Abstract
Nitric oxide (NO) has been implicated in pancreatic β-cell death in the development of diabetes. The mechanisms underlying NO-induced β-cell death have not been clearly defined. Recently, receptor-interacting protein-1 (RIP1)-dependent necrosis, which is inhibited by necrostatin-1, an inhibitor of RIP1, has emerged as a form of regulated necrosis. Here, we show that NO donor-induced β-cell death was inhibited by necrostatin-1. Unexpectedly, however, RIP1 knockdown neither inhibited cell death nor altered the protective effects of necrostatin-1 in NO donor-treated β-cells. These results indicate that NO donor induces necrostatin-1-inhibitable necrotic β-cell death independent of RIP1. Our findings raise the possibility that NO-mediated β-cell necrosis may be a novel form of signal-regulated necrosis, which play a role in the progression of diabetes.
Keywords: Pancreatic β-cells, Cell death, NO donor, Necroptosis, Regulated necrosis, Necrostatin-1, RIP1
1. Introduction
Insulin insufficiency resulting from pancreatic β-cell failure is a major causative factor in both type 1 and type 2 diabetes [1]. The spectrum of β-cell failure responsible for these life-threatening diseases ranges from functional impairment and growth arrest to frank death of β-cells. The demise of β-cells not only contributes to insufficient β-cell mass in the pancreas, but also triggers infiltration and activation of macrophages in the islets by releasing high mobility group box 1 (HMGB1) and cyclophilin A, which, in turn, enhances inflammation and accelerates attack by immune cells.
Nitric oxide (NO) and inducible NO synthase (iNOS) have been implicated in the demise of β-cells in type 1 and type 2 diabetes [2,3]. NO and iNOS can induce both apoptosis and necrosis in various cell types, including pancreatic β-cells. Some studies have shown that NO mediates both apoptosis and necrosis of β-cells induced by proinflammatory cytokines [4]. Yet other works have demonstrated that NO preferentially induces necrosis rather than apoptosis in cultured β-cells and islet cells [2,5]. Regardless, our knowledge remains limited about the molecular mechanisms by which NO induces β-cell death.
Previously, necrosis was thought to be an accidental, uncontrolled form of cell death [6], in contrast to apoptosis which is defined as the genetically programmed cell death. Recently, accumulating evidence clearly indicates that there exists necrotic cell death which is finely regulated by a set of signal transduction pathways and designated as regulated necrosis [7, 8]. Specifically, death domain receptors (e.g., Fas/CD95, tumor necrosis factor-α [TNF-α] receptor) have been shown to elicit a form of regulated-necrosis, termed necroptosis, particularly in the presence of pan-caspase inhibitor, zVAD-fmk. Receptor-interacting protein-1 (RIP1, also known as RIPK1), an immediate downstream signaling molecule of the death domain receptors, is a serine/threonine protein kinase. The inhibition of RIP1 by a pharmacological inhibitor, necrostatin-1 (Nec-1), or knockout/knockdown of RIP1 can block necroptosis induced by anti-Fas antibody-plus-zVAD-fmk or TNF-α-plus-zVAD-fmk. Nec-1 was originally identified as a small compound inhibitor of necroptotic cell death induced by TNF-α-plus-zVAD-fmk in human Jurkat and U937 leukemia cells [9]. Later it was discovered to be a specific allosteric inhibitor of RIP1 kinase [10]. The prevention of necrotic cell death by Nec-1 has been attributed, therefore, to RIP1 inhibition.
Recent studies have shown that Nec-1 can inhibit cell death in many pathological conditions, such as ischemia/reperfusion injury and excitotoxicity, as well as in various cell types, including neuronal cells and cardiomyocytes [9,11–21]. Based on these findings, Nec-1-inhibitable necroptosis has emerged as a contributor to a wide range of pathological cell death paradigms. Several of these studies, however, did not examine the effects of RIP1 knockdown/knockout in Nec-1-inhibitable cell death [11–16,18–21], and this represents a gap in knowledge about RIP1 in the necroptotic cell death paradigm. A previous study has shown that both Nec-1 and its analogue, Nec-1i, which is incapable of inhibiting RIP1 [9], reduce infarct size to a similar extent after ischemia/reperfusion in isolated mouse hearts [15]. Other studies have shown that anti-necroptotic effect was specific for Nec-1, whereas Nec-1i was ineffective in the prevention of cell death [12]. Taken together, these findings raise the possibility that under certain circumstances Nec-1 and Nec-1i may elicit RIP1-unrelated pro-survival actions, although RIP1-dependent anti-necroptotic effects are Nec-1-specific. This possibility has not yet been investigated. Hence, it remains an open question whether RIP1 is required for all types of Nec-1-inhibitable necroptotic cell death. Moreover, it is not known whether signal-regulated necrotic cellular demise is involved in pancreatic β-cell death. Here, we show that both Nec-1 and Nec-1i inhibit NO donor-induced necrotic β-cell death independent of RIP1.
