Abstract
Objective
Burn injury is associated with inflammatory responses and metabolic alterations including insulin resistance. Impaired insulin receptor substrate-1 (IRS-1)-mediated insulin signal transduction is a major component of insulin resistance in skeletal muscle following burn injury. To further investigate molecular mechanisms that underlie burn injury-induced insulin resistance, we study a role of inducible nitric oxide synthase (iNOS), a major mediator of inflammation, on burn-induced muscle insulin resistance in iNOS-deficient mice.
Materials/Methods
Full-thickness third-degree burn injury comprising 12% of total body surface area was produced in wild-type and iNOS-deficient C57BL/6 mice. Insulin-stimulated activation (phosphorylation) of insulin receptor (IR), IRS-1, and Akt was assessed by immunoblotting and immunoprecipitation. Insulin-stimulated glucose uptake by skeletal muscle was evaluated ex vivo.
Results
Burn injury caused induction of iNOS in skeletal muscle of wild-type mice. The increase of iNOS expression paralleled the increase of insulin resistance, as evidenced by decreased tyrosine phosphorylation of IR and IRS-1, IRS-1 expression, insulin-stimulated activation of phosphatidylinositol 3-kinase and Akt/PKB, and insulin-stimulated glucose uptake in mouse skeletal muscle. The absence of iNOS in genetically engineered mice significantly lessened burn injury-induced insulin resistance in skeletal muscle. In wild-type mice, insulin tolerance test revealed whole-body insulin resistance in burned mice compared to sham burned controls. This effect was reversed by iNOS deficiency. Unexpectedly, however, blood glucose levels were depressed in both wild-type and iNOS-deficient mice after burn injury.
Conclusions
Gene disruption of iNOS ameliorated the effect of burn on IRS-1-mediated insulin signaling in skeletal muscle of mice. These findings indicate that iNOS plays a significant role in burn injury-induced skeletal muscle insulin resistance.
Keywords: IRS-1, nitric oxide, phosphatidylinositol 3-kinase, Akt, glucose uptake
1. Introduction
Critical illness including burn injury is associated with inflammatory response and deranged metabolism [1–3]. Despite advancements in the resuscitation and surgical treatment of burn patients, metabolic dysfunction remains a significant cause of morbidity and mortality [4]. Metabolic aberrations in critically ill patients cause muscle wasting and reduced lean body mass, which in turn leads to decreased mobility, hypoventilation, difficulties in weaning-off respirators, prolonged rehabilitation and hospitalization, and even death [5–8]. A major common denominator of critical illness-associated metabolic derangements is insulin resistance, which causes protein catabolism and glycogenolysis as well as impaired insulin-stimulated glucose uptake in muscle [9,10]. The molecular bases of insulin resistance in critical illness, however, remain to be investigated.
Binding of insulin to its receptor results in activation of insulin receptor (IR) kinase, which in turn phosphorylates tyrosine residues of IR itself and insulin receptor substrates (IRSs). When phosphorylated by IR, IRSs binds and transduces the insulin signal downstream to the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K). The IRSs–PI3K–Akt pathway plays a central role in the metabolic actions of insulin, including stimulation of glucose uptake, and synthesis of glycogen and protein [11]. Gene knock-out of IRS-1 causes insulin resistance in skeletal muscle in mice [12], while gene disruption of IRS-2 induces insulin resistance in liver, but not in skeletal muscle [13]. Tissue-specific gene disruption of IRS-1 and IRS-2 also reveals that IRS-1, but not IRS-2, plays a prominent role in the metabolic actions of insulin in skeletal muscle [14–16]. Hence, impaired IRS-1-mediated insulin signaling has been recognized as a major contributor to obesity-induced insulin resistance in skeletal muscle. Consistently, we and others have demonstrated that IRS-1-mediated insulin signal transduction in response to insulin is attenuated in skeletal muscle after burn injury in rats [17–19]. However, the molecular mechanisms by which IRS-1-mediated signaling are impaired are yet to be elucidated.
Chronic inflammatory response has been implicated in the pathogenesis of obesity-related insulin resistance [20–22]. However, our knowledge remains limited about how this inflammatory response causes and/or exacerbates insulin resistance. Inducible nitric oxide synthase (iNOS) is a major mediator of inflammation. Although iNOS plays an important role in the immunological defense mechanisms against microorganisms, iNOS also exerts detrimental effects in various tissues including skeletal muscle in critical illness [23–25]. We and others have shown that iNOS plays an important role in obesity and lipopolysaccharide-induced insulin resistance in skeletal muscle and liver [26–30]. To investigate the role of iNOS in burn injury-induced insulin resistance, we examined the effects of burn injury on IR–IRS-1-mediated insulin signaling in skeletal muscle of iNOS knockout (−/−) mice.
2. Materials and methods
2.1. Animals
Adult male C57BL/6 mice and iNOS knockout (−/−) mice at 14 weeks of age, purchased from the Jackson Laboratory (Bar Harbor, ME), were used for this study. iNOS−/− mice were backcrossed onto wild-type C57BL/6 mice by at least 10 generations. The study was approved by the Institutional Animal Care Committee. The animal care facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The mice were housed in a pathogen-free animal facility maintained at 25°C and illuminated by 12:12-h light-dark cycles. The mice were provided with standard rodent chow and water ad libitum. Both wild-type and iNOS−/− mice were divided into four groups (sham-burn with saline or insulin, and burn with saline or insulin). Each group consisted of 8 animals, unless otherwise indicated in the figure legends. Full-thickness third-degree burn injury comprising 12% of total body surface area was produced, as described previously [31]. Briefly, mice were treated by immersing the abdomen for 8 sec in 80°C water under anesthesia (pentobarbital sodium, 50 mg/kg BW, ip). Buprenorphin (0.1 mg/kg BW, sc) was administered every 8 h up to 48 h after burn or sham-burn. Sham-burned mice were immersed in lukewarm water. Just before, and at one, three, and seven days after burn or sham-burn injury, the rectus abdominis muscle was excised under anesthesia for biochemical analyses. The animals were then immediately euthanized with an overdose of pentobarbital sodium (200 mg/kg BW, ip). The study was performed in pair-fed burn and sham-burned mice. The average food intake was 1.9 g/day.
2.2. Tissue homogenization
Burned or sham-burned mice were anesthetized with pentobarbital sodium (50 mg/kg BW, ip). Following an overnight fasting, insulin (1 or 5 U/kg BW, Humulin R, Eli Lilly, Indianapolis, IN) or saline was injected via the portal vein, and tissues were harvested at 90 sec or 5 min thereafter, as indicated in the figure legends. Tissue samples were homogenized as described previously [32]. Briefly, tissues were powdered under liquid nitrogen and homogenized in ice-cold homogenization buffer (50 mM HEPES-NaOH, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 10% glycerol, 10 mM sodium fluoride, 2 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium pyrophosphate, 10 µg/ml aprotinin, and 10 µg/ml leupeptin). After incubation on ice for 30 min, the homogenized samples were centrifuged at 13,000 g for 30 min. Aliquots of the supernatant containing equal amounts of protein, determined by the Bradford protein assay, were subjected to immunoprecipitation or SDS-PAGE.
