Abstract
Among a range of cognitive functions of the amygdala, recent studies suggest its involvement in identification of the pupil size. To further address its role, we investigated the response of the amygdala to human and cat faces with varied pupil size, taking into account the effect of the gender and subjective attractiveness ratings. Twenty-seven subjects underwent functional magnetic resonance imaging while viewing faces with large and small pupils. Large pupil faces induced increased activation in the amygdala, without interactions with either subject or stimuli gender, although no equivalent activation differences were seen for cat face stimuli. The activation differences were irrespective of the perceived attractiveness, and without explicit knowledge about the manipulation of the pupil size. These data support the idea that the amygdala is responsive not only to explicit or implicit fear, abhorrence or preference, but also to other elements that might suggest heightened vigilance of biologically relevant stimuli, which does not necessarily require subjective awareness.
Keywords: Amygdala, fMRI, pupil, biological relevance
INTRODUCTION
Face perception is one of the most developed visual perceptual skills in humans, and extensive neuroscience research has focused on visual processing of faces and facial expressions (for review, see Haxby et al., 2000). Among the structures of the face, the eyes are considered to provide much information about a person’s state of mind, intentions and identity. Larger ratio of exposed sclera area in the human eyes (Kobayashi and Kohshima, 1997) allows a better gaze direction discrimination or detection of emotion and human infants shift their attention in the direction of another’s gaze as early as 3 months of age (Hood et al., 1998). The human amygdala is known to be involved in the process of redirecting gaze toward the eye region (Adolphs et al., 2005), and that its activity is enhanced for emotionally laden (Breiter et al., 1996; Morris et al., 1996; Blair et al., 1999; Hariri et al., 2002; Gläscher et al., 2004; Reinders et al., 2005), or unfamiliar (Dubois et al., 1999) faces. In addition to the researches focused on the eye widening and eye gaze (for review, see Itier and Batty, 2009), recent studies suggest that the human amygdala is engaged in identification of more subtle changes in the eyes that are not considered to necessarily convey emotional expressions, such as the pupil size of others (Harrison et al., 2006; Demos et al., 2008). In studies investigating whether observed pupil size modulates our perception of other's emotional expressions, Harrison et al. (2006) showed that the amygdala is more responsive to smaller pupil faces with sad expressions, but not to those with other expressions. Another study tested the response of the amygdala in male subjects, using female faces as stimuli (Demos et al., 2008), on the contrary, showed that the amygdala is more responsive for faces with larger pupils. The results were inconsistent, and it is less clear under what kind of circumstances the pupil size evokes the response in the amygdala.
While pupil dilation is considered a facial signal indicating heightened vigilance on the part of a conspecifics (Ursin and Kaada, 1960; Applegate et al., 1983), it has also long been thought to convey sexual interests in conspecifics in Western cultures as reflected in an anecdote of Belladonna eye drops (extract of a plant containing atropine) that is known to be employed by women in the Victorian Era and the Italian Renaissance who purposefully dilated their pupils to appear more attractive to male suitors (Demos et al., 2008). Behavioral studies have indeed shown positive correlations between the pupil size of female faces and attractiveness rated by male subjects (Hess, 1965, 1975; Stass and Willis, 1967; Tomlinson et al., 1978; Bull and Shead, 1979), which was not always replicable in corresponding studies in female subjects (Stass and Willis, 1967; Tomlinson et al., 1978; Bull and Shead, 1979). Although Demos et al. (2008) excluded the possible influence of the attractiveness (Winston et al., 2007; Sergerie et al., 2008) in their deliberate study, it is still possible to attribute greater responses in the amygdala of male subjects for larger pupil women faces to implicit preference that is primarily based on sexual interests as indicated by the literature. We, therefore, hypothesized that the amygdala sensitivity for pupil size is gender dependent, and the amygdala might not show greater activity for larger pupils when male faces are shown to male subjects or female or male faces were shown to female subjects, which can explain the incongruent results in the context on neutral facial expressions by Harrison et al. (2006) to some extent.
Alternatively, on the basis of the data showing that the amygdala is responsive to wide-eyed faces with either neutral, fearful or surprised expressions (Morris et al., 1996; Kim et al., 2003, 2004) or that subliminal presentation of larger size of fearful eye whites alone (Whalen et al., 2004) can provoke greater responses in the amygdala, faces with large pupils might induce larger responses irrespective of the gender or even for non-human faces.
Based on the findings that presentation of the smaller pupils in the context of sad facial expressions was associated with significantly greater neural activity in the amygdala, and that subjects’ pupils constricted in response to the observed smaller pupils, again, only for faces with sad expressions (Harrison et al., 2006), the activation in the amygdala might be interpreted as reflecting its role in mirroring papillary contagion of observed sad face stimuli. However, in light of the evidence that stimulation of the amygdala is associated with pupillary dilatation (Gastaut et al., 1952; Wilson et al., 1952; Koikegami and Yoshida, 1953) such a direct interpretation might be difficult to explain their findings.
To address these questions, we performed an event-related functional magnetic resonance imaging (fMRI) study, employing both male and female face stimuli shown to both sexes of the subjects. In addition, to further test the generality of its role, we also examined the response of the amygdala to cat face stimuli with large and small pupils.
By employing cat face stimuli, we can examine if the response differences in the amygdala reflect the interests in conspecifics or more general perceptional role of the amygdala for the eye or eye-like stimuli.
