Abstract
The current study takes a new approach to understand the neural systems that support emotion-congruent judgment. The bulk of previous neural research has inferred emotional influences on judgment from disadvantageous judgments or non-random individual differences. The current study manipulated the influence of emotional information on judgments of stimuli that were equivocally composed of positive and negative attributes. Emotion-congruent processing was operationalized in two ways: neural activation significantly associated with primes that lead to emotionally congruent judgments and neural activation significantly associated with judgments that were preceded by emotionally congruent primes. Distinct regions of medial orbitofrontal cortex were associated with these patterns of emotion-congruent processing. Judgments that were incongruent with preceding primes were associated with dorsomedial prefrontal cortex, ventrolateral prefrontal cortex and lateral orbitofrontal cortex activity. The current study demonstrates a new approach to investigate the neural systems associated with emotion-congruent judgment. The findings suggest that medial OFC may support attentional processes that underlie emotion-congruent judgment.
Keywords: mood, affect, attention, orbitofrontal, fMRI
INTRODUCTION
Emotions can influence judgments by directing attention to information that has the same (i.e. congruent) valence as the emotional state (Bower et al., 1983; Murphy and Zajonc, 1993; Joormann and Gotlib, 2007; Beevers et al., 2009). For example, a speaker in a negative emotional state might interpret a furrowed brow in a listener’s facial expression as critical disbelief but a speaker in a positive emotional state might interpret the expression as deep interest. Emotionally influenced judgment has been associated with a network of brain regions, but it is difficult to draw strong conclusions about this network for several reasons. Much of the previous research has not explicitly manipulated emotion which makes it difficult to conclude that neural activity is associated with the influence of emotion (Elliott et al., 2002; Paulus et al., 2003; Camille et al., 2004; Bishop et al., 2004b; Coricelli et al., 2005; Kuhnen and Knutson, 2005; Shiv et al., 2005). Other research has identified neural activity associated with emotion-conguent judgment, while simultaneously eliciting the emotional state intended to influence judgments. Therefore, neural activation may reflect judgment that is influenced by an emotional state or it might reflect the processing of emotional stimuli (Camille et al., 2004; Greene et al., 2004; Kuhnen and Knutson, 2005; Beer et al., 2006; De Martino et al., 2006). Finally, other studies make it difficult to understand whether emotion-congruence reflects increased attention to information that shares valence with emotional stimuli or reflects some other process. For example, emotion can also influence depth of processing (Forgas, 1998, 2008; Bless and Fiedler, 2006). The current study addresses the interpretation challenges in previous research by manipulating the presentation of negative or neutral information before probing judgments which indicate whether attention was prioritized in an emotion-congruent manner.
Extant research suggests that emotion-congruent judgments are associated with orbitofrontal cortex (OFC), amygdala, insula and anterior cingulate cortex (ACC) activation. These associations are broadly derived from three lines of research. In one approach, emotion-congruent judgments are operationalized as those that diverge from rational judgments specified by economic models (e.g. risk-averse decisions might indicate fear of potential loss). In other words, emotion is not manipulated; it is inferred from economic decisions. For example, decisions that are overly conservative when a greater risk would maximize financial gain are inferred to indicate fear of loss. These types of decisions are associated with OFC, amygdala, insula and ACC (Camille et al., 2004; Coricelli et al., 2005; Kuhnen and Knutson, 2005; Shiv et al., 2005). A second research approach operationalizes emotion through non-randomly selected differences in chronic emotional states such as the disorders of depression or anxiety. For example, OFC and ACC activity in depressed individuals is associated with processing negative emotional stimuli that are congruent with their depressed state (Elliott et al., 2002). Additionally, OFC and amygdala in anxious individuals are associated with processing threatening stimuli that are congruent with their anxious state (Bishop et al., 2004a; Stein et al., 2007; Goldin et al., 2009). A third research approach manipulates emotional primes that accompany or precede judgments. For example, OFC activity is associated with viewing surprised facial expressions or making more conservative risk decisions in the context of negative emotional primes (Beer et al., 2006). Taken together, these lines of research suggest that OFC, amygdala, insula and ACC may be associated with emotion-congruent judgment.
However, it is difficult to draw strong conclusions about the associations of emotion-congruent judgment and activation in OFC, amygdala, insula and ACC for several reasons. Many studies infer emotion rather than manipulate it making it difficult to strongly interpret any neural activation as a reflection of emotional influences. Research with patient populations is subject to confounds of non-random selection. Additionally, research that elicits emotion simultaneously with a decision makes it challenging to disentangle neural activation elicited in response to emotional stimuli as opposed to judgments based on emotionally influenced attention. Finally, even when previous research did manipulate emotion separately from judgment, neural associations were either not assessed in relation to any kind of judgment (Kim et al., 2004) or, if they were, could have reflected a number of different processes (Beer et al., 2006). Emotion influences attention, depth of processing, motivation and a number of other processes involved in judgment (e.g. Bower et al., 1983; Murphy and Zajonc, 1993; Forgas, 1998, 2008; Gray et al., 2002; Cunningham et al., 2005; Bless and Fiedler, 2006; Joormann and Gotlib, 2007; Beevers et al., 2009). For example, gambling decisions that become more conservative as a function of negative emotion may reflect increased attention to the negative aspects of a gamble, deeper consideration of the risk involved, both of these processes or some other process altogether. Taken together, the current research provides an important foundation for making predictions about which neural regions may be associated with emotion-congruent judgment. An important next step is to design studies which avoid some of the potential confounds of previous research to more precisely understand how these neural regions relate to emotion-congruent judgment.
