Abstract
Individuals with low self-esteem have been found to react more negatively to signs of interpersonal rejection than those with high self-esteem. However, previous research has found that individual differences in attentional control can attenuate negative reactions to social rejection among vulnerable, low self-esteem individuals. The current fMRI study sought to elucidate the neurobiological substrate of this buffering effect. We hypothesized and found that while looking at scenes of social rejection (vs negative scenes) low self-esteem high attentional control individuals engaged the rostral anterior cingulate cortex (rACC), an area of the brain associated with emotional control, more than their low self-esteem low attentional control peers. Furthermore, we found that low self-esteem high attentional control individuals evaluated social rejection as less arousing and less rejecting in a separate behavioral task. Importantly, activation in the rACC fully mediated the relationship between the interaction of self-esteem and attentional control and emotional evaluations, suggesting that the rACC activation underlies the buffering effects of attentional control. Results are discussed in terms of individual differences in emotional vulnerability and protection and by highlighting the role of rACC in emotion regulation.
Keywords: attentional control, self-esteem, social rejection, rostral anterior cingulate cortex, emotion regulation
INTRODUCTION
Securing interpersonal acceptance is a fundamental human motive (Leary et al., 1995; Hart et al., 2005). Thwarted acceptance strivings, therefore, result in a prototypical sequelae of negative reactions that include heightened negative emotions (Nezlek and Plesko, 2001), elevated cortisol stress responses (Dickerson et al., 2004) and behavioral aggression (Leary et al., 2006). Individual differences in sensitivity to rejection can further exacerbate these response patterns. Low self-esteem—the chronic, enduring sense of low self-worth (Leary and Baumeister, 2000)—appears to make people particularly vulnerable in the face rejection. Specifically, low self-esteem individuals have been shown to experience elevated levels of cortisol stress responses (Pruessner et al., 1999) and negative affect (Nezlek and Plesko, 2001) when faced with rejection.
Owing to the potency of rejection as an interpersonal stressor, people normatively engage regulatory efforts to manage the impact of social threat (Eisenberger et al., 2003; Wager et al., 2009). One potential mechanism through which reactions to threat in general and social rejection specifically might be dampened is attentional control (Derryberry and Reed, 2002)—the ability to direct and maintain attentional focus. Attentional control has been related to faster disengagement from threatening stimuli in anxious individuals (Derryberry and Reed, 2002), and to lower negative affect after a laboratory induction of negative mood (Compton, 2000). Infants with high attentional control tend to be less reactive in threatening, novel situations (Rothbart and Bates, 1998) and show better psycho-social adjustment later in life as adults (Eisenberg et al., 2000; Kochanska and Knaack, 2003). Finally, among adults who are prone to borderline personality disorder, higher attentional control is related to lower likelihood of exhibiting symptomatology (Ayduk et al., 2008).
In our previous work, we hypothesized that attentional control might act as a protective mechanism for vulnerable low self-esteem individuals when faced with social rejection; individuals otherwise prone to display heightened threat reactions (Gyurak and Ayduk, 2007). We assessed physiological threat as the strength of the startle eye-blink responses to loud noise bursts paired with rejection stimuli. The startle eye-blink is one component of the biologically based defensive response system, generated by the amygdalae as a reaction to threatening stimuli (Davis et al., 1982). However, startle responses can be modulated by regulatory attempts (Jackson et al., 2000). Thus, we hypothesized that individual differences in attentional control might buffer low self-esteem individuals from heightened reactivity in response to rejection. In line with this, we found that self-esteem and attentional control interacted, and attentional control moderated the effects of low self-esteem, such that low self-esteem was related to smaller eye blink responses to rejection among those high in attentional control.
In our previous work (Gyurak and Ayduk, 2007), we speculated that this lower physiological reactivity might occur via the engagement of the rostral, pregenual portion (BA24/32) of the anterior cingulate cortex (ACC) given its extensive connections to emotional centers in the brain (Devinsky et al., 1995; Lewis and Todd, 2007). More specifically, a growing body of imaging studies indicates that attentional control is associated with the recruitment of brain areas that enact cognitive control over behavior and emotions (rostral ACC, rACC and dorsal ACC, dACC; Mathews et al., 2004; Etkin, et al., 2006; Carter and van Veen, 2007; Egner et al., 2008). Furthermore, there appears to be some specialization in the ACC, such that the dACC subserves cognitive regulation and expression of negative emotional stimuli (Egner et al., 2008; Etkin, et al., 2011), whereas the rACC is more closely linked to modulation of control and regulatory functions in emotional contexts (Bush et al., 2000; Etkin et al., 2006, 2011). In line with this, better attentional control—measured as an individual difference using a questionnaire measure (Derryberry and Reed, 2002) has been found to relate to activation in the ACC. For example, Bishop and colleagues (Bishop et al., 2007) found stronger blood oxygenation level-dependent (BOLD) signal in the left dACC as a function of attentional control in a task where participants had to disregard threatening negative faces to complete a cognitive digit-recognition task. Importantly, individual difference in attentional control were found to be related to activation in the left rACC in a task where participants were asked to passively view facial expressions of fear vs neutral faces (Mathews et al., 2004). These authors concluded that individual differences in the recruitment of the left rACC index engagement of control processes over emotional threat and the ability to maintain attention in the face of threatening stimuli.