2. Materials and Methods
2.1. Materials
Necrostatin-1 (Nec-1, Enzo Life Sciences, Plymouth Meeting, PA), Nec-1i, cycloheximide, Akt inhibitor (1L6-Hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecyl- sn-glycerocarbonate) (EMD Biosciences, San Diego, CA), S-nitroso-L-glutathione (GSNO), S-nitroso-N-acetyl-D,L-penicillamine (SNAP), Dea NONOate, staurosporine (Cayman Chemical, Ann Arbor, MI), reduced glutathione, oxidized glutathione, zVAD-fmk, Hoechst 33342, trypan blue, Histopaque 1077 (Sigma, St. Louis, MO), mouse tumor necrosis factor-α (TNF-α, R&D systems, Minneapolis, MN), SYTOX® Green (Invitrogen, Carlsbad, CA), siRNA oligonucleotides for mouse RIP1 (M-040150, Thermoscientific, Waltham, MA), and anti-HMGB1 (Novus Biologicals, Littleton, CO), anti-cyclophilin A, anti-Akt, anti-phosphorylated Akt (serine 473) (Cell Signaling Technology, Danvers, MA), anti-Fas/CD95 (Beckman Coulter, Brea, CA), anti-RIP1 (BD Transduction Laboratories, Lexington, KY), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Trevigen, Gaithersburg, MD) antibodies were purchased commercially.
2.2. Cell Culture
Rat INS-1/832 insulinoma cells, a kind gift of Dr. C. Newgard [22], were grown in RPMI 1640 supplemented with 10% FBS, 10 mM HEPES, 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin. Mouse βTC-6 cells (ATCC, Manassas, VA) were cultured in Dulbecco’s Modified Eagle’s Medium with 15% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. βTC-6 cells were transfected with siRNA for RIP1 or control siRNA [23] using Lipofectamine RNAi MAX (Invitrogen). RIP1-deficient and control human Jurkat T-cell lymphoma cells, a kind gift of Dr. B. Seed [24], were grown in RPMI 1640 supplemented with 10% FBS, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin.
2.3. Mouse islet isolation
The study protocol was approved by the Institutional Animal Care Committee of Massachusetts General Hospital. The animal care facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Islets were isolated from male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) by collagenase digestion followed by centrifugation over a Histopaque gradient, and cultured in RPMI1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, as previously described [25].
2.4. Treatment of cells and islets
Cells and mouse islets were treated with Nec-1 (10–100 μM) or Nec-1i (100 μM) in the presence or absence of GSNO (100–600 μM), SNAP (800 μM), anti-Fas/CD95 antibody (200 ng/mL), zVAD-fmk (30 μM), cycloheximide (3 μg/mL), staurosporine (10 μM) for 24 h unless otherwise indicated.
2.5. Evaluation of cell viability
Cell viability of INS-1/832, βTC-6, and Jurkat cells was assessed using Sytox green and Hoechst 33342 according to the manufacturers’ instructions or by the Trypan blue exclusion test. The viability of islet cells was assessed using Sytox green and fluorescence intensities were quantified by NIH Image J 1.410 (National Institutes of Health, Bethesda, MD). DNA fragmentation was determined by ELISA (Roche Diagnostics, Indianapolis, IN). The metabolic activities of Jurkat cells were measured by TOX-8 (Sigma).
2.6. Immunoblot analysis
Immunoblotting was performed to evaluate the efficiency of RIP1 knockdown and release of HMGB1 and cyclophilin A to the culture media, and the effects of GSNO, Nec-1, and Nec-1 on phosphorylation of Akt, as previously described [26]. Bands of interest were scanned using HP Scanjet 4850 (Hewlett-Packard, Palo Alto, CA) and were quantified by NIH Image J 1.410.
2.7. Measurement of cellular ATP
Cellular ATP content was determined using commercial kits from Promega (Madison, WI) and Bioassay Systems (Hayward, CA). Our pilot experiments showed that the most profound reductions in cellular ATP levels were observed at 2 h after the addition of GSNO in INS-1/832 and βTC-6 cells.
2.8. Statistical analysis
The data were compared using One-way ANOVA followed by Tukey’ s least significant difference test. A value of p<0.05 was considered statistically significant. All data are expressed as mean ± SEM.