2.3. Immunoblotting
Immunoblot analysis was performed as described previously [33]. Briefly, tissue homogenates containing equal amounts of protein or immunoprecipitates were subjected to 10% or 7.5% SDS-PAGE. After electrophoretical transfer to nitrocellulose membrane (Bio-Rad, Hercules, CA), the membranes were blocked in 5% nonfat dry milk for 2 h at room temperature, followed by incubation for 2 h at room temperature or overnight at 4°C with anti-IR, anti-IRS-1, anti-iNOS, anti-p85 (Upstate, Lake Placid, NY), anti-phosphotyrosine (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Akt, anti-phospho-Akt (Ser473), anti-phospho-GSK-3β (Ser 9) (Cell Signaling, Beverly, MA), anti-GSK-3β (Invitrogen, Carlsbad, CA), or antiglyceraldehydes-3-phosphate dehydrogenase (GAPDH) (Treviden, Gaithersburg, MD) antibody. The membranes were then incubated with anti-rabbit or -mouse IgG antibody conjugated with horseradish peroxidase for 1 h at 4°C. Western blotting chemiluminescence luminol reagent (Perkin-Elmer, Boston, MA) was used to visualize the blots. Bands of interest were scanned with the use of Power Look (UMAX Technologies, Dallas, TX) and quantified by NIH Image 1.62 software (NTIS, Springfield, VA).
2.4. Immunoprecipitation
Immunoprecipitation was performed by incubating the lysates with the antibody at 4 °C for 5 h. The immune complexes were collected by incubation with protein A- or protein G-agarose beads for 1.5 h at 4 °C, washed three times with wash buffer B (50 mM HEPES-NaOH, pH 7.5, 150 mM NaCl, 2 mM EDTA, 0.1% Nonidet P-40, 10% glycerol, 10 mM sodium fluoride, 2 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium pyrophosphate, 10 µg/ml aprotinin, 10 µg/ml leupeptin), and boiled in Laemmli sample buffer.
2.5. Phosphatidylinositol 3-kinase assay
PI3K activity in the immunoprecipitates with anti-IRS-1 antibody was measured by in vitro phosphorylation assay using phosphatidylinositol (Sigma) as a substrate as described previously [33]. Briefly, 5 µl of 100 mM MgCl2 and 10 µl of phosphatidylinositol (2 mg/ml) sonicated in kinase buffer (10 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.1 mM sodium vanadate) were added to the immunoprecipitates. The PI3K reaction was initiated by the addition of 1.5 µl of 1.5 mM ATP containing 10 µCi of [γ-32P] ATP. After incubation for 10 min at 37 °C, the reaction was stopped by the addition of 10 µl of 6 N HCl and 80 µl of CHCl3/methanol (1:1). The samples were centrifuged, and the lower organic phase was applied to a silica gel TLC plate (Sigma), which had been prebaked for 1 h. The plate was developed in CHCl3/CH3OH/H2O/NH4OH (100:78:19:3.3), dried, and visualized by autoradiography.
2.6. Insulin tolerance test
At 3 days after burn or sham-burn, following 4-h fasting, blood glucose levels were measured just before and at 15, 30, 60, 90, and 120 min after insulin injection (1 U/kg BW, Humulin R). Insulin concentrations in plasma samples obtained just before the insulin injection were measured by ELISA (Crystal Chem, Downers Grove, IL).
2.7. Insulin-stimulated glucose uptake in isolated skeletal muscle
Glucose uptake was measured as previously described [34]. Briefly, at 3 days after burn or sham-burn, mice were anesthetized following an overnight fasting, and the rectus abdominis was excised and rapidly split by mid-incision into two muscle strips. After the muscle strips were rinsed briefly in 5 mL Krebs-Henseleit bicarbonate (KHB) buffer supplemented with 32 mM mannitol, they were incubated in 5 mL of KHB buffer supplemented with 8 mM glucose and 32 mM mannitol in the presence or absence of insulin (2 mU/mL, Humulin R) in a shaking water bath at 37°C for 20 min. The muscles were then rinsed for 10 min at 37°C in KHB buffer containing 36 mM mannitol and 0.1% bovine serum albumin with or without insulin (2 mU/mL). Next, the muscles were incubated for 20 min at 37°C in 2 mL of KHB buffer containing 2-deoxy-[3H] glucose (2.5 Ci/mL, PerkinElmer, Waltham, MA) and 36 mM [14C] mannitol (0.3 µCi/mL, PerkinElmer) with or without insulin in a shaking incubator. Buffers were gassed continuously with 95% O2: 5% CO2 throughout the experiment. The muscles were then rinsed with KHB buffer, rapidly blotted, weighed, and solubilized by incubation at 60°C for 1 h in 0.5 mL of 1N NaOH. Radioactivity was counted in the sample using a scintillation counter. 2-Deoxy-[3H] glucose uptake rates were corrected for extracellular trapping using [14C] mannitol counts [34]. Insulin-stimulated glucose uptake was calculated based on differences in glucose uptake in the presence and absence of insulin.
2.8. Isolation of total RNA and quantitative RT-PCR
Total RNA was isolated with an RNeasy Mini kit (Qiagen, Valencia, CA). The first-strand cDNA was synthesized from 1 µg of total RNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Real-time RT-PCR analyses were performed as previously described [35] using 10 ng cDNA and TaqMan probes (Applied Biosystems) for mouse phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) conducted with Mastercycler® ep realplex (Eppendorf, New York City, NY). mRNA expression level of mouse 36B4 [36,37] with SYBR Green (Applied Biosystems). mRNA levels of PEPCK and G-6-Pase were normalized to those of 36B4.
2.9. Statistical analysis
The data were compared with one-way ANOVA followed by Fisher's protected least significant differences test for the analysis of time-dependent effects of burn injury in wild-type mice. To compare the effects of burn in wild-type and iNOS knockout mice, two-way ANOVA was performed using genotype (wild-type or iNOS knockout), treatment (burn or sham-burn), and injection (insulin or saline) as individual variables. When the two-way ANOVA indicated a significant overall difference, significance between the pairs of the groups was determined by Bonferroni post hoc multiple comparison test. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). A value of P < 0.05 was considered statistically significant. All values are expressed as means ± SEM.