The current study builds on previous research by using a new approach to test the neural regions associated with emotionally congruent judgments. The study manipulates the influence of emotion on judgments of stimuli that include equal amounts of positive and negative information. Therefore, judgments that match the valence of the emotion reflect emotion-congruent processing. An additional contribution of the current study is the ability to probe emotion-congruence in two ways. First, what neural activity elicited by an emotional prime predicts whether a subsequent judgment will be emotion-congruent? Second, what neural activity elicited by a valenced judgment distinguishes whether it was made in the context of an emotion-congruent prime?
MATERIALS AND METHODS
Participants
The results from 17 participants [right handed; 12 females; ages 20–38 years, mean age = 24.7, standard deviation (s.d.) = 5.5] are reported. Data from three additional participants were excluded from analysis because the participants failed to perceive the rapidly presented stimuli. All participants provided informed consent and the study was approved by the institutional review board of the University of Texas at Austin. Participants were recruited for an experiment described as a study of emotional processing in the brain and compensated $15/h or course credit for their participation. All participants were native English speakers screened for psychological and neurological conditions as well as for medications that might influence the measurement of cerebral blood flow.
Behavioral paradigm
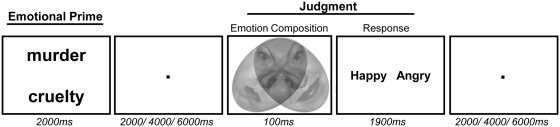
Participants performed a primed-judgment task (Figure 1) while undergoing functional magnetic resonance imaging (fMRI). Each task trial consisted of a prime, an interstimulus interval (ISI) and a judgment. For each prime screen (2 s), two negative words or two neutral words were flashed on the top/bottom and left/right of the screen. The stimuli set included eight negative (e.g. cruelty) and eight neutral (e.g. copper) words previously used in neural research on emotion (Ochsner et al., 2009).
Fig. 1.
Primed-judgment task. Participants viewed a negative emotion or neutral word prime, and then judged whether they saw an angry or happy facial expression in a composition of emotive facial expressions. Judgments of ambiguous compositions (composed of one angry face and one happy face) constituted the critical trials for analysis of emotion-congruence effects. Judgments indicating the angry expression after a negative prime were classified as emotion-congruent, whereas judgments indicating the happy expression after a negative prime were classified as emotion-incongruent.
An ISI separated each prime from a subsequent judgment. For each ISI, participants were instructed to clear their minds while viewing a blank screen with a fixation point. The length of ISI screens were jittered between events to maximize independence of neural activity estimates related to primes and judgments (variable length ISI: 50% of trials 2 s, 25% 4 s, 25% 6 s: see Donaldson et al., 2001).
After the ISI, participants then made judgments about ambiguous facial stimuli. Each judgment consisted of a rapidly presented composition of emotive facial stimuli (100 ms) followed by a probe for participants to judge whether they saw a happy or angry facial expression in the facial stimuli (response: 1900 ms). The compositions of emotive facial stimuli were two emotional faces tilted 45° toward each other so that they were partially overlaid on each other with 50% transparency (Figure 1). This arrangement of the faces made it possible to direct attention to one face or the other but difficult to direct attention to both facial expressions simultaneously. Therefore, people were likely to prioritize processing of one facial expression over the other much the way they perceive ambiguously oriented cubes as having different dominant orientations depending on where they focus their attention (Ellis and Stark, 1978). Each ambiguous facial stimulus was composed of one angry and one happy closed-mouth expression in grayscale from the NimStim image set (Tottenham et al., 2009) cropped to an oval shape to remove the hair and neck. The left–right placement of angry and happy faces were counter-balanced and gender was equally represented in stimuli. The facial stimuli were presented briefly (100 ms) and then participants pressed a button to indicate which emotion, happy or angry, they identified first in the composition of faces. Participants were explicitly instructed not to make any guesses and to only respond when they identified a facial emotion in the composition. Participants were told that the purpose of their responses was to let the experimenter know what facial expressions they saw on each trial and there were no incorrect answers. Participants were also specifically instructed that they should only look at the word primes but not respond to them. The experimenter verified that participants understood the instructions by asking about their responses after 10 practice trials. If participants asked the experimenter about the purpose of the word primes, they were told that the study examines how the brain processes different kinds of emotion and they should pay attention to both the words and the faces but only respond to the faces.