Current study
The goal of the current study was twofold. (i) In a functional imaging study, we sought to explore the role of the rACC in individual differences in self-esteem and attentional control when processing social rejection stimuli; and (ii) assess the mediating role of rACC activation on emotional evaluations of social rejection. Similar to our previous work (Gyurak and Ayduk, 2007) we chose to investigate self-esteem and attentional control as they naturally vary along continuous dimensions in a regression framework to increase generalizability and power.
Drawing on a meta-analysis conducted by Bush and colleagues (Bush et al., 2000) a recent review by Etkin and colleagues (Etkin et al., 2011) and several imaging studies (Mathews et al., 2004; Etkin et al., 2006) who found that activation in the rACC is related to regulatory control over emotional information we defined the rACC as our a priori region of interest (ROI). However, given that there is still an ongoing debate about regional specialization of emotional functions in the ACC, statistical analysis was conducted in a bilateral ACC mask (see Etkin et al., 2011).
Participants were presented with previously validated stimuli in the form of evocative paintings proven to elicit feeling of social rejection (Downey et al., 2004; Gyurak and Ayduk, 2007; Kross et al., 2007). We controlled for neural activation associated with processing negative paintings by creating activation contrasts between rejection and negative control trials—and thus isolated rejection related processing. We hypothesized that individuals who are vulnerable to over-react to social rejection (low self-esteem), but able to control these responses (high attentional control) would show spontaneous engagement in rACC when processing social rejection stimuli but low self-esteem individuals low in attentional control would not. We expected high self-esteem individuals to engage rACC in the presence of social rejection cues regardless of their attentional control levels. This expectation is based on previous findings showing that people who are not sensitive to social rejection, such as those high in self-esteem, automatically engage regulation related brain areas when processing social rejection information (Kross et al., 2007).
To assess the mediating role of rACC activation on emotional evaluations of rejection, participants rated the social rejection stimuli (vs negative stimuli) outside the scanner on several relevant emotional dimensions. We created an emotional evaluation composite and conducted a mediational analysis between self-esteem and attentional control on emotional evaluation of social rejection with rACC activation as a mediator.
METHODS
Participants and procedures
English-speaking, right-handed undergraduate students were recruited as participants for a larger study on emotional processing in ongoing dating relationships on the UC Berkeley campus (see Ayduk et al., 2009 for details on the larger study). A subset of these participants took part in the imaging study (n = 23, 12 males, 11 couples, 1 individual) and the separate behavioral assessment. All participants (in the larger study) completed a laboratory session in which they filled out self-report questionnaires and other tasks unrelated to the present study. At that time, an invitation to participate in the fMRI scan was extended. Interested participants were then screened for fMRI procedure contraindications (e.g. metal in the body, claustrophobia, etc.) and history or current diagnosis of neurological or psychiatric illness. Eligible participants were scheduled and scanned. The mean age of the participants in the current study was 20.61 years (s.d. = 1.77). Thirty-two percent of the sample was Asian, 40% Caucasian, 28% other race. 12% of the sample indicated being Hispanic.
Participants were scheduled for the fMRI session individually. During the fMRI session participants completed the painting task (described below) and other previously reported tasks (Hooker et al., 2010) that are not pertinent to the current study. Following the fMRI scan, participants were scheduled for the separate behavioral session within 1–2 weeks where they completed the rejection perception task (described below). Participants were compensated $50 for the fMRI scan and $15/h for the behavioral session.
Questionnaire measures
Self-esteem
Participants completed Rosenberg’s (Rosenberg, 1989) 10-item questionnaire to assess subjective feelings of self-esteem. The scale is highly face valid and internally consistent and asks participants to rate their self worth on items like: I feel that I have a number of good qualities. Ratings were made on a scale from 1 (does not describe me at all) to 7 (describes me very well). Items were reverse-scored when appropriate and then averaged (M = 5.30, s.d. = 0.80).
Attentional Control
Participants completed the 20-item Attentional Control Scale (Derryberry and Reed, 2002) on a 4-point scale (1 = almost never to 4 = always). The Attentional Control Scale is internally consistent scale and is designed to tap into subjective self-reports of attentional and concentration difficulties that are believed to be biological in their origins. It is unique among self-report measures in that it tries to capture biologically based individual differences in effortful control at the self-report level. The scale taps into two inter-related aspects of effortful control; the ability to focus attention (e.g. When trying to focus my attention on something, I have difficulty blocking out distracting thoughts.) and the ability to shift attention (e.g. It takes me a while to get really involved in a new task.). After appropriate items were reverse scored, we computed each participants overall attentional control score by averaging the 20 items (M = 2.77, s.d. = 0.29).
fMRI painting task
Participants were asked to appraise how ‘negative–positive’ they felt while viewing artwork depicting negative, positive, social rejection, social acceptance and neutral themes. The current study focused on the rejection and negative paintings to isolate the neural networks associated with the processing of social rejection specifically while controlling for the effects of general negativity. Artwork used in this task was identical to the set used in our previous work (Gyurak and Ayduk, 2007) and similar to other imaging studies (e.g. Kross et al., 2007). This set of paintings represents a number of improvements over previously used stimuli sets (e.g. Downey et al., 2004; Kross et al., 2007). Negative paintings used here are representational and paintings are produced by a variety of artists, as opposed to a single source artist used in previous studies. Paintings were pilot tested to be negative, positive or neutral or to elicit social rejection, or acceptance. Results of the pilot were previously reported in Gyurak and Ayduk (2007). The paintings were scanned from high-quality slides and digitally presented on a computer screen using E-prime software (Psychology Software Tools, Pittsburgh, PA, USA). As in our previous work, we controlled for neural activation associated with processing negative paintings by creating activation contrasts between rejection and negative control trials—and thus isolated rejection related processing.