METHODS
Subjects
After excluding two subjects for structural abnormalities detected on T2-weighted images acquired in prior to fMRI scans, a total of 32 volunteers (16 males and 16 females) who gave written informed consent participated in the fMRI study. All were heterosexual by self-report, right handed as measured by the Edinburgh handedness inventory (Oldfield, 1971), free of abnormal neurological history, taking no medication and had normal or corrected-to-normal visual acuity. Approval for the study was obtained from the institutional review board of the University of Tokyo. Five subjects were excluded from the analyses; three for severe head motion during the scanning and the other two for non-compliance with instructions. The remaining 27 subjects (13 males and 14 females) aged 20–31 years male, 22.8 ± 3.4; female, 22.0 ± 2.2. All female subjects had regular menstrual cycles without taking any hormonal medications.
Stimuli
A total of 100 unfamiliar colored face images (50 males and 50 females faces of no identical person) and 50 cat faces with neutral expressions, sampled from the media, cropped below the neck and around the outer hairline, presented on a solid white background were prepared. Each image was digitally edited using Adobe Photoshop 7.0 (San Jose, CA, USA) to create a set of large and small pupil images with a diameter of pupils as large as 75 and 25% of the iris diameter, respectively (Figure 1).
Fig. 1.
An example of a pair of large- and small-pupil faces. Bilateral pupils were manipulated to create a set of large and small pupil faces that are otherwise identical from a source image.
All human faces were preliminary normalized on explicit measures of attractiveness and arousal in a separate set of 10 males and 10 females volunteers (age range 21–30 years, 25.5 ± 2.1). In this pilot study, each subject viewed a total of 50 male and 50 female and 50 cat faces with either large or small pupils (25 for each). Stimuli were counterbalanced such that half of the subjects viewed the large pupil version of a given face, while the remaining half viewed the small pupil version of that face. Explicit ratings of attractiveness and arousal for human faces were; attractiveness: large pupil, 4.50 ± 0.68 (mean ± s.d.); small pupil, 4.50 ± 0.56; arousal: large pupil, 5.21 ± 0.75; small pupil, 5.16 ± 0.67, respectively, and they did not differ as a function of the pupil size when tested with a paired t-test (attractiveness = large pupil: 4.50 ± 0.67; small pupil: 4.50 ± 0.56; t = 0.47, P = 0.32; arousal: = large pupil: 5.21 ± 0.75; small pupil: 5.16 ± 0.67; t = 0.14, P = 0.45). A three-way mixed analysis of variance (ANOVA) (pupil size × subject gender × stimuli gender) with a subject-specific random effect found no main effect or any interactions.
Attractiveness and arousal for cat faces were: attractiveness = large pupil: 5.89 ± 0.94; small pupil: 5.09 ± 0.99; arousal = large pupil: 4.89 ± 0.82; small pupil: 4.70 ± 0.77, respectively. A paired t-test found a significant difference on attractiveness according to pupil size (t = 6.58, P < 0.001) but not on arousal (t = 1.35, P = 0.10). A two-way mixed ANOVA (pupil size × subject gender) also found a main effect of pupil size on attractiveness [F(1, 18) = 41.0, P < 0.001] without interaction with subject gender but not on arousal. Because attractiveness of cat faces were hard to normalize and because change in pupil size was more noticeable, cat face session was performed in the last.
Event-related fMRI study
Prior to scanning, subjects were informed that they would see 100 human faces in each of the first two runs, and 100 cat faces in the third run, presented one at a time for 2000 ms, followed by a central fixation cross on a black background. Subjects were asked to make an age judgment for human stimuli and sex judgment for cat stimuli using a right index finger button press for >25 years or male cat, and a right middle finger button press for <25 years or female cat by using a button box held in the right hand. They were also informed that the images would be repeatedly presented, but no further details were explained. In each of the first two runs, 50 male and 50 female faces with either large or small pupils (25 faces of each version randomly intermixed) were presented. No identical face, irrespective of the pupil size was repeated in a single run. In the second run, subjects viewed small or large pupil counterparts of all the faces presented in the first run. Human face stimuli were counterbalanced such that a large pupil version of a source image was presented in the first run for a half of the subjects, while the other half viewed the same image in the second run, and vice versa. Cat face stimuli were also presented in counterbalanced design. Face trials were pseudorandomly intermixed with jittered periods of fixation, creating a variable interstimulus interval ranging from 2000 to 7500 ms (Ollinger et al., 2001). Each run included 90 s of resting period in the middle of the run, during which subjects were instructed to passively view the fixation cross. Presentation (Neurobehavioral Systems, Inc., Albany, CA, USA) was used for stimulus presentation and response recording. Stimuli were presented through Visua Stim XGA goggles (Resonance Technology, Inc., Northridge, CA, USA) with a resolution of 800 × 600 pixels.
Imaging was performed on a 3.0 Tesla scanner (GE Signa HDx; GE Healthcare, Milwaukee, WI, USA) using an eight-channel phased-array head coil. Before starting the functional runs, whole-brain T2-weighted images (TR: 4400 ms, TE: 80 ms, flip angle: 90°, matrix: 256 × 256, slice thickness: 2.5 mm) were acquired to confirm if there were no structural abnormalities.
Functional data were obtained from 33 transverse slices covering the whole brain using a single-shot gradient echo-planar sequence (TR: 2500 ms, TE: 30 ms, flip angle: 90°, matrix: 64 × 64, FOV: 240 × 240 mm, slice thickness: 5 mm, interslice gap: 0 mm). A total of 312 volumes were acquired for each participant in a single run lasting 13 min, with the first four volumes subsequently discarded to allow for T1 equilibration effects.