Each trial was followed by a blank screen with a fixation point [variable length intertrial interval (ITI): 50% of trials: 2 s, 25%: 4 s, 25%: 6 s]. The length of ITI screens were jittered between events to maximize independence of neural activity estimates (Donaldson et al., 2001). Participants completed 64 ambiguous judgment trials (32 negative prime followed by ambiguous facial stimuli, 32 neutral prime followed by ambiguous facial stimuli) and eight check trials (described more fully below: four neutral prime followed by only angry faces, four negative prime followed by happy faces). Trials were pseudo-randomly ordered and equally distributed across two consecutive functional scanning runs that lasted 8 min each.
Additionally, we conducted two manipulation checks. First, participants’ processing of the rapidly presented stimuli (rather than just answering based on the valence of the preceding emotional prime) was verified by ‘check trials’. In these trials, participants were presented with face compositions composed of two faces from the same emotion category. If participants were not actually processing the facial stimuli, then they would respond that they had seen a face from an emotion category that was not actually presented. Responses on ‘check’ trials were significantly higher than chance [93.36% correct, t(16) = 22.91, P < 0.05]. Check trials were not included in the measure of emotion-congruent judgment and were modeled as events of non-interest in fMRI analysis. Second, behavioral data was collected from an additional group of participants to understand how judgments in our primed conditions compared to unprimed judgments. In previous research, unprimed facial emotion identification tasks have shown that happy faces tend to be identified before angry faces (Leppänen and Hietanen, 2004; Leppänen et al., 2003). This effect also characterized the unprimed version of our judgment paradigm. Eighteen new participants (14 females; ages 18–24 years, mean age = 19.4, s.d. = 1.4) recruited from the same community as the fMRI study participants performed only the judgment portion of our experimental task. Each stimulus included equivocal amounts of happy and angry facial information. Compared to chance, participants indicated they saw the happy expression more of the time [M = 53.37%, s.d. = 7.90%, t(17) = 1.81, P < 0.05, one tailed, with the remaining 46.63% of the stimuli being identified as angry]. The Results section reports comparisons of primed and unprimed judgments.
MR data acquisition
All images were collected on a 3.0-T GE Signa EXCITE scanner at the University of Texas at Austin Imaging Research Center. Functional images were acquired with a GRAPPA sequence (TR = 2000 ms, TE = 30 ms, FOV = 240, 96 × 96 matrix, voxel size 2.5 × 2.5 × 3.3 mm) with each volume consisting of 35 axial slices. Functional volume acquisitions were time-locked to the onset of the first screen at the beginning of each trial. A high resolution SPGR T1-weighted image was also acquired from each subject.
fMRI data analysis
All statistical analyses were conducted using SPM2 (Wellcome Department of Cognitive Neurology, London). Functional images were corrected for slice-timing skew using temporal sinc-interpolation and for movement using rigid-body transformation parameters. Structural and functional volumes were normalized to T1 and EPI templates, respectively, using a 12-parameter affine transformation together with a nonlinear transformation involving cosine basis functions that resampled the volumes to 2-mm cubic voxels. Templates were based on the Montreal Neurological Institute (MNI) atlas space. The functional images were then smoothed with an 8-mm FWHM Gaussian kernel. To remove drifts within sessions, a high-pass filter with a cutoff period of 128 s was applied.
A fixed-effects analysis modeled event-related responses to primes and judgments for each participant. Neural activity related to the prime and judgment for each trial type were modeled as events using a canonical hemodynamic response function with a temporal derivative entered into a general linear model analysis. The trial events were modeled to reflect the combination of the prime (neutral or negative) and each participant’s judgment (happy or angry). Therefore, neural activity related to primes was distinguished by: (i) the emotional content of the prime and (ii) the subsequent judgment of the stimuli that followed the prime (prime screen: negative prime followed by an angry judgment, negative prime followed by a happy judgment, neutral prime followed by an angry judgment, neutral prime followed by a happy judgment). Similarly, neural activity related to judgments was distinguished by: (i) the result of the judgment and (ii) the emotional content of the prime that preceded the judgment (judgment screen: angry judgment preceded by a negative prime, happy judgment preceded by a negative prime, angry judgment preceded by a neutral prime, happy judgment preceded by a neutral prime). Interstimulus and intertrial intervals were modeled as baseline activity and the primes and judgments from unambiguous ‘check’ trials were modeled as regressors of non-interest. Contrast images were calculated for each participant and used in a second-level analysis treating participants as a random effect. Group average SPM t-statistic maps were created for each contrast of interest.