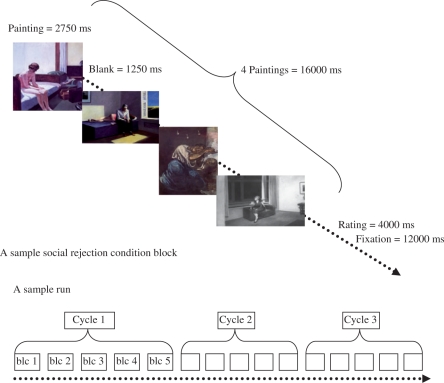
The fMRI task (Figure 1) consisted of two 8-min runs. Presentation of the painting stimuli was blocked by condition type (negative, positive, social rejection, social acceptance and neutral). Four paintings of the same category were presented within a condition block. Each painting was presented for 2750 ms, followed by a 1250 ms blank screen creating 16 s condition blocks. At the beginning of the task, participants were instructed that ‘while viewing the paintings, allow yourself to really feel whatever feelings come up for you’ to draw attention to internal feelings. After each block of trials, participants rated how ‘negative/positive the previous pictures made you feel’ on a 1 to 4 scale (1: very negative to 4: very positive). Each condition block was followed by 12 s of ‘rest’ to ensure that the hemodynamic response returned to baseline.
Fig. 1.
Schematic depiction of the fMRI task.
Across the two runs, each condition block was presented six times. Participants saw each individual painting three times during the task. Within each condition block the paintings were pseudo-randomized so that no two blocks were composed of the same four paintings. The category blocks were presented in a fixed random order. The order of presentation remained the same across participants.
Similar to our previous work, valence rating during the Painting Task (Table 1) indicated that rejection and negative pictures were perceived as equally negative.
Table 1.
Valence ratings of different painting category blocks in the fMRI task
| Scale | Category |
||||
|---|---|---|---|---|---|
| Neutral M (s.d.) | Negative M (s.d.) | Positive M (s.d.) | Rejection M (s.d.) | Acceptance M (s.d.) | |
| Valence | 2.50b (0.45) | 1.49a (0.42) | 3.46c (0.42) | 1.46a (0.50) | 3.54c (0.40) |
Note: Ratings were made on a 4-point scale. Higher ratings on valence indicate greater positivity. Means with different subscripts differ from each other at P < 0.05.
fMRI image acquisition
All images were acquired at 4 Tesla using a Varian INOVA MR scanner (Palo Alto, CA, USA) equipped with echo-planar imaging. A standard radiofrequency head coil was used, and a memory foam pillow restricted head motion. E-Prime software controlled the stimulus display and recorded participant responses via a 4-button fiber-optic keypad. An LCD projector (Epson, Long Beach, CA, USA) projected stimuli onto a backlit projection screen (Stewart, Torrance, CA, USA) within the magnet bore, which the participant viewed via a mirror mounted on the head coil. Functional images were acquired during two runs. Each run included five dummy scans (with no data acquisition) and four scans at the beginning of the run which were subsequently dropped from analysis to insure steady state magnetization for all analyzed data. This resulted in 242 whole-brain volumes per run (total of 484 volumes). Images were acquired with a set of parameters used to optimize signal in regions susceptible to drop-out due to magnetic field inhomogenities (i.e. frontal cortices). Each volume acquisition included 40, 3.5 mm thick coronal slices with a 0.5 mm inter-slice gap, with a phase encode direction oriented in the superior–inferior direction. A one-shot T2* weighted echo-planar image (EPI) sequence (TR = 2000 ms, TE = 28 ms, FOV = 22.4 cm2, matrix size = 64 × 64) was used to acquire blood-oxygenation dependent (BOLD) signal. EPI voxel size at acquisition is 3.5 × 3.5 × 4 mm. A high resolution 3D T1-weighted structural scan (MPFLASH sequence) and an in-plane low resolution T2-weighted structural scan (GEMS) were acquired for anatomical localization.