Results
Burn injury caused iNOS induction in association with impaired insulin signaling in wild-type mice
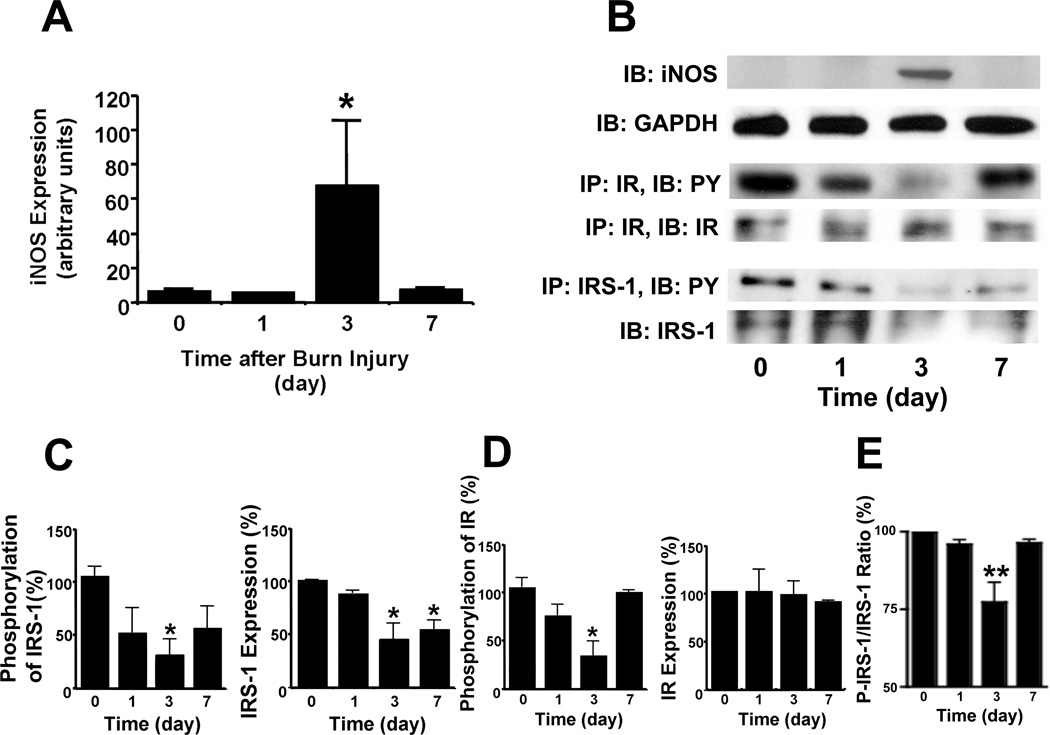
First, we examined the effects of burn injury on iNOS expression in wild-type mice. Immunoblot analysis revealed robust expression of iNOS in skeletal muscle at 3 days after burn injury (Fig. 1A). GAPDH protein expression was not affected by burn injury and iNOS was not detected in skeletal muscle before burn injury (Fig. 1B). Concomitant with iNOS induction, there was a prominent decrease in insulin-stimulated tyrosine phosphorylation of IR and IRS-1. This effect was most pronounced at 3 days after burn injury (Figs. 1B, C, D). Although IR expression was not altered by burn injury, IRS-1 expression was significantly reduced at 3 and 7 days after burn injury (Fig. 1D). Even after normalized to IRS-1 protein expression, insulin-stimulated phosphorylation was significantly decreased at 3 days after burn injury (Fig. 1E). These findings are consistent with our previous study in rats which showed that IRS-1-mediated signaling was impaired in skeletal muscle after burn injury [17]. Of note, burn injury-induced impairment in IRS-1-mediated insulin signaling paralleled iNOS induction in skeletal muscle in mice. As expected, iNOS induction was not observed in sham-burned wild-type controls (Fig. 2A).
Fig. 1.
Induction of iNOS paralleled attenuated IR- and IRS-1-mediated insulin signaling in skeletal muscle after burn injury in wild-type mice. A, Immunoblotting (IB) revealed a robust induction of iNOS expression at 3 days after burn injury. The protein expression of GAPDH was not altered following burn injury. B, C, D, Before (0) or at 1, 3 and 7 days after burn injury, insulin (5 U/kg BW) was injected and muscle was excised 90 sec thereafter. Immunoprecipitation (IP) followed by immunoblotting demonstrated that insulin-stimulated tyrosine phosphorylation (PY) of IR and IRS-1 was significantly decreased at 3 days after burn injury. The protein expression of IR was unaltered by burn injury. In contrast, IRS-1 protein expression was significantly decreased at 3 and 7 days after burn injury as compared with before burn injury. E, The ratio of insulin-stimulated IRS-1 phosphorylation normalized to IRS-1 protein expression was significantly decreased at 3 days after burn injury. *P<0.05, **P<0.01 vs before burn injury. n = 3 per group.
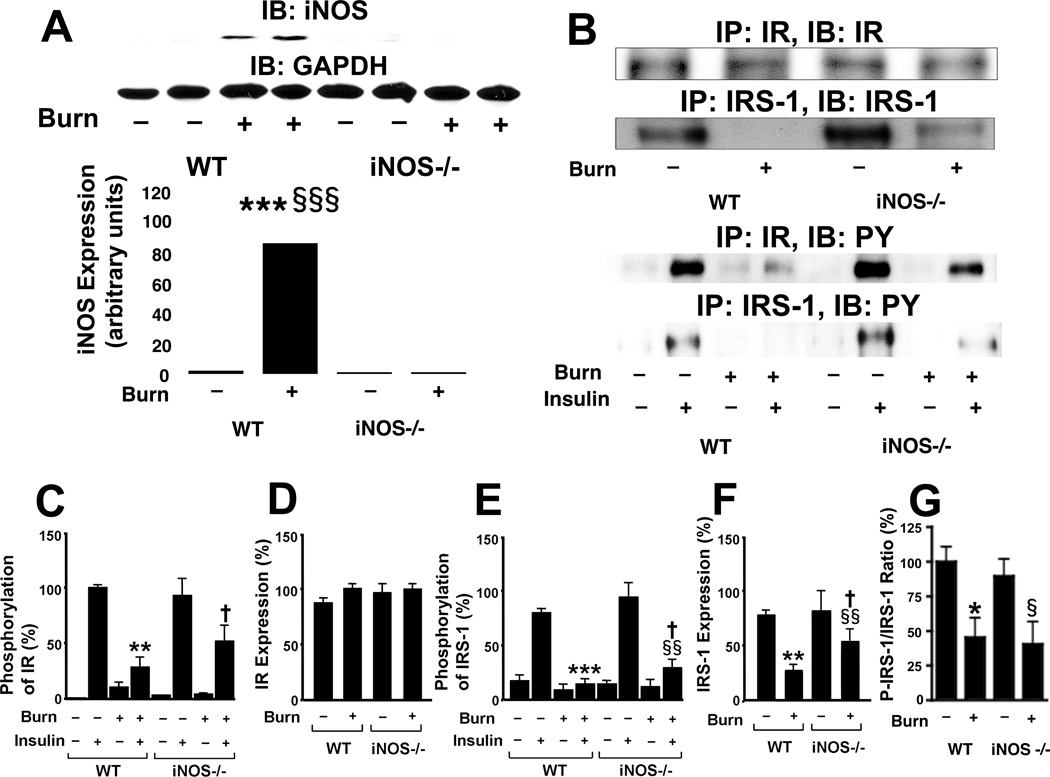
Fig. 2.