Following scanning, subjects were asked to report if they noticed any experimental manipulation of the images, and if they saw images of any identical person repeatedly during a run or during the whole sessions. To assess the effect of perceived attractiveness, subjects were further asked to rate each face on a 9-point Likert scale of attractiveness (1 = extremely unattractive; 5 = average; 9 = extremely attractive). Faces were again presented in random order for 2000 ms followed by a 1000 ms fixation crosshair. Subjects were given 3000 ms to respond. Subjects were also asked to report which type of the stimuli was subjectively more interesting. Statistical analyses were performed using JMP 8.0 (SAS Institute Inc., Cary, NC, USA). Statistical threshold was set at P = 0.05.
Imaging data analysis
Functional MRI data were processed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB R2006b (The MathWorks, Inc., Natick, MA, USA). Data were corrected for differences in acquisition time between slices for each whole-brain volume, realigned to the first volume within and across runs to account for movement artifact, normalized into standard stereotactic space using standard EPI template provided by the Montreal Neurological Institute, and spatially smoothed with a Gaussian kernel with 8 mm full-width at half-maximum. The time-series in each voxel were high-pass filtered to 1/128 to remove low-frequency noise.
Statistical analysis was performed in two stages in a mixed-effects model. In the first-level analysis, four main conditions for human faces: male with large (M-large) or small (M-small) pupils, and female with large (F-large) or small (F-small) pupils, and two main conditions for cat faces: cat with large (C-large) and small pupils (C-small) were defined, and attractiveness ratings for each of the face trials were included in each condition as modulation parameters. The BOLD impulse response to events of each type was modeled by a canonical hemodynamic response function (HRF) (Friston et al., 1998). This function was convolved with a sequence of delta functions for events of each type in a high-resolution time space and down sampled at the midpoint of each scan to form covariates for the general linear model. For each session, six covariates to capture residual movement-related artifacts (three rigid-body translations and rotations determined from the realignment stage) and a single covariate representing the mean over scans were also included. Parameter estimates for each covariate were determined by a least mean square fit of the model to the data. The parameter estimates for the canonical HRF comprised the data for the second stage of analyses.
In the second-level analysis, statistical parametric maps (contrast images) for each condition and for each subject were tested with a three-way mixed ANOVA (subject gender × stimuli gender x pupil size) for human face session and a two-way mixed ANOVA (subject gender × pupil size) for cat face session to detect the regions showing significantly different hemodynamic responses in response to the pupil size, and to see if there were any effect of the gender. For whole-brain analyses, the AlphaSim program included in AFNI (http://afni.nimh.nih.gov/afni) was used to correct for multiple comparisons. A minimum cluster size of 73 voxels was used to achieve a corrected significance of P < 0.05 as determined by a Monte Carlo simulation with a voxel-wise threshold of P < 0.001.
Impact of the attractiveness was also evaluated with a whole-brain parametric analysis with the attractiveness ratings as a modulation parameter. Contrast images for modulation parameters of attractiveness ratings, obtained in the aforementioned first-level analysis, were tested with one-sample t-test to find regions exhibiting linearly increasing or decreasing hemodynamic responses across the subjects.
To further investigate the response in the amygdala, region-of-interests (ROI) analyses were performed. ROIs for the bilateral amygdala were derived from Talairach definitions of the WFU PickAtlas software (Maldjian et al., 2003). Hemodynamic responses, measured as averaged percent signal changes, were calculated for each condition and for each subject using MARSeille Boîte À Région d’Intérêt (MarsBaR, Brett et al., 2002), and were subjected to a three-way mixed ANOVA (subject gender × stimuli gender × pupil size) and a two-way mixed ANOVA (subject gender × pupil size) for human face and cat face task, respectively.
RESULTS
Post-scan debriefing of subjects
Post-scan debriefing of the subjects revealed that no subject was consciously aware of the change in human pupil size across images. One male subject reported that he noticed changes in pupil size in cat face stimuli. No one correctly reported that they saw each face for two times. All male subjects reported that they were subjectively most interested in female faces. Two of the female subjects reported that female faces were more interesting than male faces. All subjects reported that cat faces were least interesting.
Ratings of attractiveness
Subjective ratings of attractiveness for large and small pupil male, female and cat face stimuli (mean ± s.d.) were: female subjects = 4.67 ± 0.78 (M-large), 4.58 ± 0.83 (M-small), 4.78 ± 0.79 (F-large), 4.69 ± 0.82 (F-small), 6.04 ± 1.07 (C-large), 5.00 ± 1.24 (C-small); male subjects = 4.91 ± 0.85 (M-large), 4.85 ± 0.77 (M-small), 4.86 ± 0.76 (F-large), 4.82 ± 0.83 (F-small), 5.72 ± 0.88 (C-large), 5.03 ± 0.82 (C-small), respectively.
A three-way mixed ANOVA (subject gender × stimuli gender × pupil size) on attractiveness ratings of human stimuli revealed no significant main effect or interactions, while a two-way mixed ANOVA (subject gender × pupil size) of cat stimuli revealed a significant main effect of the pupil size [F (1, 50) = 9.02, P = 0.0057] without interactions with subject gender, reflecting higher attractiveness ratings for large pupil cat faces (C-large, 5.89 ± 0.98; C-small, 5.01 ± 1.04).
Explicit categorization task
Averaged numbers of human faces categorized as <25 years old were: female subjects = 22.1 ± 4.89 (M-large), 27.4 ± 9.61 (M-small), 26.1 ± 3.51 (F-large), 25.8 ± 5.95 (F-small); male subjects = 27.6 ± 9.09 (M-large), 22.2 ± 6.45 (M-small), 27.2 ± 6.96 (F-large), 28.0 ± 4.69 (F-small), respectively. A three-way mixed ANOVA (subject gender × stimuli gender × pupil size) revealed a significant main effect of stimuli gender [F (1, 100) = 4.06, P = 0.048] without any interactions, reflecting larger number of female faces judged as being <25 years old (female face = 26.7 ± 0.92; male face = 24.7 ± 0.92).