MRI data processing
MRI data was processed and analyzed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK) software. Each EPI volume was realigned in space to the first scan, using a six parameter, rigid body, least-squares transformation algorithm. None of the participants in the sample had >3 mm of movement across the two runs. After realignment, we re-sliced the coronal EPI data to the axial plane, and smoothed the data using 8 mm (FWHM). We then created and estimated a general linear model (GLM), and created contrast images of the difference between neural activity during rejection vs negative paintings. We focused on the rejection vs negative contrast to isolate neural mechanisms associated with processing of social rejection related information for theoretical reasons. Additionally, past research indicated that rejection-related (but not negative) stimuli to be diagnostic of self-esteem (Gyurak and Ayduk, 2007). In the creation of the GLM, the BOLD response for each painting category block was modeled with a boxcar function from the onset of the block. We defined each painting category block as a covariate of interest. We then convolved the canonical hemodynamic response function (hrf) with brain activity at the onset of the block with duration of 16 s. Brain activity was high-pass filtered at 200 s, scaled by the global mean, and corrected for serial autocorrelation. Finally, contrast images were co-registered to the individual participant’s co-planar (GEMS) and high resolution (MPFLASH) anatomical images, re-sliced to 2 × 2 × 2 mm isotropic voxels and then normalized to the Montreal Neurological Institute (MNI) atlas space.
fMRI data analysis
The analysis proceeded in several steps. First, to evaluate task-related activation, in SPM2 (Wellcome Department of Cognitive Neurology, London, UK) we created a whole brain, random-effects analysis across the group of participants to examine significant group level activation in the rejection vs negative contrast. This analysis was thresholded at t(22) = 3.5, P < 0.001 (uncorrected) with an extent threshold of 10 voxels.
Next, in separate regression analyses we entered grand mean centered self-esteem and attentional control scores as predictors using the rejection vs negative contrasts. We used an anatomically defined mask of bilateral ACC (WFU PickAtlas; Maldjian et al., 2003) in these analyses.1 Areas of BOLD activation associated with the main effect of each predictor (i.e. self-esteem and attentional control separately) were modeled in a t-contrast by setting the weight of the predictor at 1 and the other two predictors at 0 (e.g. for modeling self-esteem, we set the weight of self-esteem at 1, and the weight of attentional control and the self-esteem × attentional control interaction term at 0). Areas of BOLD activation associated with the interaction term (i.e. self-esteem x attentional control) in the rejection vs negative contrast was modeled in a t-contrast by setting the weight of self-esteem and attentional control at 0 and thus holding them constant and setting the weight of the self-esteem × attentional control interaction at −1. We used −1 as the weight to isolate areas of significant BOLD activation associated with ‘interference’ interactions (Cohen et al., 2003) where the value of the outcome variable (i.e. areas of BOLD activation) is significant when the predictors are of opposite signs (e.g. low self-esteem and high attentional control).2 Voxel-level family-wise error (FWE P = 0.05) correction with extent threshold of 10 was used to correct for multiple comparisons. Where significant effects were found, in order to decompose the pattern of the interaction, we used ‘MarsBaR’ (Brett et al., 2002; http://marsbar.sourceforge.net/) to extract the mean contrast values of each participant from the cluster of voxels showing a significant interaction effect. These effects were analyzed in the SAS statistical package (Version 9.1) and the pattern of interaction was graphed for visualization purposes.
Emotional evaluation task
The emotional evaluation task was designed to assess emotional-evaluative processing of the same pictures used in the ‘fMRI Painting Task’ section. Participants completed the task in a separate session scheduled at the earliest available opportunity, usually 1–2 weeks after the MRI scan in a sound-attenuated behavioral testing room. Paintings were presented using E-prime, and were displayed for 6 s. The order of the paintings was randomized across participants. After picture offset, participants were asked to evaluate how the painting made them feel on three scales. Valence: unhappy/bad-happy/good (1: very bad—5: very good), arousal: calm-aroused (1: very calm—5: very alert), and rejection: rejecting–accepting (1: highly rejected—5: highly accepted). Ratings were self-paced, each question and corresponding scale appeared on the screen until the participant submitted their response via key press on the key board. To parallel the method used in the fMRI task, we focused on the rejection and negative paintings (by creating a difference score on all three scales separately).
RESULTS
Main effects of task
Areas of BOLD activation in the rejection vs negative contrast appear in Table 2 (P < 0.001 uncorrected, extent threshold 10). Areas that survived whole brain FWE correction included a cluster in the right middle temporal gyrus/extending to the precuneus.
Table 2.
Significant activations at the group level in the rejection vs negative contrast
| Brain region | Voxels | BA | x, y, z | t-value |
|---|---|---|---|---|
| Right parahippocampal gyrus | 2249 | 27 | 16, −40, −4 | 5.95 |
| Right middle temporal gyrus/extending to precuneus | 1320 | 21 | 58, −50, 14 | 7.61a |
| Left middle temporal gyrus | 472 | 21 | −54, −56, 18 | 4.20 |
| Left lateral occipital cortex | 139 | 19 | −18, −52, 0 | 4.02 |
Note: BA = Brodmann Area; x, y, z = center of mass according to MNI coordinates, P < 0.001 uncorrected, extent threshold 10.
aThe only area that survives whole brain FWE correction is the Right middle temporal gyrus/extending to precuneus, P = 0.016.
Brain areas associated with the main effects of self-esteem and attentional control
There were no areas of significant activation associated with the main effects of self-esteem or attentional control. Next, we examined the effect of the interaction term.