Contrasts of interests focused on neural activity in relation to prime screens and judgment screens. First, we examined whether neural activation associated with prime screens differentiated whether subsequent judgements were emotion-congruent or not. We compared activation from the negative prime screen for trials in which participants indicated they later saw the angry face to activation from the negative prime screen for trials in which participants indicated they later saw the happy face (prime screen: negative prime angry judgment > negative prime happy judgment). Therefore, this contrast and its inverse (prime screen: negative prime happy judgment > negative prime angry judgment) yielded activation associated with the prime screen that was differentiated by subsequent emotion-congruent (or emotion-incongruent) judgment rather than a difference of prime valence. Second, an analogous approach tested neural activation associated with judgment screens. To identify activity associated with making an emotion-congruent judgment, we compared activation from judgment screens in which participants indicated they saw an angry face following a negative prime to activation from angry judgments following a neutral prime (judgment screen: negative prime angry judgment > neutral prime angry judgment). In this way, activity differences to angry judgments indicated a relation to whether the preceding prime was emotion-congruent rather than a difference in the valence of the judgment. To identify activity associated with making an emotion-incongruent judgment, we compared activation from judgment screens in which participants indicated they saw a happy face following a negative prime to activation from happy judgments following a neutral prime (judgment screen: negative prime happy judgment > neutral prime happy judgment). Similarly, neural activity differences to happy judgments indicated a relation to whether the preceding prime was emotion-incongruent rather than a difference in the valence of the judgment. A final contrast compared activation to negative primes with activity to neutral primes, regardless of the subsequent response. This contrast identified neural regions that were involved in processing emotional primes but that were not specific to emotion-congruent processing. Results were interpreted using regions of interest (ROIs) derived from previous research on emotion-congruence and emotion interference in judgment (Bechara et al., 2000; Elliott et al., 2002; Camille et al., 2004; Bishop et al., 2004b; Coricelli et al., 2005; Kuhnen and Knutson, 2005; Beer et al., 2006; Etkin et al., 2006; Egner et al., 2008; Ochsner et al., 2009). Specifically, activation clusters were corrected for the size and shape of the relevant neuroanatomical volume of interest in the Automated Anatomical Labelling map (Tzourio-Mazoyer et al., 2002) [family-wise error corrected (FWE) P < 0.05 threshold, search volumes: lateral OFC, medial OFC, ventrolateral prefrontal (VLPFC: triangular and opercular part of inferior frontal gyrus), dorsolateral prefrontal (DLPFC: middle frontal gyrus), dorsomedial prefrontal (DMPFC: medial superior frontal gyrus), ACC, striatum, insula and amygdala]. Marsbar software was used to extract region of interest (ROI) parameter estimates from significant clusters for each event type (Brett et al., 2002). Furthermore, we tested these ROIs for an interaction between prime (negative or neutral) and judgment (angry or happy) to verify that activity was driven up in emotion-congruent trials (negative prime angry judgment) compared to the other conditions. Similarly, for regions associated with emotion-incongruent judgment, we tested the same interaction of Prime and Judgment to verify that activity was driven up in emotion-incongruent trials (negative prime happy judgment).
Finally, conjunction analyses were conducted using the minimum statistic compared to the conjunction null (Nichols et al., 2005) to test whether any of our significantly activated regions held for both prime screen and judgment screen contrasts.
RESULTS
Task performance
Consistent with previous research, negative primes influenced judgments in an emotion-congruent pattern (Murphy and Zajonc, 1993; Lewis et al., 2005; Joormann and Gotlib, 2007; Beevers et al., 2009). Participants indicated that they saw the angry expressions more frequently in the negative prime condition (M = 53.72% of judgments, s.d. = 10.04%) compared to the neutral prime condition [M = 43.83%, s.d. = 10.61%; t(16) = 3.83, P < 0.05] and unprimed judgments [M = 46.63%, s.d. = 7.90%, t(33) = 2.33, P < 0.05]. Judgments in the neutral prime condition were not significantly different from unprimed judgments [t(33) = 0.89, P = 0.38]. Whereas participants who performed judgments without primes were significantly less likely than chance to indicate the angry face, participants in the primed study showed a marginally significant trend to indicate the angry face more often than chance in the negative prime condition [t(16) = 1.53, P = 0.07, one tailed].
Response times in the fMRI study did not significantly differ according to prime valence [F(1, 16) = 0.20, P > 0.05], judgment valence [F(1, 16) = 2.21, P > 0.05], or the interaction of prime valence and judgment valence [F(1, 16) = 2.95, P > 0.05; negative prime angry response mean latency = 900 ms, s.d. = 169; negative prime happy response mean latency = 886 ms, s.d. = 148; neutral prime angry response mean latency = 932 ms, s.d. = 161; neutral prime happy response mean latency = 869 ms, s.d. = 161].