Averaged numbers of cat faces categorized as female were: female subjects = 26.9 ± 4.29 (C-large), 21.6 ± 7.19 (C-small); male subjects = 30.2 ± 4.55 (C-large), 21.8 ± 8.24 (C-small), respectively. A two-way mixed ANOVA (subject gender x pupil size) revealed a significant main effect of the pupil size [F (1, 50) = 21.59, P < 0.001] without any interactions, reflecting larger number of large pupil faces judged as being female cat (large pupil face = 28.5 ± 4.66; small pupil face = 21.7 ± 7.56).
Response time
Response time for large and small pupil faces was 1318.7 ± 183.4 ms, and 1324.9 ± 193.7 ms for human faces, and 1263.3 ± 225.0 and 1282.1 ± 191.5 ms for cat faces, respectively. A three-way mixed ANOVA (subject gender × stimuli gender × pupil size) for human faces, and a two-way mixed ANOVA (subject gender × pupil size) for cat faces on response time difference revealed no significant main effect or interactions.
fMRI results
Whole-brain analysis
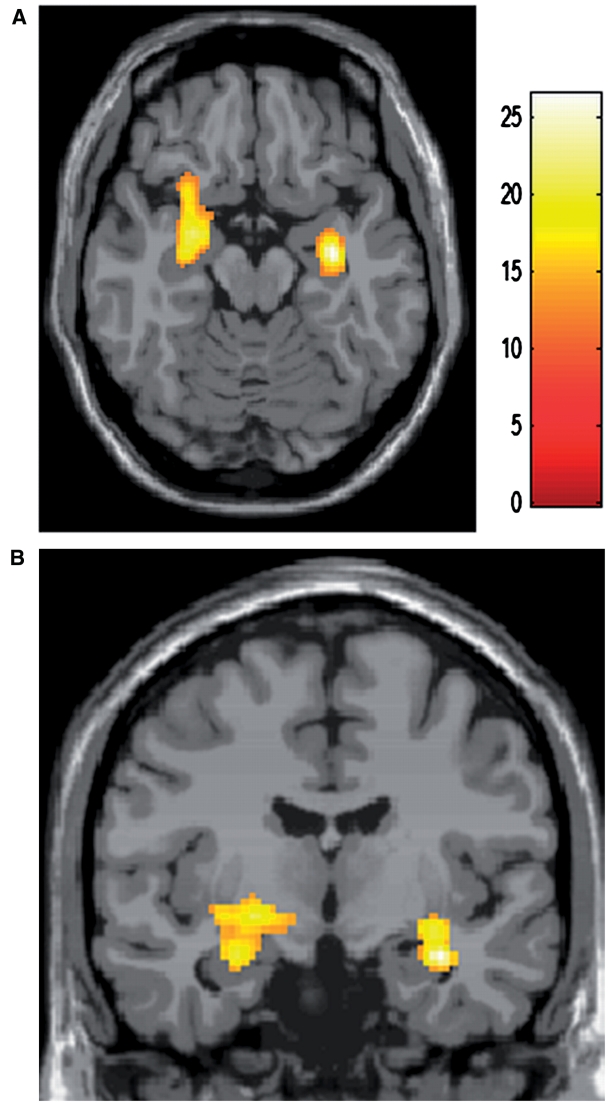
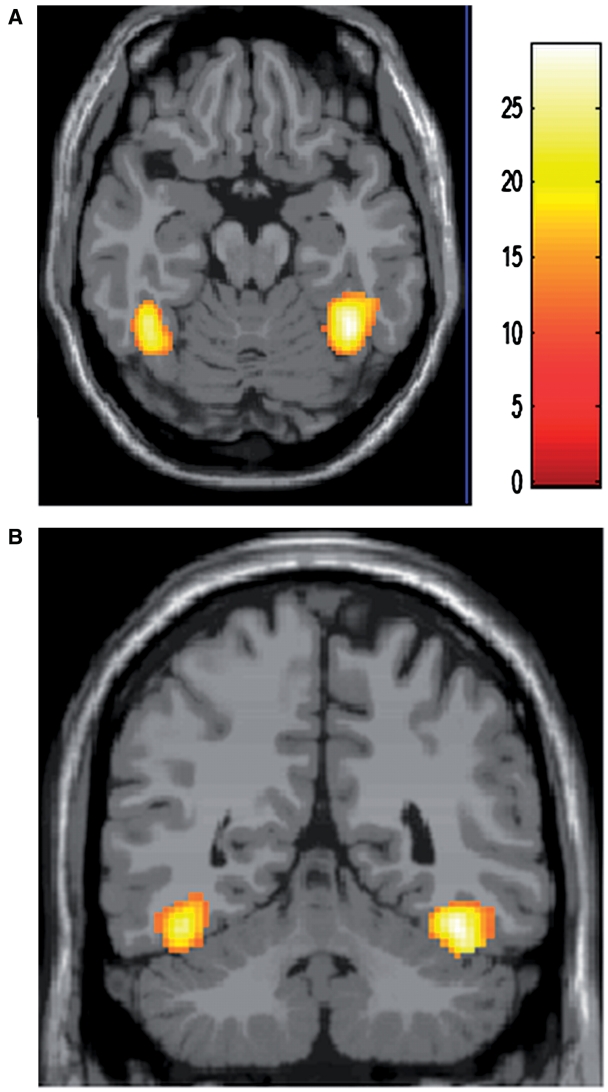
A three-way mixed ANOVA (subject gender × stimuli gender × pupil size) for human face task revealed an effect of the pupil size [F(1, 100) = 11.50, uncorrected P < 0.001 at voxel and cluster level of P < 0.05] in the regions including the left amygdala, and the border between the right amygdala and hippocampus, reflecting greater responses to large pupil faces (T100 > 3.17, uncorrected P < 0.001 at voxel and cluster level of P < 0.05), but no effect of the subject gender or of the stimuli gender, or any interactions among the three or between any two factors were found in the amygdala (Table 1 and Figure 2). Other regions exhibiting significantly greater activation for large vs small pupils included the insula, putamen, perirolandic area, middle frontal gyrus and superior parietal gyrus (Table 1). No areas showed significantly greater activation for small vs large pupils. Significant effect of the subject gender [F(1, 100) = 11.50, uncorrected P < 0.001 at voxel and cluster level of P < 0.05] reflecting greater responses in female subjects (T100 > 3.17, uncorrected P < 0.001 at voxel and cluster level of P < 0.05) was seen in the bilateral temporal lobe, presumably corresponding to the so called fusiform face areas and the visual cortex (Kanwisher et al., 1997; Gauthier et al. 1999), without any interactions with other factors (Table 1 and Figure 3).