Brain areas associated with the self-esteem × attentional control interaction
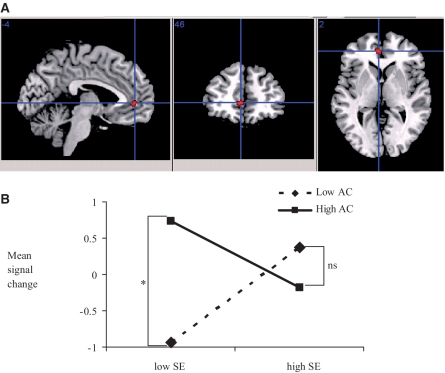
Consistent with our predictions, a cluster in the rACC on the left side was associated with the interaction term of self-esteem × attentional control, x, y, z = −4, 46, 2, t(21) = 4.72, FWE P = 0.03, (see Figure 2A). Mean contrast values were extracted using ‘MarsBaR’. Analyses of distribution conducted in SAS indicated that one participant's contrast value was more extreme than the rest, though it did not qualify as an outlier (over 2.5 s.d. away from the group’s mean), thus we kept this person in the analyses. Re-analyses of the data without this observation revealed the same pattern of results, and the main interaction findings remained significant but some of the simple slope tests did not reach conventional level of significance. Figure 2B depicts the pattern of interaction where contrast values from the rACC region were entered as dependent variables, centered self-esteem, attentional control and their interaction term as predictors in a multiple regression analysis. Results show that rACC activation was significantly predicted by the self-esteem x attentional control interaction term, F(1, 19) = 20.51, P = 0.0004, while none of the main effects were significant. Follow-up simple slopes analyses 1 s.d. above and below the respective means of the centered predictors were conducted to examine the pattern of interaction (Aiken and West, 1991).
Fig. 2.
(A) BOLD activation significantly related to the interaction term of self-esteem and attentional control in the left rACC area [x, y, z = −4, 46, 2, t(20) = 4.73, FWE P = 0.03] in the fMRI painting task in the rejection vs negative contrast. Crosshairs are aligned at center of mass. (B) Contrast values were derived from the left rACC area and predicted values were graphed at 1 s.d. above and below the respective means of the centered predictors. Images for display were thresholded at t(21) = 3.78, P < 0.001, extent threshold 10 voxels. Self-esteem (SE), attentional control (AC). *P < 0.05.
First, we examined the association between attentional control and BOLD activation separately for participants low and high in self-esteem. These analyses showed that consistent with our prediction, among vulnerable low self-esteem participants, those higher in attentional control showed significantly more BOLD activation in the rACC in response to the rejection than to the negative paintings, β = 0.85, t(21) = 4.94, P < 0.0001, whereas among high self-esteem participants, the slope of attentional control did not reach significance, β = −0.23, t(21) = −1.10, P = 0.30. Next, we examined the slope of self-esteem separately for low and high attentional control. Among low attentional control participants, the slope of self-esteem was significant such that higher self-esteem was related to more rACC activation, β = .63, t(21) = 3.56, P = 0.002, whereas among high attentional control participants, the slopes of self-esteem was significantly related to less rACC activation, β = −0.46, t(21) = −2.19, P = 0.04. These results demonstrate that in the face of rejection threat low self-esteem-high attentional control individuals and high self-esteem individuals as a group (regardless of their level of attentional control) engage the rACC to a greater degree than low self-esteem-low attentional control individuals (Figure 2 panel B).
Emotional evaluation task
We examined the relationship between mean contrast values from the rACC and valence, arousal and rejection appraisals of rejection stimuli (vs negative stimuli) using the SAS package. Ratings of valence were in the expected direction (indicating more positive perceptions) but did not reach significance, r(21) = 0.28, P = 0.19. Importantly, correlations indicated that higher rACC recruitment was related to lower arousal, r(21) = −0.45, P = 0.03, and more acceptance appraisals, r(21) = 0.48, P = 0.01. We thus created a composite emotion evaluation score of arousal (reverse scored) and acceptance ratings for subsequent mediational analyses, Cronbach’s α 0.64. Correlation between emotional evaluation of rejection (vs negative) stimuli and activation in the rACC was significant, r(21) = 0.73, P < 0.001, indicating that more rACC activation was related to more favorable (less arousal and more acceptance) evaluations of rejection stimuli.3
Does activation in the rACC mediate the relationship between self-esteem and attentional control on emotional evaluation of rejection?