Medial OFC emotion prime and judgment activity distinguishes emotion-congruent judgments
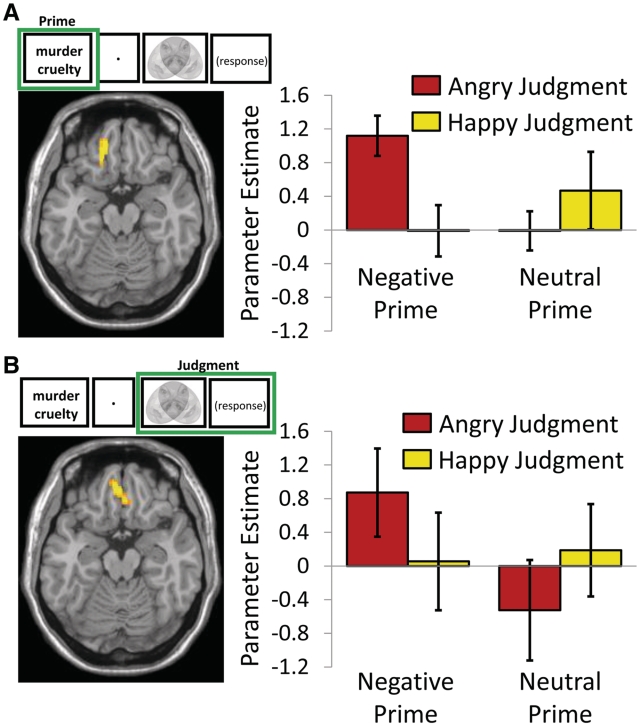
Medial OFC activity is related to emotion-congruent judgment regardless of whether emotional congruence is operationalized by prime or judgment. Medial OFC activity was greater for primes that led to emotion-congruent judgments [prime screen: negative prime angry judgment > negative prime happy judgment: peak = −16, 36, −18, Brodmann area (BA) 11, t(16) = 4.15, P < 0.05 FWE]. Additionally, medial OFC activity during Judgments was associated with emotion-congruent influences from preceding emotional cues. Medial OFC activity was greater for angry judgments influenced by the negative prime compared to the neutral prime [judgment screen: negative prime angry judgment > neutral prime angry judgment: peak = −2, 44, −18, BA 11, t(16) = 4.91, P < 0.05 FWE]. Further analyses confirmed that medial OFC activity is driven up by emotion-congruent judgment in comparison to all experimental conditions. Parameter estimates from the prime contrast showed a significant interaction between prime (negative or neutral) and subsequent judgment (angry or happy) [F(1, 16) = 7.84, P < 0.05]. Figure 2A shows that medial OFC derived from the prime contrast was most engaged by negative primes when they led to emotion-congruent judgments. Parameter estimates from the judgment contrast showed a significant interaction between prime (negative or neutral) and judgment (angry or happy) interaction [F(1, 16) = 15.58, P < 0.05]. Figure 2B shows that medial OFC derived from the judgment contrast was most engaged in relation to emotion-congruent angry judgments on negative primed trials. Finally, a conjunction analyses did not show significant overlap in theses medial OFC regions. That is, the regions of medial OFC that showed emotion-congruent activity related to the prime (peak: 16, 36, −18) were distinct from those showing emotion-congruent activity related to judgment (peak: 2, 44, −18).
Fig. 2.
Medial OFC emotion-congruent activity related to processing emotional primes and judging equivocally positive and negative stimuli. (A) Left column: a cluster of left medial OFC (peak = 16, 36, −18) shows increased activity to negative primes that lead to emotion-congruent judgments (prime screen: negative prime angry judgment > negative prime happy judgment, P < 0.05, FWE). Right column: parameter estimates for activity in this region related to negative and neutral primes, split by whether the subsequent judgment was angry or happy. (B) Left column: A cluster of bilateral medial OFC (peak = 2, 44, −18) shows increased activity during emotion-congruent judgments (judgment screen: negative prime angry judgment > neutral prime angry judgment, P < 0.05, FWE). Right column: parameter estimates for activity in this region related to judgments following negative and neutral primes, split by whether the judgment was angry or happy. Error bars represent standard error.
In summary, analyses of prime- and judgment-related neural activity suggests that medial OFC regions are involved in emotion-congruent judgment in two ways: processing emotional stimuli such that they predict subsequent emotion-congruence of judgment and in making valence judgments that are congruent with emotional primes.
DMPFC and right lateral prefrontal cortex are associated with emotion-incongruent judgment
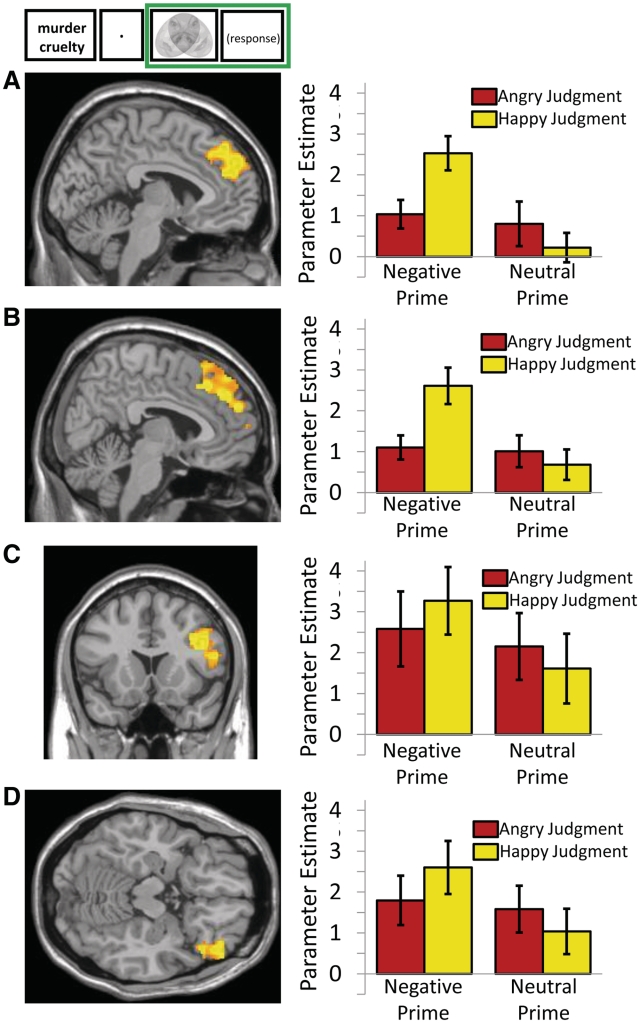
Bilateral DMPFC, right VLPFC and right lateral OFC activity was greater for emotion-incongruent judgments [judgment screen: negative prime happy judgment > neutral prime happy judgment: left DMPFC peak = −4, 54, 34, BA 9, t(16) = 5.77, P < 0.05 FWE; right DMPFC peak = 10, 54, 24, BA 9, t(16) = 5.22, P < 0.05 FWE; right VLPFC peak = 44, 16, 26, BA 44, t(16) = 5.65, P < 0.05 FWE; right lateral OFC peak = 46, 36, −16, BA 47, t(16) = 5.29, P < 0.05 FWE]. Further analyses confirmed that activity in these regions is driven up by emotion-incongruent judgment in comparison to all experimental conditions. Parameter estimates of judgment activity derived from these regions were characterized by an interaction of prime (negative or neutral) and judgment (happy or angry) [left DMPFC: F(1, 16) = 11.28; right DMPFC: F(1, 16) = 11.83; right VLPFC: F(1, 16) = 5.27; right lateral OFC: F(1, 16) = 7.75; all P < 0.05]. Activity in these bilateral DMPFC, right VLPFC and right lateral OFC regions was most engaged for emotion-incongruent happy judgments on negative primed trials. Figure 3 shows that activity derived from the judgment contrast was most engaged in relation to emotion-incongruent happy judgments following negative primes. In summary, bilateral DMPFC, right VLPFC and right lateral OFC activity was associated with making valence judgments that were incongruent with emotional primes.