Table 1.
Results of a three-way mixed ANOVA (subject gender × stimuli gender × pupil size), human face session
| Region | Laterality | Stereotactic coordinates (MNI) | Z-score |
|---|---|---|---|
| Effect of pupil size [F(1, 100)] | |||
| Amygdala/Hip | R | (32, −12, −18) | 4.7 |
| Amygdala | L | (−28, −10, −18) | 4.09 |
| Putamen | R | (20, 10, −8), (22, 0, −4) | 3.47, 3.15 |
| L | (−22, 0, −10), (−32, −4, −6) | 4.41, 4.07 | |
| Insula (BA13) | L | (−40, 14, 10) | 3.74 |
| MFG (BA46) | R | (36, 32, 26) | 4.02 |
| postCG (BA43) | L | (56, −10, 22) | 3.42 |
| (BA44) | R | (56, 4, 22) | 3.86 |
| SPG (BA46) | L | (−28, −64, 58) | 3.48 |
| Effect of subject gender [F(1, 100)] | |||
| Fusiform (BA37) | R | (40, −54, −20), (24, −80, −14) | 4.91, 3.94 |
| (30, −76, −6) | 3.42 | ||
| L | (−42, −50, −18), (−34, −66, −14) | 4.52, 3.87 | |
| Interactions, pupil size × stimuli gender [F(1, 100)] | |||
| Tri (BA45) | R | (40, 26, 6) | 3.9 |
| Positive effect of pupil size (big > small) (T100) | |||
| Amygdala/Hip | R | (32, −12, −18) | 4.84 |
| Amygdala | L | (−28, −10, −18) | 4.25 |
| Putamen | R | (20, 10, −8), (22, 0, −4) | 3.66, 3.34 |
| L | (−22, 0, −10), (−32, −4, −6) | 4.56, 4.22 | |
| MFG (BA 46) | R | (36, 32, 26) | 4.18 |
| postCG (BA 43) | L | (56, −10, 22) | 3.61 |
| preCG (BA3) | L | (−18, −22, 60) | 3.39 |
| (BA44) | R | (56, 4, 22) | 4.03 |
| SPG (BA46) | L | (−28, −64, 58) | 3.66 |
| SFG (BA6) | L | (−24, −12, 60), (−16,−6, 58) | 3.5, 3.23 |
| Op (BA44) | R | (58, 0, 10) | 3.36 |
| Interaction, pupil size × subject gender (T100) | |||
| Insula (BA13) | L | (−38, −8, −4), (−48, −4, −2) | 3.56, 3.14 |
| MTG (BA21) | L | (−58, −10, −4) | 3.34 |
| Interaction, pupil size × stimuli gender (T100) | |||
| Tri (BA45) | R | (40, 26, 6) | 4.06 |
Activations significant at uncorrected P < 0.001 at voxel and cluster level of P < 0.05.
ACC, anterior cingulate cortex; BA, Brodmann area; MFG, middle frontal gyrus; MTG, middle temporal gyus; Hip, hippocampus; Op, pars opercularis; preCG, precentral gyrus; postCG, postcentral gyrus; SFG, superior frontal gyrus; SPG, superior parietal gyrus; Tri, pars triangularis.
Fig. 2.
Result of a whole-brain analysis (three-way ANOVA for human face session). Regions showing main effect of pupil size including the left amygdala are shown in an axial (A) and a coronal section (B) of a statistical parametric map superimposed onto a stereotactically normalized T1-weighted image (provided by Brain Web: Simulated Brain Database, http://www.bic.mni.mcgill.ca/brainweb/).
Fig. 3.
Result of a whole-brain analysis (three-way ANOVA for human face session). An axial (A) and a coronal (B) section demonstrate regions exhibiting main effect of subject gender in the bilateral fusiform gyri.
A two-way mixed ANOVA (subject gender × pupil size) for cat face task found an effect of the subject gender [F(1, 50) = 12.22, uncorrected P < 0.001 at voxel and cluster level of P < 0.05] reflecting greater responses in female subjects (T50 > 3.26, uncorrected P < 0.001 at voxel and cluster level of P < 0.05) in the left fusiform gyrus, but no other effect or interactions were found (Table 2).