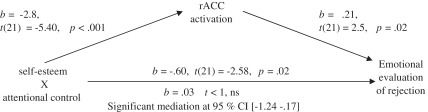
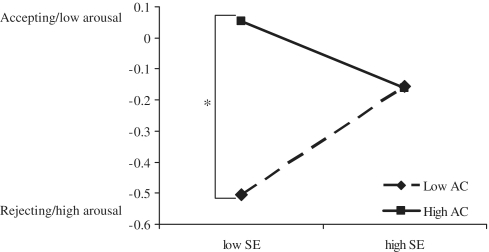
In order to address this question, we conducted a mediational analysis using bootstrapping (Preacher and Hayes, 2008) implemented in SAS. Emotional evaluation of rejection stimuli was entered as the outcome variable, rACC activation served as the mediator and the interaction term between self-esteem and attentional control was the predictor while the main effects of self-esteem and attentional control were entered as control variables. In order for a mediation to work, first we had to establish three links between our respective predictors (Figure 3). The first critical link between self-esteem and attentional control and emotional evaluation of rejection was significant, b = −0.57, t(21) = −2.58, P = 0.02. Because the predictor in this case is the interaction between self-esteem and attentional control, we ran follow-up simple slopes analysis to visualize these effects (Figure 4). As expected, among low self-esteem participants, attention control was related to more accepting/low arousal evaluations of rejection stimuli, b = 0.92, t(21) = 3.81, P = 0.001. Among high self-esteem participants attentional control had no effect, b = 0.01, t < 1, ns. Furthermore, among low attentional control, self-esteem was related to more accepting/low arousal evaluations, b = 0.20, t(21) = 2.29, P = 0.03. Finally, among high attentional control, the slope of self-esteem was not significant, b = −0.12, t(21) = −1.17, P = 0.26. These results closely parallel the effects of self-esteem and attentional control on rACC activation and reveal a more positive evaluation of rejection stimuli among low self-esteem individuals who are high on attentional control relative to those with low self-esteem and low attentional control.
Fig. 3.
Mediational analysis showing that rACC activation fully mediates the relationship between self-esteem × attentional control and emotional evaluations of rejection stimuli.
Fig. 4.
Emotional evaluation of rejection (vs negative) stimuli as a function of self-esteem and attentional control.
The second critical link was between self-esteem and attentional control and rACC activation. Results for this link have been reported above, but were confirmed again using bootstrapping, b = −2.8, t(21) = −5.40, P < 0.001. This relationship is visualized in Figure 2B.
The third critical link was between rACC activation and emotional evaluations of rejection, also reported above and confirmed by bootstrapping, b = 0.21, t(21) = 2.5, P = 0.02. In the final step of the mediation the predictors (self-esteem × attentional control) were entered simultaneously with the mediator (rACC). These results indicated that rACC remained a significant predictor, but the relationship between self-esteem and attentional control on emotional evaluation was no longer significant, b = 0.03, t < 1, ns and a significant mediation was confirmed at 95% CI (−1.2460 to −0.1713).4
DISCUSSION
Social rejection, a ubiquitous source of threat in human life poses an emotion regulatory challenge to most people. Managing social rejection effectively is likely to depend on top-down control processes that enable regulation of immediate responses and better anticipation of future outcomes (Wager et al., 2009). Results of the current study demonstrate that individual differences in self-esteem and regulatory abilities can alter the nature of these top-down neurobiological responses to social rejection. Our results show that those low self-esteem individuals who are also low in attentional control capacity show a less advantageous pattern of neural responses to rejection than their low self-esteem high regulatory capacity counterparts. Specifically, we find that low self-esteem individuals who are also low in attentional control show less activity in emotion control related regions in the rACC while appraising their personal feelings in response to rejection-themed images.
On the flip side, our results show that high attentional control alters the neural signature among low self-esteem individuals resulting in higher engagement of the rACC—a neural response indicative of more adaptive responding to threat (Mathews et al., 2004). Similar to rACC activation, we also found that self-esteem and attentional control modified emotional evaluations of rejection stimuli. Low self-esteem but high attentional control participants perceived rejection-themed images as more arousing and accepting than their low attentional control counterparts. Since these evaluations were assessed in a separate behavioral session, we have convincing evidence that the constellation self-esteem and attentional control reliably shapes recruitment of emotion control-related brain areas and perceptions of rejection. Most importantly, activation in the rACC mediated the relationship between personality and emotional evaluation of rejection, indicating that more favorable perceptions of rejection come about via the engagement of critical regulatory regions.
These results are consistent with our earlier findings and hypotheses on the buffering role of attentional control among low self-esteem individuals (Gyurak and Ayduk, 2007). In the current study we found that those who showed the strongest physiological reactivity to rejection (i.e. low self-esteem low attentional control individuals) in our prior work (Gyurak and Ayduk, 2007), demonstrated the lowest levels of rACC activation and the least favorable emotional evaluation of rejection. Lack of activation in the rACC thus appears to result in rating rejection as more arousing and more rejecting. These perceptions, in turn might pave the way for heightened physiological reactions and negative behavioral responses down the road.
The results of this study are also consistent with findings that implicate the rACC in situations when the organism needs to meet the demands of a heightened emotion regulatory challenge (Bush et al., 2000; Mathews et al., 2004; Etkin et al., 2006; Bishop et al., 2007; Wager et al., 2009). Furthermore, these results underscore findings by animal and neuroimaging studies that show that the rACC is located at a critical regulatory juncture (for a review see, Etkin et al., 2011) and is optimally positioned to modulate emotional responses. Collectively, the results argue that the activation of the rACC during social threat serves an important regulatory role and enables a more beneficial profile of responding. Adding further to this argument, there is support from a recent PET study that more rACC activation during the Trier Social Stress Task, a potent elicitor of rejected feelings, can buffer prototypical stress responses, such as elevated cortisol level, that typically ensue after social rejection (Kern et al., 2008).