Fig. 3.
Emotion-incongruent neural activity related to judging equivocally positive and negative stimuli. Left column shows clusters with increased activity during emotion-incongruent judgments (judgment screen: negative prime happy judgment > neutral prime happy judgment, P < 0.05, FWE). Right column shows parameter estimates for activity in each region related to judgments following negative and neutral primes, split by whether the judgment was angry or happy. (A) Left DMPFC (peak = −4, 54, 34). (B) Right DMPFC (peak = 10, 54, 24). (C) Right VLPFC (peak = 44, 16, 26). (D) Right lateral OFC (peak = 46, 36, −16). Error bars represent standard error.
Lateral OFC is associated with negative compared to neutral primes
Neural activity might relate to the processing of emotional primes even if it is not associated with emotion-congruent judgment. This pattern of activity was examined with a contrast of activity during negative primes compared to activity during neutral primes for all trials. Left lateral OFC activity was greater for negative compared to neutral primes [peak = −38, 22, −10, BA 47, t(16) = 5.62, P < 0.05 FWE]. No significant activity was detected showing greater activity for neutral compared to negative primes.
DISCUSSION
The current study builds on previous research by taking a new approach to investigating the neural systems that support emotional influences on judgment. An association between medial OFC activation and emotion-congruent judgment was found both when emotion-congruence was operationalized by primes leading to subsequent emotion-congruent judgments and by judgments that shared valence with their preceding emotional prime. Additionally, bilateral DMPFC, right VLPFC and right lateral OFC are associated with judgments that do not share valence with their preceding prime. These findings have implications for refining our understanding of the neural systems associated with emotion-congruent judgment.
Instead of inferring emotional influences from disadvantageous judgments or non-randomly assigned individual differences, the current study took a new approach to emotion-congruent judgment by examining neural activation in relation to the effect of manipulated emotional primes on judgment. The new approach found an association between emotion-congruent judgment and one of the previously identified regions, the medial OFC (Bechara et al., 2000; Camille et al., 2004; Coricelli et al., 2005; Shiv et al., 2005; Wang et al., 2006; Goldin et al., 2009). More specifically, two relations between medial OFC and emotion-congruent judgment were identified. One region of medial OFC is engaged by emotional primes that lead to emotion-congruent judgments and another region of medial OFC is engaged by judgments that are preceded by emotion-congruent primes. These findings suggest that medial OFC supports emotion-congruent judgment in cases where negative emotional information influences judgments drawn from equivocal sources of negative and positive attributes. Although previous research has associated other regions with emotion-congruent judgment (e.g. amygdala, insula and ACC: Sanfey et al., 2003; Bishop et al., 2004a; Camille et al., 2004; Coricelli et al., 2005; Kuhnen and Knutson, 2005; Shiv et al., 2005; Stein et al., 2007; Goldin et al., 2009), they were not significantly activated in the current study even in contrast between the negative and neutral primes. Future research that broadly adopts the approach of the current study will be helpful in refining our understanding of the roles these regions play in emotion-congruent judgment. For example, it may be that the involvement of certain regions are dependent on factors that differed unsystematically across previous studies such as whether judgments are influenced by positive, negative or more specific emotions or the type of judgment itself. Future research that manipulates the influence of emotional information on judgments is needed to more systematically understand the neural systems that support emotion-congruent judgment.
