific phobia) or from elaborate, biased cognitions (e.g. ruminative depression). The present results open the possibility that different therapeutic interventions are called for in order to effectively down-regulate negative emotion in these different types of disorders. For example, interventions such as exposure may best weaken the perceptual punch of excessive emotion that has been generated from the bottom-up, while interventions that reply on using cognitive reappraisal to decrease negative emotions may be most effective to quell an excess of top-down generated emotion.
Limitations and future directions
In the present study, we avoided group differences and emotion regulation by restricting our sample to young, healthy, female volunteers. This allowed us to make contact with the earlier literature without attempting to reconcile the heterogeneity of emotional responding and emotion regulation that has been observed earlier between men and women. However, future studies should test the possibility that the interaction between emotion generation and regulation observed here is also true of men. In addition, participants in our sample were screened to be clear of past and present mood and anxiety disorders. If evident, the same interaction between emotion regulation and emotion generation in those with mood and anxiety disorders would have important implications for treatment. Future work should systematically characterize individual and group differences in the regulation of top-down and bottom-up emotions.
Our goal in designing this study was to compare two types of emotion generation, representing them as they have been used in previous affective neuroscience research. We chose to do this in a relatively conservative fashion, employing stimuli that would elicit emotions that were generated in a relatively top-down vs bottom-up fashion. We believe the differences we report between top-down and bottom-up generated emotion may be consequential, considering our conservative effort to separate these processes. Future work should examine the explanatory power of these differences in emotion generation while characterizing the regulation of blended experiences, which are more characteristic of real-world emotional encounters.
The theoretical framework for the present study includes the notion that a match between the processes involved in emotion generation and subsequent emotional regulation should result in maximally effective regulation. We chose to balance generalizability and novelty by employing a tight parallel of an earlier design (Kim et al., 2004) to include both top-down and bottom-up generated emotions, as well as added an emotion regulation manipulation. Therefore, time and complexity restraints did not allow us to employ a fully crossed design, comparing top-down and bottom-up emotion generation with top-down and bottom-up emotion regulation. Future designs should more completely test the idea of a match between processes employed during emotion generation and regulation.
In addition, our hypothesis specified that a match between generation and regulation should facilitate regulation. It is possible that if processes are too tightly matched, or happen at the exact same time, the resources required for generation and regulation would be completely overlapping, and regulation could be hindered. This notion, consistent with an ego-depletion account of self-regulation (Baumeister et al., 1998), would predict that top-down processes be depleted if used to a great extent during emotion generation, such that no more top-down resources are available for regulation. Future studies should investigate whether the same interaction between emotion generation and regulation is true at all degrees of overlap between processes.
Acknowledgments
The authors would like to acknowledge the generous help of Ana Draghici, Ben Edwards, Stephen Allison and Evan Kelso in assisting with creation of the experimental task and portions of fMRI data analysis, as well as Gal Sheppes for his comments on a previous version of this article. In addition, we thank Gary Glover and acknowledge grant National Institutes of Health R01 MH058147 (to J.J.G.).
Footnotes
1To facilitate interpretation of the main finding (the predicted interaction between generation and regulation), amygdala parameter estimates for all comparisons presented here are from the ROI identified in the hypothesized interaction seen in Figure 2. However, the same pattern of results is true if parameter estimates are extracted from anatomical amygdala ROIs (right or left). In addition, the voxels identified in the interaction ROI are a subset of the voxels identified in the other comparisons reported (e.g. bottom-up > top-down during the emotion presentation period) and show the same activation pattern as these larger ROIs.
2The interval between the generation and bias periods was jittered so that estimates of amygdala activity to the neutral face are separable from estimates of amygdala activity to the preceding fearful face of the same identity. The lesser amygdala activity observed here after two faces in a row is likely not due to purely perceptual phenomena such as repetition suppression, as the signal is no lower than that observed during a neutral face that was preceded by a scrambled sentence or face (Table 1).
3When two outliers with Z-scores exceeding ±3 were removed from this comparison, it reached statistical significance (t(23) = 2.67, P < 0.02). The removal of these two outliers did not affect the significance of the top-down comparison.
4It should be noted that amygdala parameter estimates are statistics that represent data that have been rescaled for each participant while computing the GLM. Therefore, to summarize across participants, we consider our comparison of amygdala parameter estimates between conditions to be more comparable to the percent change of self-reported negative affect than the raw difference scores in negative affect.
REFERENCES
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Bar M, Neta M. Visual elements of subjective preference modulate amygdala activation. Neuropsychologia. 2007;45:2191–2200. doi: 10.1016/j.neuropsychologia.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74:1252–65. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. NeuroImage. 2006;31:906–19. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Frijda NH. The laws of emotion. American Psychologist. 1988;43:349–58. doi: 10.1037//0003-066x.43.5.349. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Gross JJ. Reappraisal. In: Sander D, Scherer. KR, editors. Oxford Companion to the Affective Sciences. New York: Oxford University Press; 2009. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2009;65:170–80. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: approach -withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–52. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross, J.J., editor. (2007). Handbook of Emotion Regulation. New York, NY: Guilford Press.
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. NeuroReport. 2002;21:15–9. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Lima SD, Carroll PJ. Contextual Effects on Metaphor Comprehension in Reading. Vol. 12. Austin, TX, ETATS-UNIS: Psychonomic Society; 1984. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neuroscience & Biobehavioral Reviews. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: expression, experience, and physiology. Journal of Social and Personality Psychology. 1998;74:686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report. The University of Florida: The Center for Research in Psychophysiology; 2001. International affective picture system (IAPS). Instruction manual and affective ratings. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, et al. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using meg. NeuroImage. 2007;34:839–47. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, et al. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JDE, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations. 2008;11:143–62. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science. 2009;20:1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Social Cognitive and Affective Neuroscience. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor K J, Gatenby J C, et al. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. NeuroImage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: the cognitive costs of keeping one's cool. Journal of Personality and Social Psychology. 2000;79:410–24. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Roediger HLI, Weldon MS, Challis BH. Varieties of Memory and Consciousness: Essays in Honour of Endel Tulving. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc.; 1989. Explaining dissociations between implicit and explicit measures of retention: a processing account; pp. 3–41. [Google Scholar]
- Scherer KR, Schorr A, Johnstone T. Appraisal Processes in Emotion: Theory, Methods, Research. New York: Oxford University Press; 2001. [Google Scholar]
- Seligman ME. Phobias and preparedness. Behavior Therapy. 1971;2:307–20. doi: 10.1016/j.beth.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Better late than never? On the dynamics of online regulation of sadness using distraction and cognitive reappraisal. Personality and Social Psychology Bulletin. 2007;33:1518–32. doi: 10.1177/0146167207305537. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RSE, Piven J. Analysis of face gaze in autism using "Bubbles". Neuropsychologia. 2007;45:144–51. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, et al. Functional MRI study of the cognitive generation of affect. American Journal of Psychiatry. 1999;156:209–15. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience. 2003;6:624–31. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Hamilton JP, Gotlib IH. The neural temporal dynamics of the intensity of emotional experience. NeuroImage. 2010;49:1699–707. doi: 10.1016/j.neuroimage.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. NeuroImage. 2003;18:660–9. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]