3. Results
3.1. Nec-1 inhibited NO donor-induced pancreatic β-cell death
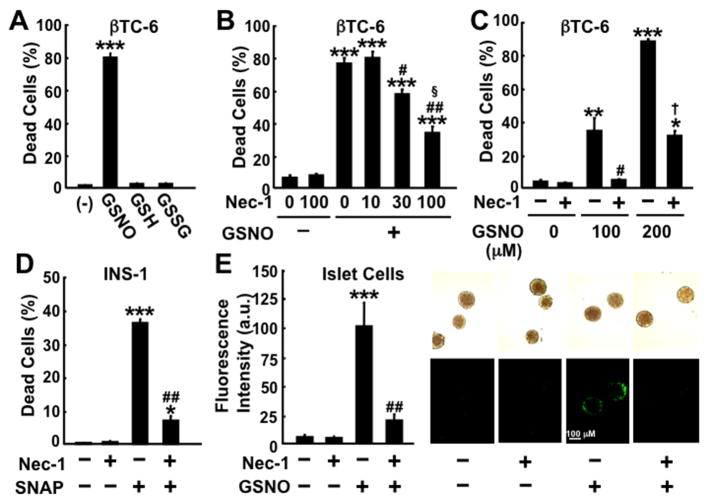
Treatment with NO donor, GSNO and SNAP, resulted in cell death in INS-1/832 and βTC-6 β-cell lines and mouse cultured islets, as evidenced by loss of membrane integrity (Fig. 1). In contrast to GSNO-induced cell death, neither reduced (GSH) nor oxidized (GSSG) glutathione affected cell viability in β-cells. These findings indicate that the effect of GSNO on β-cell viability is specific for NO. zVAD-fmk (100 μM) did not prevent GSNO-induced death of βTC-6 cells, whereas zVAD-fmk significantly inhibited staurosporine (10 μM)-induced cell death (Supplementary Fig. 1). These results indicate that activation of caspases does not have an important role in NO donor-induced β-cell death.
Fig. 1.
Effects of NO donors and Nec-1 on cell viability of pancreatic β-cells. (A) Treatment with GSNO (200 μM) for 24 h, but not equivalent amounts of reduced (GSH) or oxidized (GSSG) glutathione, increased dead cells in βTC-6 cells. (B, C, D, E) Nec-1 significantly decreased GSNO- or SNAP-induced cell death in βTC-6 (GSNO 150 μM [B]; GSNO 100 or 200 μM [C]), INS-1/832 (SNAP 800 μM [D]) and mouse islet cells (GSNO 300 μM [E]). Cell death was assessed by Sytox (A, C, D, E) or trypan blue staining (B). *p<0.01, **p<0.001 ***p<0.0001 vs without NO donor, #p<0.001, ##p<0.0001 vs NO donor without Nec-1, §p<0.001 vs GSNO+Nec-1 30 μM, †p<0.0001 vs GSNO 200 μM without Nec-1.
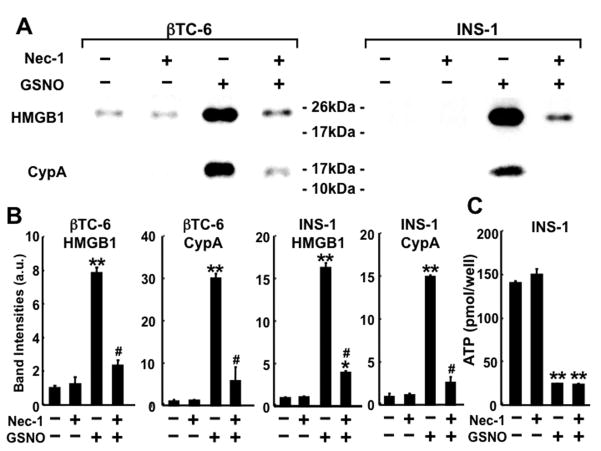
NO donor-induced β-cell death was accompanied by early release of HMGB1 and cyclophilin A into the culture media (Fig. 2. and Supplementary Fig. 2), biomarkers of necrosis [27]. In contrast, NO donor did not significantly increase DNA fragmentation, a hallmark feature of apoptosis, while robust DNA fragmentation was induced by staurosporine (10 μM) (Supplementary Fig. 3). More than 95% of INS-1/832 cells did not show nuclear fragmentation after 24-h incubation with and without GSNO (500 μM), whereas staurosporine-induced β-cell death was associated with nuclear fragmentation (data not shown). These data indicate that GSNO-induced cellular demise of cultured β-cells is necrotic cell death rather than apoptosis.
Fig. 2.