iNOS deficiency mitigated burn injury-induced impairment in IR- and IRS-1-mediated insulin signaling in skeletal muscle. At 3 days after burn injury or sham burn, insulin (5 U/kg BW) or saline was injected and 90 sec thereafter skeletal muscle was taken. A, Immunoblotting (IB) demonstrated that iNOS protein expression was induced by burn injury in wild-type, but not iNOS-deficient, mice. The protein expression of GAPDH was not affected by burn injury or iNOS deficiency. The overall interaction between genotype and burn/sham is statistically significant (P<0.001). ***P<0.001 vs sham-burned WT, §§§P<0.001 vs burned iNOS −/−. B, C, D, E, Immunoprecipitation (IP) followed by immunoblotting revealed that insulin-stimulated tyrosine phosphorylation (PY) of IR and IRS-1 was suppressed by burn injury in wild-type (WT) mice, which was ameliorated by iNOS deficiency (−/−). Neither burn injury nor iNOS deficiency altered IR protein expression. The overall interactions between genotype and other factors (burn/sham and insulin/saline) are statistically significant for phosphorylation of IR and IRS-1 (P<0.05). **P<0.01, ***P<0.001 vs sham-burned WT with insulin, §§P<0.01 vs burned iNOS −/− with insulin, †P<0.05 vs burned WT with insulin, ‡‡P<0.01 vs sham-burned WT with insulin. F, In contrast, IRS-1 protein expression was suppressed by burn injury. iNOS deficiency increased IRS-1 expression in burned mice. The overall interaction between genotype and burn/sham is statistically significant (P<0.05). **P<0.01 vs sham-burned WT, §§P<0.01 vs sham-burned iNOS −/−, †P<0.05 vs burned WT. G, The ratio of insulin-stimulated IRS-1 phosphorylation (p-IRS-1) normalized to IRS-1 protein expression was significantly decreased by burn injury in wild-type and iNOS deficient mice, as compared with sham controls. This effect of burn injury on p-IRS-1/IRS-1 ratio was not altered by iNOS deficiency. *P<0.05 vs sham-burned WT, §P<0.05 vs sham-burned iNOS −/−.
iNOS deficiency ameliorated impaired IRS-1-mediated insulin signaling in skeletal muscle of burned mice
To investigate the role of iNOS in burn-induced insulin resistance, we examined the effects of burn injury in iNOS knockout (−/−) mice at 3 days after burn injury or sham-burn injury in comparison with wild-type mice. iNOS was not detected in skeletal muscle of iNOS−/− mice regardless of burn or sham-burn. GAPDH protein expression was unaltered by either burn injury or iNOS deficiency (Fig. 2A).
In wild-type mice, insulin injection induced robust tyrosine phosphorylation of IR in skeletal muscle of sham-burned animals, whereas insulin-stimulated tyrosine phosphorylation of IR in burned mice was decreased to 28% of that in sham-burned wild-type mice (p<0.01) (Figs. 2B, C). Insulin-stimulated tyrosine phosphorylation of IR in burned iNOS−/− mice was 2-fold greater than that of burned wild-type mice (p<0.05). However, insulin-stimulated phosphorylation of IR following sham-burn injury did not differ between wild-type and iNOS−/− mice. Protein expression of IR was not affected by burn injury or disruption of iNOS (Figs. 2B, C).
Insulin induced marked tyrosine phosphorylation of IRS-1 in skeletal muscle of sham-burned mice. Although insulin failed to increase tyrosine phosphorylation of IRS-1 in burned wild-type mice, disruption of the iNOS gene partially restored tyrosine phosphorylation of IRS-1 in response to insulin in burned mice (p<0.05) (Figs. 2B, D). In contrast to unaltered IR expression in burned mice, protein expression of IRS-1 in burned wild-type mice was decreased to 36% of that in sham-burned wild-type mice (p<0.01). IRS-1 expression in burned iNOS−/− mice was increased 2-fold compared with burned wild-type mice (p<0.05) (Figs. 2B, D). When insulin-stimulated phosphorylation of IRS-1 is normalized to IRS-1 protein expression, iNOS deficiency did not significantly alter the ratio of phosphorylated IRS-1 to IRS-1 protein expression (Fig. 2E). These results suggest that the improved insulin-stimulated phosphorylation of IRS-1 by iNOS deficiency may be mainly attributable to the increased IRS-1 protein expression in burned mice. Regardless, insulin-stimulated IRS-1 tyrosine phosphorylation and IRS-1 protein expression did not differ between wild-type and iNOS−/− sham-burned mice.
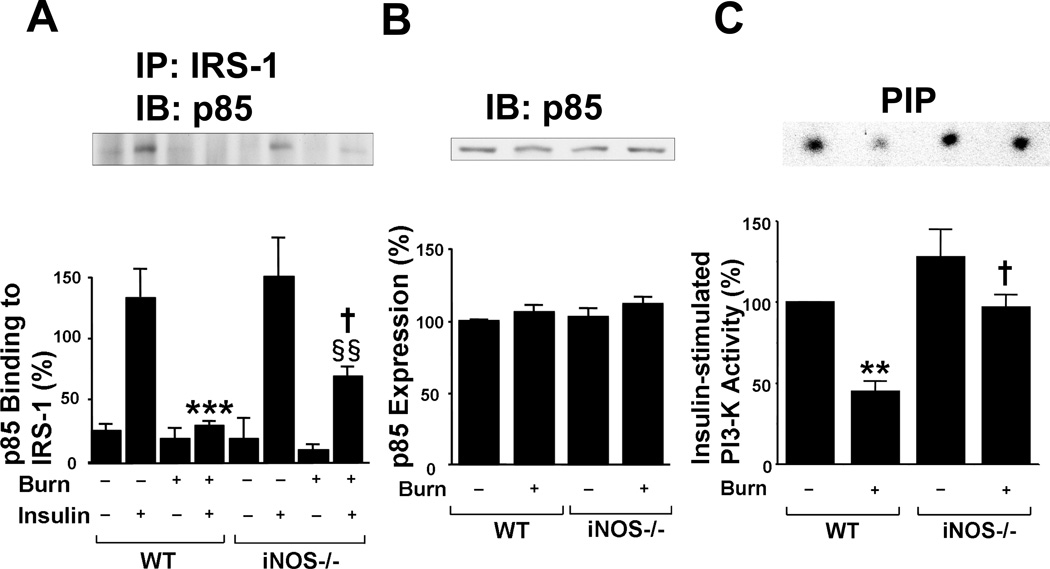
We further investigated the effects of burn and disruption of iNOS on PI3K, a key downstream molecule of the insulin signaling pathway. Insulin increased p85 PI3K binding to IRS-1 in skeletal muscle of sham-burned mice. Yet, consistent with the failure of insulin to increase IRS-1 tyrosine phosphorylation in burned wild-type mice, the binding of p85 PI3K to IRS-1 did not increase in response to insulin in burned wild-type mice (Fig. 3A). Again, as with IRS-1 tyrosine phosphorylation, disruption of iNOS restored insulin-stimulated p85 PI3K binding to IRS-1 in burned mice, albeit to a lesser extent compared with sham-burned mice (Fig. 3B). The protein expression of p85 PI3K was unaltered by burn injury or disruption of iNOS. Consistently, the burn injury-induced decrease of insulin-stimulated PI3K activity was significantly ameliorated in iNOS−/− mice (Fig. 3C). Although insulin-stimulated PI3K activity in sham-burned iNOS−/− mice appeared to be greater than sham-burned wild-type mice, this was not statistically significant.