Although previous studies have looked at how self-esteem relates to neural activity (Onoda et al., 2010; Somerville et al., 2010), none has examined the effect of self-esteem in the context of attentional control. Nonetheless, these studies suggest that the rACC and medial prefrontal cortex (MPFC) in general show heightened activation among low self-esteem individuals—an effect that was not found in our study. There are a number of differences that might account for these findings. First, unlike these previous studies, we did not subject our participants to direct social exclusion or negative feedback; rather, we showed them evocative stimuli that are shown to elicit feelings of rejection and encouraged them to freely process these while monitoring their own feelings. Viewed this way, our task may index spontaneous processing and modulation of self-generated feelings, rather than emotional reactivity and self-referential processing to receiving social rejection. Second, our analyses focused on social rejection while controlling for general negative valence. We think this is a critical distinction, given that self-esteem is typically correlated with high neuroticism, a marker of general negative affect (Judge et al., 2002). Lastly, these studies did not report correlations between perceptions of social rejection and rACC activation, thus it is not clear how activation in this area might be related to emotional evaluations of rejection.
Our results support recent advances in clinical science that characterize psychological disorders, especially anxiety disorders as an interaction of underlying dispositional vulnerability exacerbated by poor effortful control (Beevers, 2005; Carver et al., 2008; MacDonald, 2008). Even though our sample was recruited to be psychologically healthy, we found that not all low self-esteem individuals are equally reactive in the face of rejection. Those characterized with high attentional control recruit regulatory resources whereas those low in effortful control do not. The Attentional Control Scale is designed to tap into biological differences in the efficiency of effortful control that provide the foundation for personality and other more complex skills related to self and emotion regulation (Derryberry and Rothbart, 1997). In a separate study (Gyurak, 2010, Study 4), we explored the correlates of the Attentional Control Scale on the domain of executive functioning and found that the scale was related to performance on complex executive function tasks such as verbal fluency, but not to more simple executive functions such as working-memory, inhibition or task switching. Better verbal fluency performance in turn has been shown to relate to better emotion regulation in several laboratory-based emotion regulation tests (Gyurak et al., 2009, 2012). Thus, scores on the Attentional Control Scale are likely to index one’s ability to direct, alter and maintain attentional focus in the service of goal attainment—a central feature of emotion regulation. We speculate that low attentional control indexes low regulatory abilities and in combination with a vulnerability (low self-esteem) it might be a precursor of later mental health problems.
Several limitations of this research are worth mentioning. fMRI methodology, especially with the blocked task design used here does not allow us to speak to the timing and orchestration of the regulatory sequence implemented by the rACC; this requires future research. Furthermore, our results do not reveal the precise mechanism of regulation that the rACC supports. The rACC might aid several modes of emotion regulation, for example, cardiovascular regulation (Gianaros et al., 2005) or simple control operations (Bush et al., 2000) or implicit emotion regulation (Etkin et al., 2011) or perhaps all simultaneously. Further research is needed to elaborate on these mechanisms.
In conclusion, the present study contributes to our understanding of the vulnerability and protection pattern exhibited by low self-esteem but high attentional control individuals in the face or rejection. Additionally, these findings offer a more nuanced view of these personality dispositions by elucidating the brain correlates that play an important role in lower reactivity in response to rejection.
Conflict of Interest
None declared.
Acknowledgments
We thank Silvia Bunge, Robert W. Levenson, Eduardo Andrade, Ethan Kross, Amit Etkin, Ashley Chen and members of the Stanford Psychophysiology Lab for their comments. This work was part of Anett Gyurak’s PhD dissertation. This research was supported by grants from the National Institute of Mental Health RO1MH0697043 (Geraldine Downey and O.A.), T32MH20006 (Robert W. Levenson and A.G.), K08MH71746 (C.I.H.) and the NARSAD Young Investigator Award (C.I.H.).
Footnotes
1We also conducted an unmasked, whole brain P = 0.001 uncorrected regression analyses to explore any additional areas of activation outside of the ACC. In addition to the rACC activation reported in the main text, these analyses revealed a significant locus of activation in left superior frontal gyrus (SFG) in BA 10 (x, y, z:−14, 62, 20) that survived small volume correction in an anatomically defined BA10 mask, t(21) = 5.13, P < 0.05. We explored this area in similar analyses reported for the rACC and found very similar pattern of results for self-esteem and attentional control though less consistent. For example, we failed to find a significant mediation effect, because there was no relationship between brain activation in the SFG and emotional evaluations of rejection stimuli. The SFG is considered to be an important emotion regulatory region, thus it is theoretically consistent that low self-esteem high attentional control would have an effect there, but because in previous work we predicted the involvement of the rACC, and the results in the SFG did not relate to emotional evaluations of rejection we do not present detailed results for this cluster.
2We investigated areas of significant BOLD activation associated with the positive interaction term (contrast weight +1), but this analysis did not result in any significant areas of activation, even at lenient threshold.
3Similar to the non-significant relationship between valence ratings during the emotional evaluation task, correlations between valence ratings during the fMRI painting task and rACC activation were not significant, r(21) = −0.11, P = 0.60.
4We also evaluated the reverse mediation effect where self-esteem and attentional control served as a predictor, rACC activation as the dependent variable and emotional evaluations of rejection as the mediator. Results of bootstrapping indicated that there was ‘no evidence’ for mediation, 95% CI (−2.67 to 0.01). Thus it appears that rACC activation is responsible for the link between self-esteem and attentional control and emotional evaluations.