Table 2.
Results of a two-way mixed ANOVA (subject gender × pupil size), cat face session
| Region | Laterality | Stereotactic coordinates (MNI) | Z-score |
|---|---|---|---|
| Positive effect of subject gender (female > male) (T50) | |||
| Fusiform (BA37) | L | (−34, −68, −12) | 3.52 |
BA, Brodmann area.
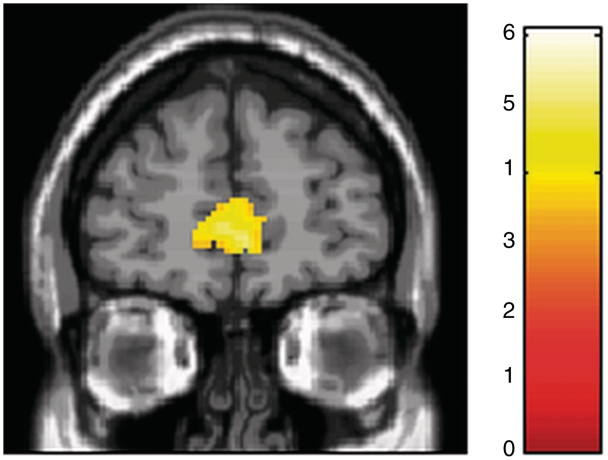
Regions showing linearly increasing hemodynamic activity in response to attractiveness ratings included the anterior cingulate cortex, middle frontal gyrus orbital part and temporal gyri (Table 3 and Figure 4). For cat face stimuli, no areas showed linearly increasing hemodynamic responses to attractiveness ratings.
Table 3.
Regions exhibiting linear hemodynamic responses to attractiveness ratings
| Region | Laterality | Stereotactic coordinates (MNI) | Z-score |
|---|---|---|---|
| Human face | |||
| ACC (BA32) | R | (2, 46, 6) | 4.1 |
| L | (−10, 56, 0) | 4.76 | |
| MFGO (BA10) | R | (6, 56, −4) | 4.51 |
| Lingular (BA18) | R | (8, −64, −10) | 3.55 |
| L | (−8, −84, −14), (−10, −82, −4) | 4.71, 4.0 | |
| (−18, −68, −16) | 4.46 | ||
| MTG (BA21) | R | (48, −4, −22), (58, −38, 0) | 4.09, 3.9 |
| STG (BA22) | R | (56, −44, 8) | 3.72 |
| Fusiform | R | (20, −64, −14) | 3.38 |
| Cerebellum | R | (18, −48, −22) | 3.43 |
Note: ACC, anterior cingulate cortex; BA, Broadmann area; MFGO, Middle frontal gyrus orbital part; MTG, middle temporal gyrus; STG, superior temporal gyrus.
Fig. 4.
Result of a whole-brain parametric analysis. A coronal section shows areas exhibiting linearly increasing hemodynamic activity in response to attractiveness ratings in the anterior cingulate cortex.
ROI analyses
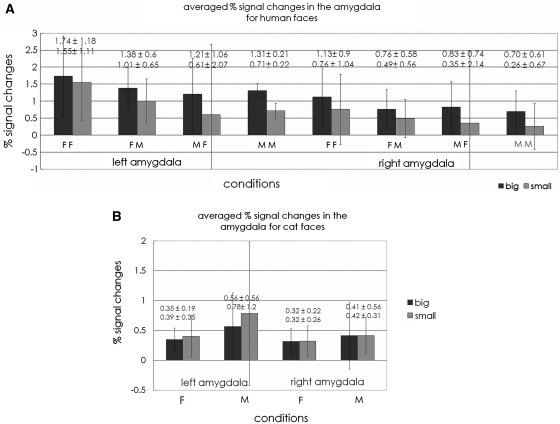
Hemodynamic responses measured as averaged percent signal changes for each condition are shown in Figure 5A and B. Hemodynamic responses in the right and left amygdala for human faces were: right amygdala—large pupil = 0.86 ± 0.14; small pupil = 0.51 ± 0.14; left amygdala—large pupil = 1.41 ± 0.15; small pupil = 0.98 ± 0.15%, respectively.
Fig. 5.
Averaged percent signal changes in the right and left amygdala for each condition for human face session (A) and cat face session (B). FF stands for female subjects seeing female face stimuli: FM, female subjects seeing male stimuli; MF, male subjects seeing female stimuli; MM, male subjects seeing male stimuli; F, female subjects; M, male subjects.
A three-way mixed ANOVA (subject gender × stimuli gender × pupil size) revealed a significant main effect of the pupil size reflecting greater responses for large pupil faces without any interactions in the bilateral amygdala [right: F (1, 100) = 4.53, P = 0.037; left: [F (1, 100) = 5.29, P = 0.024]. No other main effect or interactions were found in the amygdala.
Hemodynamic responses for cat faces in the right and left amygdala were: right amygdala—large pupil = 0.68 ± 0.33, small pupil = 0.66 ± 0.33; left amygdala—large pupil = 0.67 ± 0.28, small pupil = 0.84 ± 0.28%, respectively. A two-way mixed ANOVA (subject gender × pupil size) showed no main effect or interactions for cat faces in the amygdala.
DISCUSSION
In the present study, we confirmed significantly greater activity in response to large vs small pupil human faces in the amygdala, which is in accordance with a previous study by Demos et al. (2008). Against our assumption that the effect is gender dependent, no effect or interactions with subject or stimuli gender were found. These results confirmed that the differences in the amygdala activity induced by pupil size do not reflect preference or primarily sexual interests, but rather to facial signals that might indicate heightened vigilance on the part of conspecifics.