Fig. 3.
iNOS deficiency ameliorated attenuated IRS-1-mediated PI3K activation in skeletal muscle of burned mice. At 3 days after burn injury or sham burn, insulin (5 U/kg BW) or saline was injected and 90 sec thereafter skeletal muscle was taken. A, B, Insulin injection resulted in a robust increase in binding of p85 PI3K to IRS-1 in sham-burned mice, as judged by immunoprecipitation (IP) with anti-IRS-1 antibody followed by immunoprecipitation (IB) with anti-p85 antibody. However, insulin failed to increase p85 binding to IRS-1 in burned wild-type (WT) mice. iNOS deficiency (−/−) significantly improved insulin-stimulated binding of p85 to IRS-1 in burned mice. The protein expression of p85 was not affected by burn injury or iNOS deficiency. The overall interaction between genotype and other factors (burn/sham and insulin/saline) is statistically significant for binding of p85 PI3K to IRS-1 (P<0.05). ***P<0.001 vs sham-burned WT with insulin, §§P<0.01 vs sham-burned iNOS −/− with insulin, †P<0.05 vs burned WT with insulin. n=4 per group of animals with saline, n=8 per group of animals with insulin. C, PI3K activity was evaluated in vitro phosphorylation of phosphatidylinositol (PI). Insulin-stimulated PI3K activity was decreased in burned wild-type mice relative to sham animals. iNOS deficiency almost completely reversed decreased PI3K activity in burned mice. The overall interaction between genotype and burn/sham is statistically significant (P<0.05). **P<0.01 vs sham-burned WT, †P<0.05 vs burned WT. n=4 per group.
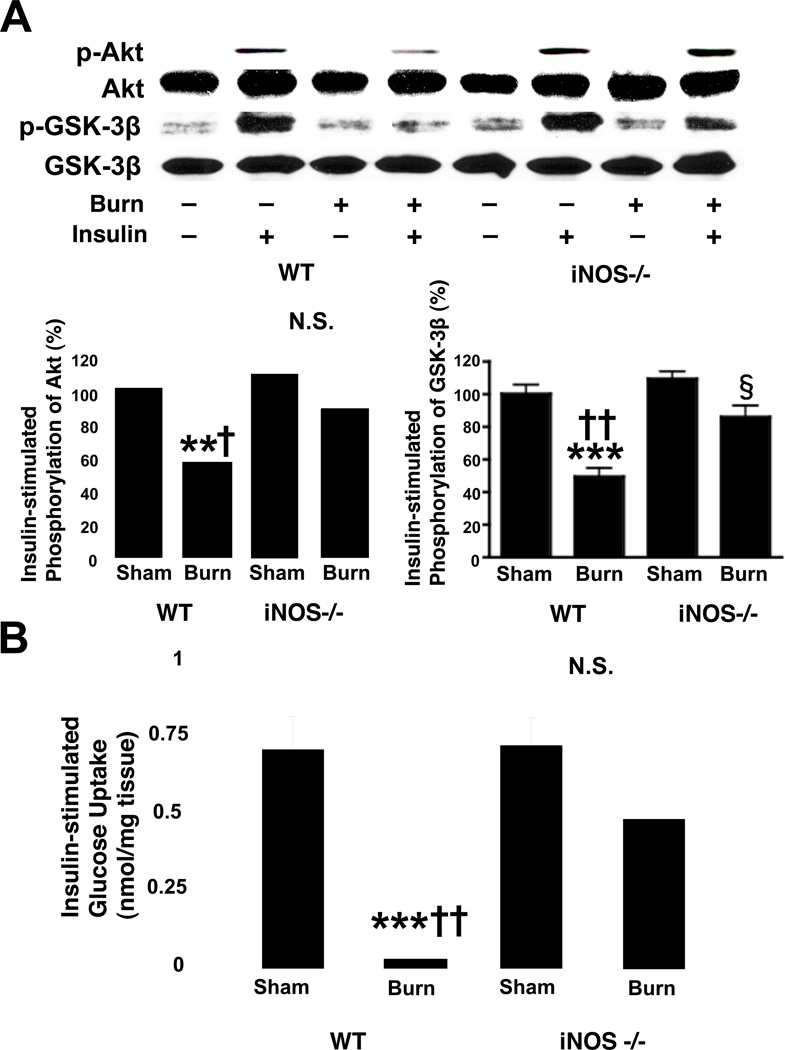
iNOS deficiency prevented burn-induced reductions in insulin-stimulated Akt phosphorylation and ex vivo glucose uptake in mouse skeletal muscle
Consistent with the effects of burn and iNOS deficiency on PI3K activity, insulin-stimulated phosphorylation of Akt was significantly reduced by burn injury in wild-type mice, and gene disruption of iNOS reverted this burn-induced decrease in insulin-stimulated Akt phosphorylation (Fig. 4A). Impaired insulin-stimulated activation by burn injury and its restoration by iNOS deficiency were corroborated by phosphorylation of GSK-3β. Burn injury decreased the insulin-stimulated phosphorylation of GSK-3β in wild-type, but not iNOS knockout mice. The protein expression of Akt and GSK-3β was not affected by burn injury or iNOS deficiency.
Fig. 4.