Effects of Nec-1 on NO donor-induced release of HMGB1 and cyclophilin A, and reduced ATP levels in β-cells. (A, B) Nec-1 (100 μM) inhibited GSNO-induced release of HMGB1 and cyclophilin A in βTC-6 cells (GSNO 150 μM) and INS-1/832 cells (GSNO 500 μM). *p<0.05, **p<0.0001 vs without GSNO, #p<0.0001 vs GSNO without Nec-1. (C) In contrast, Nec-1 (100 μM) did not alter cellular ATP levels at 2 h after the addition of GSNO (500 μM) in INS-1/832 cells. **p<0.0001 vs without GSNO.
Nec-1 inhibited GSNO-induced cell death in βTC-6 cells in a dose-dependent manner (Fig. 1B). Nec-1 (100 μM) also significantly prevented GSNO- or SNAP-induced cell death in INS-1/832 cells and mouse cultured islets. Consistently, Nec-1 (100 μM) significantly increased the number of alive cells after 24-h treatment with GSNO (150 μM) in βTC-6 cells (Alive cells [× 105/well]: control: 9.2 ± 0.2 [mean ± SEM]; Nec-1 alone: 8.9 ± 0.3; GSNO alone: 2.8 ± 0.04; GSNO+Nec-1: 4.9 ± 0.2, p<0.001 GSNO alone vs GSNO+nec-1). In contrast, Nec-1 (100 μM) did not inhibit staurosporine-induced death in βTC-6 cells (Supplementary Fig. 1).
Nec-1 blocked GSNO-induced release of HMGB1 and cyclophilin A from cultured β-cells (Fig. 2). However, Nec-1 did not affect the reductions in intracellular ATP content in GSNO-treated β-cells (Fig. 2C and data not shown). In contrast to the protective effects of Nec-1 against GSNO-induced β-cell death, Nec-1 (100 μM) failed to inhibit cell death after 24-h deprivation of serum and glucose in βTC-6 cells (Sytox-positive cells [%]: control: 2.5 ± 0.3; control+Nec-1: 2.7 ± 0.3; deprivation: 9.3 ± 0.8; deprivation+Nec-1: 10.4 ± 0.3).
3.2. Neither NO donor-induced cell death nor the protective effect of Nec-1 was blocked by RIP1 knockdown
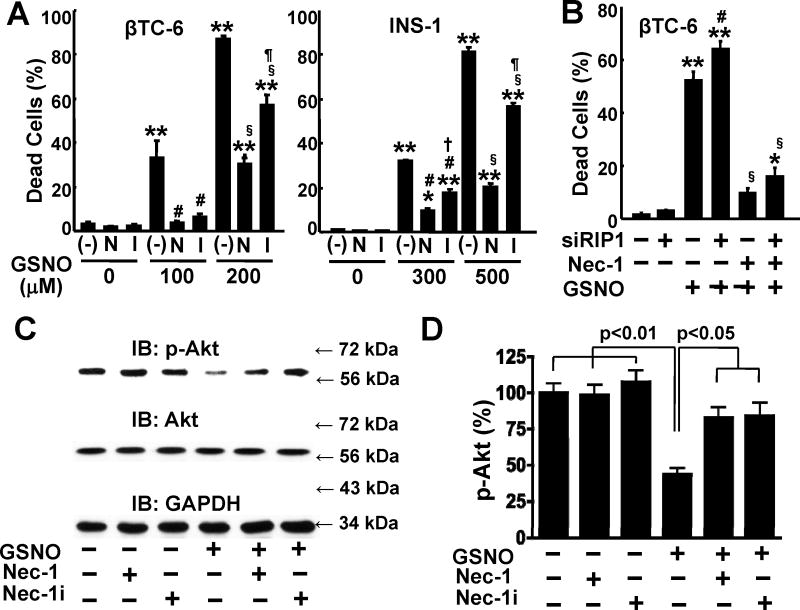
Next, we examined the effects of Nec-1i, which is an analogue of Nec-1 but incapable of inhibiting RIP1. Nec1i has been used as a negative control for Nec-1 [10]. Unexpectedly, Nec-1i significantly inhibited GSNO-induced cell death in INS-1/832 and βTC-6 cells (Fig. 3A). These results prompted us to study the effect of RIP1 knockdown. siRNA-mediated knockdown of RIP1 failed to decrease GSNO-induced β-cell death as compared with control siRNA (Fig. 3B, C and Supplementary Fig. 4). Moreover, Nec-1 effectively protected β-cells from GSNO-induced cell death even when RIP1 was knocked down. These results indicate that Nec-1 does not require RIP1 to protect β-cells from GSNO-induced death.