The current study suggests that one way the OFC may support emotion-congruent judgment is by directing attention to aspects of judgments that share valence with emotional states. Behavioral research has shown that emotions can influence a number of component processes involved in judgment. For example, emotions may increase the salience of similarly valenced aspects of judgment (Bower et al., 1983; Murphy and Zajonc, 1993; Joormann and Gotlib, 2007; Beevers et al., 2009) or influence whether the judgment is processed more or less deeply (Forgas, 1998, 2008; Bless and Fiedler, 2006). Therefore, in previous neural research, any number of processes may account for the association between OFC and viewing surprised facial expressions or making conservative gambling choices in negative contexts (Kim et al., 2004; Beer et al., 2006). The study on surprised facial expressions was not designed to measure how judgments were influenced by the negative context making it difficult to interpret underlying processes. Additionally, the influence of negative emotion on conservative gambling choices may have indicated increased attention to the potential losses versus the potential gains, thinking more carefully about the choices, or both (e.g. Bechara et al., 2000; Shiv et al., 2005). In the current study, participants had limited time to make judgments of stimuli composed of equal parts negative and positive information. Therefore, judgments that shared valence with preceding primes most likely reflected attention shaped by the prime rather than differences in how deeply the stimuli were processed before judgment. Consistent with the interpretation that effects were not accounted for by differences in depth of processing, there were not significant differences in reaction time for emotion-congruent judgments compared to emotion-incongruent judgments. If attention is one way that medial OFC supports emotion-congruent judgment, then the current findings suggest that different attentional mechanisms may be engaged when emotion leads to emotion-congruent judgment compared to when judgments are influenced by emotional states. When emotion leads to emotion-congruent judgments, medial OFC may be important for directing attention to subsequent information that shares valence with the emotional state. When judgments are influenced by an emotional state, medial OFC may be involved in selecting emotion-congruent information from competing information. The current study suggests that OFC supports attentional processes that underlie emotion-congruent judgment but does not rule out the possibility that it is also important for other processes underlying emotion-congruent judgment. Future research will be beneficial for understanding whether OFC is also important for other processes that support emotional-congruence. For example, people in negative emotional states tend to process information more deeply. Therefore, people in the negative emotional states tend to better account for situational factors that influence other people’s behavior rather than the more common judgment that a person’s behavior reflects their disposition (Forgas, 1998). If OFC is also important for mediating emotional influences on depth of processing, then it should be involved in the effect of negative emotion on behavioral attributions. Alternatively, it may be that other neural regions associated with emotion-congruent judgment underlie emotional influences on depth of processing in a judgment.
There were a number of parallels between the neural regions associated with emotion-incongruent judgment in the current study and neural regions previously associated with the inhibition of interfering information. In the current study, DMPFC, VLPFC and lateral OFC activity increased when participants made judgments that were inconsistent with the valence of a preceding prime. Regions similar to the DMPFC, VLPFC and lateral OFC emotion-incongruent processing regions have been associated with the inhibition of emotional information that interferes with a judgment task (Elliott et al., 2000; Bishop et al., 2004a; Kringelbach, 2005; Etkin et al., 2006; Egner et al., 2008; Ochsner et al., 2009). For example, in previous research, emotional interference arose when participants had to judge the valence of an angry facial expression that was imprinted with the emotionally incongruent word ‘happy’. Further research is needed to fully address whether emotion-incongruent information is processed similarly to interfering information. For example, future studies might directly contrast conditions where emotion-incongruent information interferes or does not interfere with a judgment.
Although task instructions discouraged participants from thinking about judgments in terms of correctness, it is worth considering the resemblance between the current study’s findings and a recent review which posits an association between medial OFC and reward evaluation and an association between lateral OFC and punishment evaluation (Kringelbach and Rolls, 2004). Although participants in the current study were not rewarded or punished for particular answers, activation in a medial OFC region was associated with emotion-congruent judgments, whereas activation in a lateral OFC region was associated with emotion-incongruent judgments. One possibility is that participants might have interpreted emotion-congruent judgments as correct and emotion-incongruent judgments as incorrect. In this case, it is possible that the medial and lateral OFC activations identified in the current study indicate that participants found it rewarding to direct attention to emotion-congruent stimuli or believed that emotion-congruent responses were somehow more ‘correct’ than emotion-incongruent responses. Future research will be beneficial in more directly testing this possibility because of the differences between the current study and previous research on the role of OFC in evaluating reward and punishment. The research linking medial and lateral OFC to rewards and punishments is concerned with the evaluation of explicit outcomes (such as monetary outcomes, tastes, or performance feedback; Kringelbach and Rolls, 2004) and there were no explicit outcomes or feedback that participants could evaluate in the current study. Furthermore, medial OFC activity during emotion-congruent judgments could not be driven by the reward value of the faces themselves because emotion-congruent judgments indicated negatively valenced faces. Likewise, lateral OFC activity during emotion-incongruent judgments could not be driven by the punishment value of faces because emotion-incongruent judgments indicated positively valenced faces. Nonetheless, the valence match between negative facial expressions and negative words would have been apparent to participants and it cannot be ruled out that activation during judgments might be influenced by participants making note of a match or mismatch between words and facial expressions. Future research might benefit from a task which manipulates reward and emotion-congruence.