REFERENCES
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Ayduk O, Gyurak A, Luerssen A. Rejection sensitivity moderates the impact of rejection on self-concept clarity. Personality and Social Psychology Bulletin. 2009;35(11):1467–78. doi: 10.1177/0146167209343969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Zayas V, Downey G, Cole AB, Shoda Y, Mischel W. Rejection sensitivity and executive control: joint predictors of borderline personality features. Journal of Research in Personality. 2008;42(1):151–68. doi: 10.1016/j.jrp.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG. Cognitive vulnerability to depression: a dual process model. Clinical Psychology Review. 2005;25(7):975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cerebral cortex. 2007;17(7):1595–603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox; 2002. Presented at the 8th International Conference on Functional Mapping of the Human Brain, 2–6 June 2002, Sendai, Japan. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective and Behavioral Neuroscience. 2007;7(4):367–79. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134(6):912–43. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd edn. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Compton RJ. Ability to disengage attention predicts negative affect. Cognition and Emotion. 2000;14(3):401–15. [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1982;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111(2):225–36. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9(4):633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain: a Journal of Neurology. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: shame, physiology, and health. Journal of Personality. Special Issue: Emotions, Personality, and Health. 2004;72(6):1191–216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Downey G, Mougios V, Ayduk O, London BE, Shoda Y. Rejection sensitivity and the defensive motivational system: insights from the startle response to rejection cues. Psychological Science. 2004;15(10):668–73. doi: 10.1111/j.0956-7976.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18(6):1475–84. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: their role in predicting quality of social functioning. Journal of Personality and Social Psychology. 2000;78(1):136–57. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Science. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Dearbyshire SWG, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42(6):627–35. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A. Attenuating Reactivity among Low Self-Esteem Individuals: The Role of Attentional-Control. Berkeley, –California: University of California; 2010. [Google Scholar]
- Gyurak A, Ayduk O. Defensive physiological reactions to rejection: the effect of self-esteem and attentional control on startle responses. Psychological Science. 2007;18(10):886–92. doi: 10.1111/j.1467-9280.2007.01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Kramer JH, Miller BL, Levenson RW. Executive functions and the down-regulation and up-regulation of emotion. Cognition and Emotion. 2012;26(1):103–18. doi: 10.1080/02699931.2011.557291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Madan A, Kramer JH, Miller BL, Levenson RW. Do tests of executive functioning predict ability to downregulate emotions spontaneously and when instructed to suppress? Cognitive, Affective and Behavioral Neuroscience. 2009;9(2):144–52. doi: 10.3758/CABN.9.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Shaver PR, Goldenberg JL. Attachment, self-esteem, worldviews, and terror management: evidence for a tripartite security system. Journal of Personality and Social Psychology. 2005;88(6):999–1013. doi: 10.1037/0022-3514.88.6.999. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Gyurak A, Verosky SC, Miyakawa A, Ayduk O. Neural activity to a partner's facial expression predicts self-regulation after conflict. Biological Psychiatry. 2010;67(5):406–413. doi: 10.1016/j.biopsych.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37(4):515–22. [PubMed] [Google Scholar]
- Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? Journal of Personality and Social Psychology. 2002;83(3):693–710. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–29. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Knaack A. Effortful control as a personality characteristic of young children: antecedents, correlates, and consequences. Journal of Personality. 2003;71(6):1087–112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19(6):945–56. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Leary MR, Tambor ES, Terdal SK, Downs DL. Self-esteem as an interpersonal monitor: the sociometer hypothesis. Journal of Personality and Social Psychology. 1995;68(3):518–30. [Google Scholar]
- Leary MR, Twenge JM, Quinlivan E. Interpersonal rejection as a determinant of anger and aggression. Personality and Social Psychology Review. 2006;10(2):111–32. doi: 10.1207/s15327957pspr1002_2. [DOI] [PubMed] [Google Scholar]
- Leary MR, Baumeister RF. The nature and function of self-esteem: Sociometer theory. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 32. San Diego, CA, US: Academic Press; 2000. pp. 1–62. [Google Scholar]
- Lewis MD, Todd RM. The self-regulating brain: cortical–subcortical feedback and the development of intelligent action. Cognitive Development. 2007;22(4):406–30. [Google Scholar]
- MacDonald KB. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychological Review. 2008;115(4):1012–1031. doi: 10.1037/a0013327. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mathews A, Yiend J, Lawrence AD. Individual differences in the modulation of fear-related brain activation by attentional control. Journal of Cognitive Neuroscience. Special Issue: Social Cognitive Neuroscience. 2004;16(10):1683–94. doi: 10.1162/0898929042947810. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Plesko RM. Day-to-day relationships among self-concept clarity, self-esteem, daily events, and mood. Personality and Social Psychology Bulletin. 2001;27(2):201–11. [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, et al. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Social Cognitive and Affective Neuroscience. 2010;5(4):385–391. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality and Individual Differences. 1999;27(3):477–89. [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. rev. edn. Middletown, CT: Wesleyan University Press; 1989. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. Hoboken, NJ: John Wiley and Sons, Inc; 1998. [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20(12):3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47(3):821–35. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]