Fig. 3.
RIP1-unrelated protective effects of Nec-1 against NO donor-induced β-cell death. (A) GSNO-induced cell death was significantly prevented by Nec-1 (N), a potent inhibitor of RIP1, and its analogue, Nec-1i (I), which is incapable of inhibiting RIP1, in βTC-6 and INS-1/832 cells. *p<0.01, **p<0.0001 vs without GSNO, #p<0.001 vs GSNO (100 or 300 μM) without Nec-1, §p<0.001 vs GSNO (200 or 500 μM) without Nec-1, †p<0.01 vs GSNO 300 μM+Nec-1, ¶p<0.001 vs GSNO (200 or 500 μM)+Nec-1. (B) siRNA-mediated knockdown of RIP1 (siRIP1) did not block GSNO (100 μM)-induced cell death in βTC-6 cells, as compared with control siRNA. Moreover, Nec-1 (100 μM) effectively inhibited GSNO (100 μM)-induced cell death in βTC-6 cells transfected with siRNA for RIP1 as well as control siRNA. *p<0.05, **p<0.0001 vs without GSNO, #p<0.05 vs GSNO+control siRNA without Nec-1, §p<0.0001 vs GSNO+control siRNA+Nec-1. (C, D) Nec-1 and Nec-1i almost completely reversed decreased Akt phosphorylation in GSNO-treated INS-1/832 cells. IB: immunoblotting.
Treatment with TNF-α (100 ng/mL) or Fas ligand (100 ng/mL) in combination with zVAD (10, 30, and 100 μM) for up to 24 h did not significantly increase Nec-1-inhibitable cell death in INS-1 and βTC-6 cells, regardless of the presence or absence of various concentrations of cycloheximide (0.1 to 100 μg/mL), a facilitator of necroptosis in other cell types (Trypan blue-positive cells [%] in βTC-6 cells: control: 1.3 ± 0.4; Fas ligand [100 ng/mL]+zVAD [100 μM]+cycloheximide [100 μg/ml]: 3.0 ± 0.6; Fas ligand [100 ng/mL]+zVAD [100 μM]+cycloheximide [100 μg/ml]+Nec-1 [100 μM]: 2.7 ± 0.5, p>0.10, and data not shown).
3.3 The protective effects of Nec-1 and Nec-1i were associated with restoration of suppressed Akt activity (phosphorylation) in NO donor-treated β-cells
Previous studies by others and us have shown that treatment with NO donor results in suppression of Akt activity [28,29], a major pro-survival signal, although the underlying mechanisms remain elusive. We, therefore, examined the effects of Nec-1 and Nec-1i on Akt activity (phosphorylation) in NO donor-treated β-cells. As shown previously [28,29], treatment with GSNO for 5 h decreased phosphorylation of Akt in INS-1/832 cells. Both Nec-1 and Nec-1i almost completely reversed the decreased phosphorylation of Akt in GSNO-treated β-cells (Fig. 3C, D). In contrast, neither Nec-1 nor Nec-1i altered phosphorylation of Akt in the absence of NO donor. Akt and GAPDH protein expression were not altered by GSNO, Nec-1, or Nec-1i. On the other hand, Akt inhibitor (5 μM)-induced β-cell death was not inhibited by Nec-1 (100 μM) in INS-1/832 cells (Trypan blue-positive cells [%]: control: 1.7 ± 0.4; Akt inhibitor: 69.9 ± 10.7; Akt inhibitor+Nec-1: 71.8 ± 14.5).
3.4. Both Nec-1 and Nec-1i inhibited GSNO-induced cell death in Jurkat cells
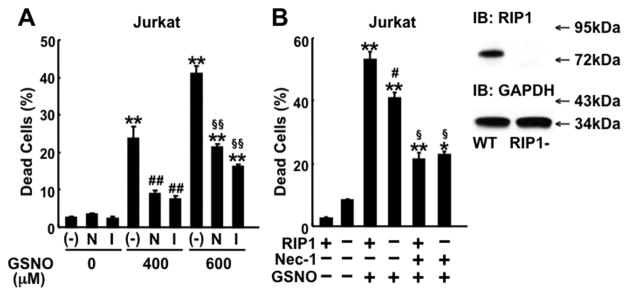
To further investigate the role of RIP1 in NO donor-induced cell death, we examined the effects of Nec-1 and Nec-1i in Jurkat cells, in which RIP1-dependent death domain receptors (e.g., Fas/CD95)-mediated necroptosis has been established [10]. Both Nec-1 and Nec-1i significantly inhibited GSNO-induced cell death (Fig. 4A). Consistently, GSNO caused cell death in both control wild-type (RIP1-proficient) and RIP1-deficient Jurkat cells (Fig. 4B and Supplementary Fig. 5), whereas RIP1 deficiency prevented anti-Fas antibody-plus-zVAD-fmk induced cell death (Trypan blue-positive cells [%]: WT without treatment: 1.7 ± 0.3; WT with Fas+zVAD: 10.2 ± 0.9; RIP1−/− with Fas+zVAD: 3.2 ± 0.2, p<0.001 WT with Fas+zVAD vs. RIP1−/− with Fas+zVAD) as shown previously [10]. RIP1-deficient Jurkat cells were slightly, but significantly less sensitive to GSNO-induced cell death as compared with wild-type Jurkat cells. Nonetheless, Nec-1 effectively inhibited GSNO-induced cell death in both wild-type and RIP1-deficient Jurkat cells.