Consistent with previous research (Kuchinke et al., 2005; Kensinger and Schacter, 2006; Lewis et al., 2007), the current study found that left lateral OFC activity was greater when participants viewed negative compared to neutral words. However, differences in left lateral OFC activation were not related to emotion-congruent judgment in the current study. Taken together, the current findings suggest that neural regions involved in processing the emotional valence of words may be somewhat distinct from the neural regions involved when emotional words direct attention to congruent information in subsequent judgments.
In conclusion, the present study took a new approach and found further support for the association between medial OFC and emotion-congruent judgment. The findings suggest that medial OFC may shape emotion-congruent judgments by directing attention to information that shares valence with emotional states. Additional research that manipulates the influence of emotion on judgment rather than inferring it from disadvantageous gambling behavior or non-random individual differences will be beneficial in refining our understanding of the neural systems that support emotional influences on judgment.
Conflict of Interest
None declared.
Acknowledgments
This research was funded by a National Science Foundation (NSF-BCS-0746017) grant to J.S.B., and University of Texas at Austin Diversity and Graduate Research Fellowships to J.P.B. We thank Callan Cooper for assistance with data collection.
REFERENCES
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beer JS, Knight RT, D'Esposito M. Controlling the integration of emotion and cognition: the role of frontal cortex in distinguishing helpful from hurtful emotional information. Psychological Science. 2006;17:448–53. doi: 10.1111/j.1467-9280.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, Fischer K. Identification of emotionally ambiguous interpersonal stimuli among dysphoric and nondysphoric individuals. Cognitive Therapy and Research. 2009;33:283–90. doi: 10.1007/s10608-008-9198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004a;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004b;24:10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bless H, Fiedler K. Mood and the regulation of information processing and behavior. In: Forgas J, editor. Affect in social thinking and behavior. New York: Psychology Press; 2006. pp. 65–84. [Google Scholar]
- Bower GH, Sahgal A, Routh DA. Affect and cognition. Philosophical Transactions of the Royal Society of London. 1983;302:387–402. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. 2002. Region of interest analysis using an SPM toolbox [abstract]. In: 8th International Conference on Functional Mapping of the Human Brain, 16(2), Sendai, Japan: Neuroimage. [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–70. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, Dolan RJ. Regret and its avoidance: a neuroimaging study of choice behavior. Nature Neuroscience. 2005;8:1255–62. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:202. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–7. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. NeuroImage. 2001;13:129–42. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18:1475–84. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Ellis SR, Stark L. Eye movements during the viewing of Necker cubes. Perception. 1978;7:575–81. doi: 10.1068/p070575. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Forgas JP. On being happy and mistaken: mood effects on the fundamental attribution error. Journal of Personality and Social Psychology. 1998;75:318–31. doi: 10.1037//0022-3514.75.2.318. [DOI] [PubMed] [Google Scholar]
- Forgas JP. Affect and cognition. Perspectives on Psychological Science. 2008;3:94–101. doi: 10.1111/j.1745-6916.2008.00067.x. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: Emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences United States of America. 2002;99:4115–20. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116:80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: Effects of valence and arousal. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:110–26. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuchinke LK, Jacobs AM, Grubich C, Vo M, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. NeuroImage. 2005;28:1022–32. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, Hietanen JK. Positive facial expressions are recognized faster than negative facial expressions, but why? Psychological Research. 2004;69:22–9. doi: 10.1007/s00426-003-0157-2. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, Tenhunen M, Hietanen JK. Faster choice-reaction times to positive than negative facial expressions. Journal of Psychophysiology. 2003;17:113–23. [Google Scholar]
- Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 2005;25:1214–23. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2007;17:742–8. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ST, Zajonc RB. Affect, cognition, and awareness: Affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology. 1993;64:723–39. doi: 10.1037//0022-3514.64.5.723. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JDE. Neural systems supporting the control of affective and cognitive conflicts. Journal of Cognitive Neuroscience. 2009;21:1841–54. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–48. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Shiv B, Loewenstein G, Bechara A, Damasio H, Damasio AR. Investment behavior and the negative side of emotion. Psychological Science. 2005;16:435–9. doi: 10.1111/j.0956-7976.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, McCarthy G. Mood alters amygdala activation to sad distractors during an attentional task. Biological Psychiatry. 2006;60:1139–46. doi: 10.1016/j.biopsych.2006.01.021. [DOI] [PubMed] [Google Scholar]