Furthermore, equivalent effect was not confirmed when cat faces were employed as stimuli, despite more explicit changes in the pupil size, suggesting that the response differences for human face stimuli according to the pupil size is not a mere response to eye stimuli, which supports the idea that the amygdala is one component of a circuit that is important for processing not only fear but rather for biological relevance (Adolphs, 2008).
Although, it was not of our primary interests, we also performed a whole-brain parametric analysis including attractiveness ratings as a modulation parameter to evaluate the impact of the attractiveness on the brain activity. The main purpose of this additional analysis was to test the validity of attractiveness ratings by examining the responses in the regions that have been shown to exhibit positive correlations with attractiveness ratings. Attractiveness ratings are subjective, and thus are fragile variables in any analysis. We, therefore, considered it possible that the rating system similarly employed by Demos et al. (2008) could not fully reflect explicit or implicit preference. Regions exhibiting linearly increasing responses to attractiveness ratings for human face stimuli included the dorsal anterior cingulate cortex, which is implicated in the process of affection. Previous studies (Winston et al., 2007; Cloutier et al., 2008), indeed, showed that increased activity in response to increased facial attractiveness was seen in the dorsal anterior cingulate cortex. Human face stimuli are, therefore, considered to have induced proper emotional responses and have been rated properly as far as we can assess. In contrast, no equivalent results were found for cat faces despite higher attractiveness ratings. A possible explanation is that it reflects comparatively less interests in biologically less relevant cat faces; although, we cannot exclude other possibilities such as methodological problem of this rating system for cat faces, or influence of the task differences or of the order of the sessions.
Despite some differences in the study design; for example, we showed both large and small pupil version of all the faces to all the subjects, a previous study by Demos et al. (2008) and the present study similarly showed that larger pupil faces induce greater responses in the amygdala, while a previous studies by Harrison et al. (2006) found no equivalent results for neutral faces. They found a trend of greater amygdala responses for larger pupil faces with happy or angry expression, and decreased responses for larger pupil faces with neutral faces, which did not reach the statistical threshold. Possible explanations for the incongruent results include differences in study design. For example, while we showed a total of 100 human faces (50 male and 50 female faces with large or small pupils), Harrison et al. used 10 male and 10 female faces with 4 expressions with 4 different sizes of pupils, and thus images of an identical person appeared 16 times. Since familiarity is known to influence the amygdala activity (Dubois et al., 1999), this might have influenced the sensitivity of the amygdala by decreasing its responses to repeatedly presented faces.
More recently, Harrison et al. (2009) evaluated the brain responses to pupils dynamically changing their size either by accurately mirroring changes in subject’s pupils (positive feedback), or the opposite (negative feedback) employing faces with neutral expressions as stimuli. In contrast to their previous study (Harrison et al., 2006) showing no significant activity differences of the amygdala in response to the varied pupil size when faces with neutral expressions were employed, they found that the discordance between observed and observer’s papillary changes enhanced activity in the left amygdala. With regard to the changes in subjects’ pupil size, no entrainment effect following changes in size of the stimulus pupil was seen (i.e. increasing pupil size of the stimuli did not increased subject’s pupil size, and vice versa). Their attempt to evaluate the implication of the amygdala activity for social interactions is interesting. However, lack of significant activity difference in the amygdala in response to observed pupil size for neutral faces in their static study (Harrison et al., 2006) make it difficult to compare our results with their findings.
In their dynamic study, the left amygdala was significantly activated only in the negative feedback, but no entrainment effect or significant difference in mean pupil size, change in pupil size or variance of pupil size across conditions between positive vs negative feedback conditions were seen (Harrison et al., 2009), while amygdala’s activity was associated with entrainment effect or significantly reduced pupil size when static sad face stimuli were employed (Harrison et al., 2006). These discrepancies might suggest that the activity of the amygdala reflect the sensitivity to different factors or mechanisms of activation between their static (Harrison et al., 2006) and dynamic (Harrison et al., 2009) studies, which is not necessarily limited to face expressions. Therefore, although it is possible that the increased amygdala activity induced by larger pupils in the study by Demos et al. (2008) or in the present study was also influenced by the discordance effect to some extent, it seems difficult to explain all the effect. Although in the context that they all support the idea that the amygdala is sensitive to the changes in the pupil size of the others, which is not necessarily due to sudden change in ambient light that would affect observer and observed pupil sizes equally, they are not contradictory to each other.
While it is well-known that the pupillary size (both baseline size and the dilatation in the dark) is reduced as a function of age (Birren et al., 1950), pupil dilatation in response to attention–arousal stimulation does not differ significantly with age (Kim et al., 2000). Larger pupils in the light could, therefore, be a facial signal indicating heightened vigilance irrespective of the age, and we consider this as the primary cause of enhanced amygdala activity for larger pupils in the present study.
Another factor that might have influenced the result is the size of the corneal light reflection in the stimuli faces that seems positively correlated with the size of the pupil in the previous study by Demos et al. (2008) and in the present study, whereas it seems negatively correlated in the study by Harrison et al. (2006). Since catchlight (a photography term for artificial corneal light reflection) is often used to draw attention to the eye and to make a photograph aesthetically desirable, it is possible that larger corneal light reflection helped activate the amygdala by directing or for directing subjects to the salient stimuli, the eyes of human faces.