Fig. 4.
Effects of NO donor and Nec-1 in wild-type and RIP1-deficient Jurkat cells. (A) GSNO induced cell death in control wild-type (RIP1-proficient) Jurkat cells in a dose-dependent manner. Both Nec-1 (N) (100 μM) and Nec-1i (I) (100 μM) inhibited GSNO-induced cell death in wild-type Jurkat cells. **p<0.0001 vs without GSNO, ##p<0.0001 vs GSNO 400 μM without Nec-1, §§p<0.0001 vs GSNO 600 μM without Nec-1. (B) GSNO (600 μM) induced cell death in both wild-type (RIP1+ or WT) and RIP1-deficient (RIP1−) Jurkat cells. Nec-1 (100 μM) significantly decreased GSNO-induced cell death in both wild-type and RIP1-deficient Jurkat cells. Immunoblotting confirmed that RIP1 was expressed in wild-type, but not RIP1-deficient, Jurkat cells. *p<0.001, **p<0.0001 vs without GSNO, #p<0.001 vs WT with GSNO alone, §p<0.0001 vs GSNO+Nec-1. Cell death was assessed by Trypan blue staining. IB: immunoblotting.
4. Discussion
Here, we demonstrate that Nec-1 significantly inhibits NO donor-induced necrotic death of pancreatic β-cells. Of interest, knockdown of RIP1 had little, if any, effect on NO donor-induced cell death in β-cells (Fig. 3B), although Nec-1 is a potent inhibitor of RIP1 [10]. Moreover, RIP1 knockdown did not block the protective effects of Nec-1 against NO donor-induced cell death in β-cells. In line with this, Nec-1i also exhibited protective effects against NO donor-induced cell death. Of note, the pro-survival effect of Nec-1i is consistent with a previous study which showed that both Nec-1 and Nec-1i protect isolated heart from ischemia/reperfusion injury [30]. Together, these results clearly indicate that Nec-1 and Nec-1i inhibit NO donor-induced necrotic cell death independent of the inhibition of RIP1 in β-cells.
Our data also indicate that RIP1 does not play a major role in NO donor-induced death of Jurkat cells. To the best of our knowledge, no study has shown that death domain receptors (e.g., Fas/CD95, TNF-α receptor) have an important role in NO donor-induced cell death in any cell type. Our findings support the argument that NO-mediated necrotic death of β-cells and Jurkat cells may be regulated by (an) as yet unidentified molecular mechanism(s), whereby RIP1-unrelated target(s) of Nec-1 and Nec-1i are involved.
The recently revised proposal of the Nomenclature Committee on Cell Death encourages not to use the term “necroptosis” as a synonym of regulated necrosis, but to limit its use to indicate RIP1- and/or RIP3-dependent regulated necrosis [8]. Based on this recommendation, NO donor-induced cell death is not classified as necroptosis although it is inhibited by Nec-1. It is important to note, however, that our data support the argument that there may exist a novel type of Nec-1-inhibitable, but RIP-1-independent regulated necrosis.
We found that Nec-1 and Nec-1i significantly inhibited NO donor-induced suppression of Akt activity in β-cells (Fig. 3C, D). Of note, Nec-1 failed to inhibit Akt inhibitor-induced β-cell death. Previous studies have shown that Akt inhibits ceramide-induced non-apoptotic programmed cell death with a necrosis-like morphology in glioma cells [31] and cytokine-induced necrosis as well as apoptosis of cultured β-cells [32]. Taken together, these findings suggest that suppression of Akt activity and its mitigation may play a role in NO donor-induced necrosis and its prevention by Nec-1 in β-cells.