Burn injury-induced decreases in insulin-stimulated Akt phosphorylation and glucose uptake in skeletal muscle were reversed by iNOS deficiency. A, At 3 days after burn injury or sham burn, insulin (1 U/kg BW) or saline was injected and 5 min thereafter skeletal muscle was taken. Insulin-stimulated Akt phosphorylation was significantly attenuated by burn injury in wild-type (WT) mice as compared with sham animals. iNOS deficiency (−/−) almost completely reversed decreased insulin-stimulated Akt phosphorylation in burned mice. Similarly, insulin-stimulated GSK-3β phosphorylation was significantly decreased by burn injury in wild-type mice. Burn injury-induced decreased GSK-3β phosphorylation was ameliorated by iNOS deficiency. Neither burn injury nor iNOS deficiency altered Akt or GSK-3β protein expression. The overall interactions between genotype and burn/sham are statistically significant for phosphorylation of Akt (P<0.01) and GSK-3β (P<0.05). **P<0.01, ***p<0.001 vs sham-burned WT, †p<0.05, ††p<0.01 vs burned iNOS −/−, §p<0.05 vs sham-burned iNOS −/−. N.S.: not significant. n=4 per group of animals with saline, n=6 per group of animals with insulin. B, Burn injury resulted in suppression of insulin-stimulated glucose uptake by skeletal muscle ex vivo. iNOS deficiency significantly improved insulin-stimulated glucose uptake in burned mice. The interaction between genotype and burn/sham is statistically significant (P<0.05). ***P<0.001 vs sham-burned WT, ††P<0.01 vs burned iNOS−/−. N.S.: not significant. n=4–6 per group.
To further investigate the biological relevance of the amelioration of impaired insulin signaling by iNOS deficiency in burned mice, we evaluated insulin-stimulated glucose uptake in skeletal muscle ex vivo. In wild-type mice, insulin-stimulated glucose uptake was suppressed by burn injury compared to sham-burn. Gene disruption of iNOS almost completely restored insulin-stimulated glucose uptake in skeletal muscle of burned mice to the levels observed in sham animals (Fig. 4B). In contrast, iNOS deficiency did not affect insulin-stimulated glucose uptake in sham-burned animals.
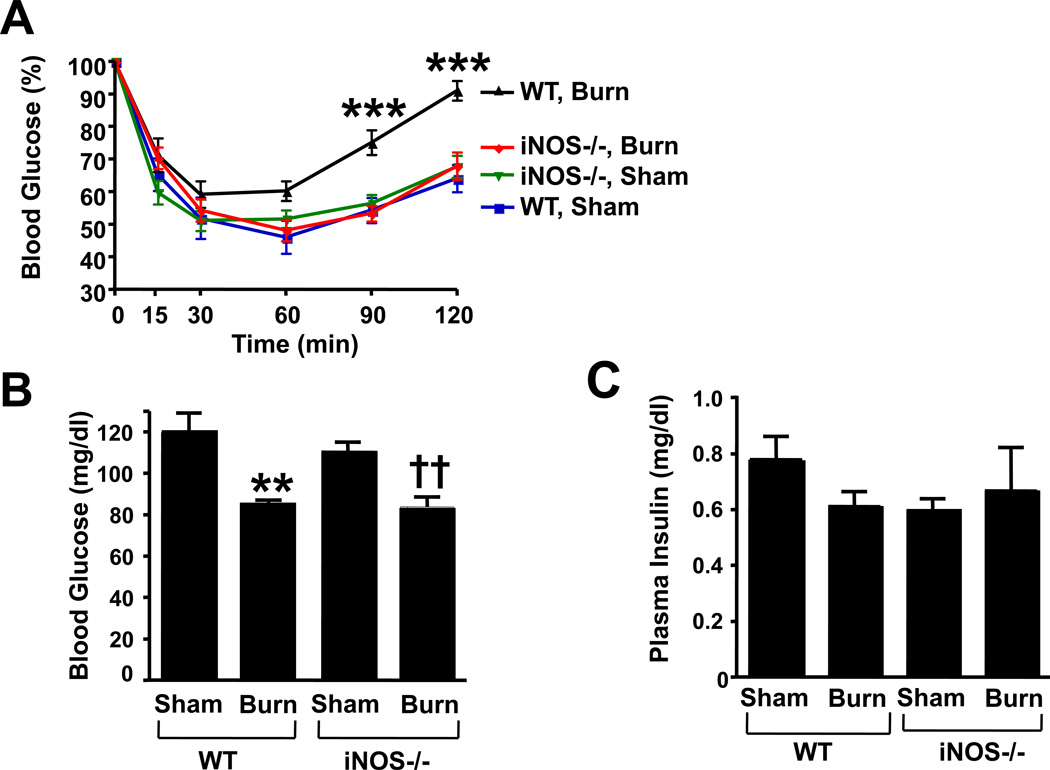
iNOS deficiency reversed whole-body insulin resistance, but did not ameliorate decreased blood glucose levels in burned mice
Burn injury was associated with whole-body insulin resistance in wild-type mice, as judged by insulin tolerance test. Gene disruption of iNOS reverted burn injury-induced insulin resistance (Fig. 5A). Unexpectedly blood glucose levels were lower in burned mice compared with sham animals (Fig. 5B). iNOS deficiency did not affect blood glucose levels in either burned or sham-burned mice. Neither burn injury nor iNOS deficiency significantly altered plasma insulin concentrations in mice (Fig. 5C). We evaluated mRNA expression levels of PEPCK and G-6-Pase, gluconeogenic genes, in the liver. PEPCK and G-6-Pase mRNA levels did not significantly differ between the groups (PEPCK mRNA [%]: WT-Sham: 100 ± 13 [mean ± SEM]; WT-Burn: 102 ± 8; KO-Sham: 101 ± 13; KO-Burn: 121 ± 9; G-6-Pase [%]: WT-Sham: 100 ± 16; WT-Burn: 85 ± 10; KO-Sham: 87 ± 11; KO-Burn: 80 ± 10).
Fig. 5.
iNOS deficiency improved whole-body insulin resistance in burned mice, but did not alter decreased blood glucose levels after burn injury. A, Insulin tolerance test revealed that hypoglycemic response to insulin injection was significantly blunted in burned wild-type (WT) compared to sham animals. Gene disruption of iNOS (−/−) restored sensitivity to insulin-stimulated decrease in blood glucose levels in burned mice. The overall interactions between genotype and burn/sham at 90 and 120 min after the insulin injection are statistically significant (P<0.005). ***P<0.001 vs sham-burned WT and sham-burned and burned iNOS−/−. n=8 per group. B, Unexpectedly, burn injury resulted in decreased blood glucose levels both in wild-type and iNOS-deficient mice. iNOS deficiency did not alter blood glucose levels either in sham-burned mice or in burned mice. **P<0.01 vs sham-burned WT, ††P<0.01 vs sham-burned iNOS−/−. n=8 per group. C, Neither burn injury nor iNOS deficiency significantly affected plasma insulin concentrations.