In parallel to previous studies (Harrison et al., 2006; Demos et al., 2008), subjects did not notice the pupil size manipulation in the present study. Responses in the brain to facial expressions can be so rapid that they could not plausibly be based on conscious awareness of the stimulus (Adolphs, 2006), and lines of evidence indicate involvement of the amygdala in such automatic neural responses to stimuli (Morris et al., 1998a, b, 2001; Whalen et al., 1998, 2004).
Finally, it might also be noteworthy that all the subjects in the present study were young adult Japanese who have grown up in a ‘pupil-naïve’ culture; Most Japanese have black to very dark brown irises that makes their pupil size hardly noticeable to each other. They are therefore considered to have comparatively fewer chances to acquire or maturate the sensitivity to pupil size of others. Still, the amygdala was sensitive to differences of the observed pupil size of human faces, but not of cat faces. These results support the generality of the finding across cultures.
In conclusion, in the present study, we confirmed that large pupil size of human faces is such a salient stimuli that provokes greater amygdala responses in the absence of explicit knowledge. The effect is irrespective of the gender of observed face or of observer, suggesting that this is not primarily based on sexual interests. Rather, based on the results that corresponding activity difference was not seen for cat faces, these data, together with previous studies, support the idea that the amygdala is responsive not only to explicit or implicit fear, abhorrence or preference, but also to other elements that might suggest heightened vigilance of biologically relevant stimuli.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank Drs Satoshi Hirose and Seiki Konishi for their helpful technical advices.
REFERENCES
- Adolphs R. Perception and emotion: how we recognize facial expressions. Current Directions in Psychological Science. 2006;15:222–26. [Google Scholar]
- Adolphs R. Fear, faces, and the human amygdala. Current Opinion in Neurobiology. 2008;18:166–72. doi: 10.1016/j.conb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiology and Behaviour. 1983;31:353–60. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Birren JE, Casperson RC, Botwinick J. Age Changes in Pupil Size. Journals of Gerontology. 1950;5:216–21. doi: 10.1093/geronj/5.3.216. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16 abstract 497 (available on CD-ROM) [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bull R, Shead G. Pupil dilation, sex of stimulus, and age and sex of observer. Perceptual and Motor Skills. 1979;49:27–30. doi: 10.2466/pms.1979.49.1.27. [DOI] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience. 2008;20:941–51. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cerebral Cortex. 2008;18:2729–34. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Rossion B, Schiltz C, et al. Effect of familiarity on the processing of human faces. NeuroImage. 1999;9:278–89. doi: 10.1006/nimg.1998.0409. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gastaut H. Corrélations entre le système nerveux végétatif et le système de la vie de relation dans le rhinencéphale. Journal of Physiology. 1952;44:431–40. [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area' increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–73. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Tüscher O, Weiller C, Büchel C. Elevated responses to constant facial emotions in different faces in the human amygdala: an fMRI study of facial identity and expression. BMC Neuroscience. 2004;5:45. doi: 10.1186/1471-2202-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Singer T, Rotshtein P, Dolan RJ, Critchley HD. Pupillary contagion: central mechanisms engaged in sadness processing. Social Cognitive and Affective Neuroscience. 2006;1:5–17. doi: 10.1093/scan/nsl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Critchley HD. Dynamic pupillary exchange engages brain regions encoding social salience. Society for Neuroscience. 2009;4:233–43. doi: 10.1080/17470910802553508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for perception. Trends in Cognitive Sciences. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hess EH. Attitude and pupil size. Scientific American. 1965;212:46–54. doi: 10.1038/scientificamerican0465-46. [DOI] [PubMed] [Google Scholar]
- Hess EH. The role of pupil size in communication. Scientific American. 1975;233:110–9. doi: 10.1038/scientificamerican1175-110. [DOI] [PubMed] [Google Scholar]
- Hood MB, Willen JD, Driver J. Adult's eyes trigger shifts of visual attention in human infants. Psychological Science. 1998;9:131–4. [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neuroscience and Biobehavioral Review. 2009;33:843–63. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuro Report. 2003;14:2317–22. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kim M, Beversdorf DQ, Heilman KM. Arousal response with aging: pupillographic study. Journal of International Neuropsychological Society. 2000;6:348–50. doi: 10.1017/s135561770000309x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye. Nature. 1997;387:767–8. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- Koikegami H, Yoshida K. Pupillary dilatation induced by stimulation of amygdaloid nuclei. Folia Psychiatrica et Neurologica Japonica. 1953;7:109–26. doi: 10.1111/j.1440-1819.1953.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Büchel C, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998a;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998b;393:467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Morris JS, deGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124:1241–52. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. NeuroImage. 2001;13:210–7. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Reinders AA, den Boer JA, Büchel C. The robustness of perception. European Journal of Neuroscience. 2005;22:524–30. doi: 10.1111/j.1460-9568.2005.04212.x. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience Biobehavioral Review. 2008;32:811–30. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Stass J, Willis F. Eye contact, pupil dilation, and personal preference. Psychonomic Science. 1967;7:375–6. [Google Scholar]
- Tomlinson N, Hicks R, Pellegrini R. Attributions of female college students to variations in pupil size. Bulletin of the Psychonomic Society. 1978;12:477–8. [Google Scholar]
- Ursin H, Kaada BR. Functional localization within the amygdaloid complex in the cat. Electroencephalography and Clinical Neurophysiology. 1960;12:1–20. doi: 10.1016/0013-4694(60)90058-4. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wilson WC. Analysis of cerebral control of reflex pupillary dilation in cat and monkey. Archives of Neurology and Psychiatry. 1952;68:333–97. doi: 10.1001/archneurpsyc.1952.02320210103012. [DOI] [PubMed] [Google Scholar]