Apparently contradictory results have been also reported concerning the role of RIP1 in NO donor-induced necrotic cell death. Contrary to our findings, a previous study showed that NO donor, Dea NONOate (10 μM)-induced necrotic death of cultured rat pulmonary microvascular endothelial cells is completely blocked by knockdown of RIP1 as well as by Nec-1 [33]. This discrepancy might be explained by differences in cell types and NO donors. Treatment with Dea NONOate (10 μM) for 24 h did not increase cell death in INS-1, βTC-6, or Jurkat cells. Less than 3% of the INS-1, βTC-6, and Jurkat cells were dead following 24-h incubation with or without 10 μM of Dea NONOate, whereas approximately 90% of rat pulmonary microvascular endothelial cells undergo necrosis after 14-h treatment with Dea NONOate (10 μM) in that study [33]. Consequently, it is possible that the role of RIP1 in NO-mediated necrotic cell death may vary depending on the cell types, the quantitative and qualitative differences between NO donors, and the cellular context.
RIP1-deficient Jurkat cells were slightly, but significantly less sensitive to GSNO-induced cell death (Fig. 4B). Although we do not have clear explanation, it is possible that signaling pathways other than RIP1, which are important for cell fate determination, might be altered by RIP1 deficiency. Our data, however, do not exclude an alternative possibility that RIP1 could play a role in NO donor-induced death of Jurkat cells. Regardless, our results clearly indicate that RIP1 is not necessary for the protective effects of Nec-1 in Jurkat cells.
Earlier studies have proposed that ATP depletion, which results from NO-mediated inhibition of ATP production, plays an important role in NO-induced necrosis [34,35]. Consistent with previous studies [29, 30], we found that treatment with NO donor decreased intracellular ATP content in β-cells. Importantly, however, the inhibition of necrotic cell death by Nec-1 was not associated with reversal of GSNO-induced reductions in intracellular ATP levels (Fig. 2C). These findings raise the possibility that NO can cause necrotic cell death via signaling pathways that are unrelated to ATP reduction. Nonetheless, it is important to note that our results do not exclude the possibility that under certain conditions NO can cause necrosis by ATP depletion, as well.
Necrosis is associated with early release of HMGB1 and cyclophilin A, which has been used as a biomarker of necrotic cell death [2]. HMGB1 is a ligand of receptor for advanced glycation endproducts (RAGE) and Toll-like receptors, and therefore acts as a potent inducer of inflammation [36]. Previous studies have shown that extracellular HMGB1 enhances autoimmune insulitis and progression of diabetes in non-obese diabetic mice [37] and promotes early graft failure during islet transplantation in mice [38]. In addition, secreted cyclophilin A functions as a proinflammatory cytokine and potent chemoattractant of immune cells, including macrophages and T-cells [39]. Release of HMGB1 and cyclophilin A from NO donor-treated β-cells was prevented by Nec-1. Taken together, one can reasonably speculate that NO-mediated necroptotic β-cell death might trigger or enhance infiltration of macrophages and T-cells into the islets, which in turn leads to the aggravated inflammation, immune attack, and resultant β-cell dysfunction in diabetes. In aggregate, our findings suggest that signal-regulated necrotic cell death may play a role in the NO-involved pathogenesis of β-cell failure in diabetes.
Supplementary Material
Research Highlights.
Nitric oxide (NO) donor-induced necrotic pancreatic β-cell death was inhibited by Nec-1, a potent inhibitor of receptor-interacting protein-1 (RIP1) and RIP1-mediated necroptosis.
siRNA-mediated knockdown of RIP1, however, neither inhibited necrotic cell death nor altered the protective effects of Nec-1 in NO donor-treated β-cells.
These findings provide evidence that NO can mediate Nec-1-inhibitable necrotic β-cell death independent of RIP1.
Acknowledgments
We thank Drs. C. Newgard and B. Seed for providing INS-1/832 and RIP1-deficient Jurkat cells, respectively. We also thank Dr. C. Waeber for his superb technical assistance. This work was supported by research grants from the National Institutes of Health to M.K. (R01 DK05827) and to the Microscopy and Image Analysis Core of Massachusetts General Hospital (P30NS045776), and from American Diabetes Association to M.K. (7-08-RA-77).