Discussion
We found that gene disruption of iNOS significantly ameliorated impaired IRS-1-mediated insulin signaling in skeletal muscle of burned mice. Consistent with our previous studies in rats [17,32], burn injury caused decreases in insulin-stimulated tyrosine phosphorylation of IR and IRS-1 (Figs. 1, 2), and insulin-stimulated activation of PI3K (Fig. 3) and Akt (Fig. 4A) in skeletal muscle compared with sham-burn. Burn injury caused a marked induction of iNOS at 3 days after burn (Fig. 1A). Importantly, the impairment of IR–IRS-1-mediated insulin signaling in skeletal muscle paralleled iNOS induction following burn injury (Fig.1). The insulin-stimulated tyrosine phosphorylation of IR and IRS-1, insulin-stimulated PI3K activity, insulin-stimulated Akt phosphorylation, and IRS-1 expression were significantly greater in burned iNOS−/− mice compared with burned wild-type mice (Figs. 1–4). Likewise, burn injury resulted in suppressed insulin-stimulated glucose uptake by muscle, which was reverted by iNOS deficiency (Fig. 4B). These findings clearly indicate that iNOS plays an important role in burn injury-induced insulin resistance in mouse skeletal muscle.
Mitigation of impaired insulin signaling by gene disruption of iNOS was observed at the levels of both IR and IRS-1 in skeletal muscle of burned mice. In insulin-resistant states, the impaired insulin-stimulated tyrosine phosphorylation of IRS-1, a major component of insulin resistance, may result from decreased tyrosine phosphorylation of IRS-1 by IR kinase, reduced IRS-1 expression, or a combination of both. Both attenuated IR kinase activity, reflected by reduced insulin-stimulated tyrosine phosphorylation of IR, and decreased IRS-1 expression were associated with insulin resistance in skeletal muscle of burned mice. It should be noted, however, that when normalized to IRS-1 protein expression, the ratio of insulin-stimulated IRS-1 phosphorylation vs IRS-1 protein expression was not altered by iNOS deficiency in either sham or burned animals (Fig. 2E). These findings suggest that the improved insulin-stimulated phosphorylation of IRS-1 by iNOS deficiency may be mainly attributable to the increased IRS-1 protein expression in skeletal muscle of burned mice.
Improvement of reduced IRS-1 expression by iNOS deficiency was in agreement with our previous study which showed that iNOS and NO donor reduce IRS-1 expression in cultured skeletal muscle cells, and that iNOS deficiency restored the reduced IRS-1 expression in skeletal muscle of genetically obese, diabetic (ob/ob) mice [28]. Taken together, these observations suggest that iNOS expressed in skeletal muscle may contribute to reduced IRS-1 protein expression following burn injury, as seen in obesity.
With respect to tyrosine phosphorylation of IR, the effects of iNOS deficiency on insulin signaling seem to differ between burn injury- and obesity-induced insulin resistance. In burned mice, gene disruption of iNOS significantly improved insulin-stimulated phosphorylation of IR as well as IRS-1 (Fig. 2). In contrast, iNOS and NO donor did not attenuate insulin-stimulated tyrosine phosphorylation of IR in cultured skeletal muscle cells [28]. Consistent with the observation in cultured cells, iNOS deficiency did not significantly alleviate the reduced insulin-stimulated tyrosine phosphorylation of IR in obese, diabetic (ob/ob) mice [28]. One may speculate, therefore, that the improvement in insulin-stimulated tyrosine phosphorylation of IR in burned iNOS−/− mice may not be fully attributable to locally expressed iNOS in the skeletal muscle. Our preliminary observation revealed that iNOS expression was also induced in adipose tissue and liver at 3 days after burn injury in mice (unpublished observation 2009, M. Kaneki). Hence, it is possible that iNOS induction in tissues other than skeletal muscle, including adipose tissue, might also contribute to insulin resistance in skeletal muscle, particularly, attenuated insulin-stimulated tyrosine phosphorylation of IR in burned mice. Alternatively, differences in the degree of iNOS induction in skeletal muscle of burned mice vs. obese, diabetic (ob/ob) mice might contribute to the distinct effects of iNOS on IR phosphorylation. iNOS expression following burn injury is much greater than that observed in skeletal muscle of obese, diabetic (ob/ob) mice.
Insulin resistance of critical illness lasts longer [1,2,38] than the three day period observed in the present study. In fact, reduced IRS-1 expression was also observed at 7 days after burn injury (end of observation period of this study) (Fig. 1). The magnitude and size of the body surface burned area in this study were quite small (12%), where the inflammatory process was modest and self-limiting. A longer lasting effect on insulin signaling would be demonstrated in a more serious injury model [18,19].
In combination with insulin insufficiency (pancreatic β-cell dysfunction), insulin resistance is a major causative factor for hyperglycemia. It is interesting to note, however, that whole-body and skeletal muscle insulin resistance was accompanied by decreased blood glucose levels in burned wild-type mice compared to sham animals. While gene disruption of iNOS reversed insulin resistance, it did not affect blood glucose levels in burned mice. The decreased blood glucose level in burned mice contrasts with our previous findings of burn injury-induced hyperglycemia and hyperinsulinemia in rats [32]. A recent study has also shown that burn injury results in a modest increase in blood glucose levels at 7 days after burn injury in CD1 mice [39]. This apparent discrepancy in the effects of burn injury on blood glucose levels could be explained by differences in the experimental procedures, including burn injury, and differences in species and strains of rodents. It is important to note that hypoglycemia also occurs in burn patients without insulin treatment or other hypoglycemic agents [40,41], although in many cases burn injury is associated with hyperglycemia in humans. We do not presently have an explanation why the burned mice developed hypoglycemia but since the reduced blood glucose levels were not influenced by iNOS deficiency, the hypoglycemia was probably unrelated to the burn-induced increase in iNOS expression. Further studies are required to clarify the etiology of hypoglycemia in burns.
Our data clearly indicate that iNOS plays an important role in burn injury-induced skeletal muscle insulin resistance. These results suggest that iNOS may exert insulin-desensitizing effects, at least in part, by suppressing the protein expression of IRS-1 in skeletal muscle after burn injury.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (R01-DK-058127 to M. Kaneki, R01-GM-31569, R01-GM-055082, R01-GM-61411, and GM-21700 [Project IV] to J. A. J. Martyn) and the Shriners Hospitals for Children Grants 8540 and 71000 (to M. Kaneki), 8530 and 8510 (to J. A. J. Martyn), and 8830 (to S. Yasuhara).