Abbreviations
- NO
nitric oxide
- Nec-1
necrostatin-1
- RIP
receptor-interacting protein
- HMGB1
high mobility group box 1
- iNOS
inducible nitric oxide synthase
- TNF-α
tumor necrosis factor-α
- GSNO
S-nitrosoglutathione
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 2.Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3:e17. doi: 10.1371/journal.pmed.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimabukuro M, Ohneda M, Lee Y, Unger RH. Role of nitric oxide in obesity-induced beta cell disease. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 2002;190:75–82. doi: 10.1016/s0303-7207(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 5.Fehsel K, Kolb-Bachofen V, Kroncke KD. Necrosis is the predominant type of islet cell death during development of insulin-dependent diabetes mellitus in BB rats. Lab Invest. 2003;83:549–559. doi: 10.1097/01.lab.0000063927.68605.ff. [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2011 Jul 15; doi: 10.1038/cdd.2011.96. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 10.Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Chua CC, Kong J, Kostrzewa RM, et al. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 12.Han W, Li L, Qiu S, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 13.You Z, Savitz SI, Yang J, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Yang X, Ma C, et al. Necroptosis contributes to the NMDA-induced excitotoxicity in rat's cultured cortical neurons. Neurosci Lett. 2008;447:120–123. doi: 10.1016/j.neulet.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum DM, Degterev A, David J, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Chua KW, Chua CC, et al. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu S, Zhang Y, Bai G, Li H. Necrostatin-1 ameliorates symptoms in R6/2 transgenic mouse model of Huntington's disease. Cell Death Dis. 2011;2:e115. doi: 10.1038/cddis.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SY, Davidson SM, Mocanu MM, et al. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northington FJ, Chavez-Valdez R, Graham EM, et al. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang QL, Niu Q, Niu PY, et al. Novel interventions targeting on apoptosis and necrosis induced by aluminum chloride in neuroblastoma cells. J Biol Regul Homeost Agents. 2010;24:137–148. [PubMed] [Google Scholar]
- 21.Asare N, Tekpli X, Rissel M, et al. Signalling pathways involved in 1-nitropyrene (1-NP)-induced and 3-nitrofluoranthene (3-NF)-induced cell death in Hepa1c1c7 cells. Mutagenesis. 2009;24:481–493. doi: 10.1093/mutage/gep032. [DOI] [PubMed] [Google Scholar]
- 22.Hohmeier HE, Mulder H, Chen G, et al. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 23.Ota H, Tokunaga E, Chang K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 24.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. Embo J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura T, Kido Y, Nef S, et al. Preserved pancreatic beta-cell development and function in mice lacking the insulin receptor-related receptor. Mol Cell Biol. 2001;21:5624–5630. doi: 10.1128/MCB.21.16.5624-5630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasukawa T, Tokunaga E, Ota H, et al. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 27.Christofferson DE, Yuan J. Cyclophilin A release as a biomarker of necrotic cell death. Cell Death Differ. 2010;17:1942–1943. doi: 10.1038/cdd.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storling J, Binzer J, Andersson AK, et al. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia. 2005;48:2039–50. doi: 10.1007/s00125-005-1912-2. [DOI] [PubMed] [Google Scholar]
- 29.Tanioka T, Tamura Y, Fukaya M, et al. iNOS and NO donor decrease IRS-2 protein expression by promoting proteasome-dependent degradation in pancreatic {beta}-cells: Involvement of GSK-3{beta} J Biol Chem. 2011;286:29388–96. doi: 10.1074/jbc.M110.192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith CC, Davidson SM, Lim SY, et al. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki T, Asai A, Saito N, et al. Akt protein kinase inhibits non-apoptotic programmed cell death induced by ceramide. J Biol Chem. 2002;277:2790–7. doi: 10.1074/jbc.M106361200. [DOI] [PubMed] [Google Scholar]
- 32.Li L, El-Kholy W, Rhodes CJ, Brubaker P/L. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia. 2005;48:1339–49. doi: 10.1007/s00125-005-1787-2. [DOI] [PubMed] [Google Scholar]
- 33.Davis CW, Hawkins BJ, Ramasamy S, et al. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med. 2010;48:306–317. doi: 10.1016/j.freeradbiomed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borutaite V, Brown GC. Nitric oxide induces apoptosis via hydrogen peroxide, but necrosis via energy and thiol depletion. Free Radic Biol Med. 2003;35:1457–1468. doi: 10.1016/j.freeradbiomed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Leist M, Single B, Naumann H, et al. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp Cell Res. 1999;249:396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- 36.van Zoelen MA, Yang H, Florquin S, et al. Role of Toll-Like Receptors 2 and 4, and the Receptor for Advanced Glycation End Products (Rage) in Hmgb1 Induced Inflammation in Vivo. Shock. 2008;31:280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J, Zhong J, Wei W, et al. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57:2118–2127. doi: 10.2337/db07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuoka N, Itoh T, Watarai H, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nigro P, Satoh K, O'Dell MR, et al. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2011;208:53–66. doi: 10.1084/jem.20101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.