Abbreviations
- G-6-Pase
Glucose-6 phosphatase
- GAPDH
Glyceraldehydes-3-phosphate dehydrogenase
- iNOS
Inducible nitric oxide synthase
- IR
Insulin receptor
- IRS-1
Insulin receptor substrate-1
- KHB
Krebs-Henseleit bicarbonate
- PEPCK
phosphoenolpyruvate carboxykinase
- PI3-K
Phosphatidylinositol 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: no conflict of interest
Authors’ Contributions:
Michiko Sugita: Experimental Design, Performance of Experiments, Data Analysis
Hiroki Sugita: Experimental Design, Performance of Experiments, Data Analysis
Minhye Kim: Experimental Design, Performance of Experiments, Data Analysis
Ji Mao: Experimental Design, Performance of Experiments, Data Analysis
Yoshikazu Yasuda: Experimental Design, Performance of Experiments, Data Analysis
Nobuyuki Shimizu: Experimental Design, Performance of Experiments, Data Analysis
Mayu Habiro: Experimental Design, Performance of Experiments, Data Analysis
Shohei Shinozaki Experimental Design, Performance of Experiments, Data Analysis
Shingo Yasuhara: Experimental Design, Performance of Experiments, Data Analysis
J.A.J. Jeevandra Martyn: Experimental Design, Data Analysis, Editing Manuscript
Masao Kaneki: Experimental Design, Performance of Experiments, Data Analysis, Writing Manuscript
References
- 1.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–168. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Hart DW, Wolf SE, Zhang XJ, Chinkes DL, Buffalo MC, Matin SI, DebRoy MA, Wolfe RR, Herndon DN. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med. 2001;29:1318–1324. doi: 10.1097/00003246-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ipaktchi K, Arbabi S. Advances in burn critical care. Crit Care Med. 2006;34 doi: 10.1097/01.CCM.0000232625.63460.D4. [DOI] [PubMed] [Google Scholar]
- 5.Zifko UA. Long-term outcome of critical illness polyneuropathy. Muscle Nerve Suppl. 2000;9:S49–S52. doi: 10.1002/1097-4598(2000)999:9<::aid-mus9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Helm PA, Pandian G, Heck E. Neuromuscular problems in the burn patient: cause and prevention. Arch Phys Med Rehabil. 1985;66:451–453. [PubMed] [Google Scholar]
- 7.Hund E. Critical illness polyneuropathy. Curr Opin Neurol. 2001;14:649–653. doi: 10.1097/00019052-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bolton CF. Critical illness polyneuropathy and myopathy. Crit Care Med. 2001;29:2388–2390. doi: 10.1097/00003246-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Chang PY, Benecke H, Le Marchand-Brustel Y, Lawitts J, Moller DE. Expression of a dominant-negative mutant human insulin receptor in the muscle of transgenic mice. J Biol Chem. 1994;269:16034–16040. [PubMed] [Google Scholar]
- 10.Le Roith D, Kim H, Fernandez AM, Accili D. Inactivation of muscle insulin and IGF-I receptors and insulin responsiveness. Curr Opin Clin Nutr Metab Care. 2002;5:371–375. doi: 10.1097/00075197-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Agati JM, Yeagley D, Quinn PG. Assessment of the roles of mitogen-activated protein kinase, phosphatidylinositol 3-kinase, protein kinase B, protein kinase C in insulin inhibition of cAMP-induced phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem. 1998;273:18751–18759. doi: 10.1074/jbc.273.30.18751. [DOI] [PubMed] [Google Scholar]
- 12.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 13.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 14.Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 16.Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White MF, Kahn CR. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992;90:1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikezu T, Okamoto T, Yonezawa K, Tompkins RG, Martyn JA. Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem. 1997;272:25289–25295. doi: 10.1074/jbc.272.40.25289. [DOI] [PubMed] [Google Scholar]
- 18.Carter EA, Burks D, Fischman AJ, White M, Tompkins RG. Insulin resistance in thermally-injured rats is associated with post-receptor alterations in skeletal muscle, liver and adipose tissue. Int J Mol Med. 2004;14:653–658. [PubMed] [Google Scholar]
- 19.Zhang Q, Carter EA, Ma BY, White M, Fischman AJ, Tompkins RG. Molecular mechanism(s) of burn-induced insulin resistance in murine skeletal muscle: role of IRS phosphorylation. Life Sci. 2005;77:3068–3077. doi: 10.1016/j.lfs.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil GS. Role of Endoplasmic Reticulum Stress and c-Jun NH2-Terminal Kinase Pathways in Inflammation and Origin of Obesity and Diabetes. Diabetes. 2005;54 Suppl 2:S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 21.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 22.Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S6–S11. doi: 10.1038/sj.ijo.0802491. [DOI] [PubMed] [Google Scholar]
- 23.Lin MC, Ebihara S, El Dwairi Q, Hussain SN, Yang L, Gottfried SB, Comtois A, Petrof BJ. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med. 1998;158:1656–1663. doi: 10.1164/ajrccm.158.5.9803112. [DOI] [PubMed] [Google Scholar]
- 24.Barker JE, Knight KR, Romeo R, Hurley JV, Morrison WA, Stewart AG. Targeted disruption of the nitric oxide synthase 2 gene protects against ischaemia/reperfusion injury to skeletal muscle. J Pathol. 2001;194:109–115. doi: 10.1002/path.845. [DOI] [PubMed] [Google Scholar]
- 25.Mikawa K, Kodama SI, Nishina K, Obara H. ONO-1714, a new inducible nitric oxide synthase inhibitor, attenuates diaphragmatic dysfunction associated with cerulein-induced pancreatitis in rats. Crit Care Med. 2001;29:1215–1221. doi: 10.1097/00003246-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 27.Sugita H, Kaneki M, Tokunaga E, Sugita M, Koike C, Yasuhara S, Tompkins RG, Martyn JA. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am J Physiol Endocrinol Metab. 2002;282:E386–E394. doi: 10.1152/ajpendo.00087.2001. [DOI] [PubMed] [Google Scholar]
- 28.Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn JA, Kaneki M. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem. 2005;280:14203–14211. doi: 10.1074/jbc.M411226200. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54:1340–1348. doi: 10.2337/diabetes.54.5.1340. [DOI] [PubMed] [Google Scholar]
- 31.Tomera JF, Lilford K, Martyn JA. Diaphragm acetylcholinesterase multimeric forms in mice in response to burn trauma. J Burn Care Rehabil. 1993;14:406–419. doi: 10.1097/00004630-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Sugita H, Kaneki M, Sugita M, Yasukawa T, Yasuhara S, Martyn JA. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E585–E591. doi: 10.1152/ajpendo.00321.2004. [DOI] [PubMed] [Google Scholar]
- 33.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 34.Hansen PA, Gulve EA, Holloszy JO. Suitabillity of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- 35.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinozaki S, Chiba T, Kokame K, Miyata T, Ai M, Kawakami A, Kaneko E, Yoshida M, Shimokado K. Site-specific effect of estradiol on gene expression in the adipose tissue of ob/ob mice. Horm Metab Res. 2007;39:192–196. doi: 10.1055/s-2007-970417. [DOI] [PubMed] [Google Scholar]
- 37.Sekiya M, Osuga J, Nagashima S, Ohshiro T, Igarashi M, Okazaki H, Takahashi M, agyu H, Ohashi K, Nagai R, Kadowaki T, Furukawa Y, Shibashi ST. Ablation of neutral cholesterol ester hydrolase 1 accelerates atherosclerosis. Cell Metabolism. 2009;10:219–228. doi: 10.1016/j.cmet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 38.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 39.Bonab AA, Carter EA, Paul K, Kaneki M, Yu YM, Tompkins RG, Fischman AJ. Effect of simvastatin on burn-induced alterations in tissue specific glucose metabolism: implications for burn associated insulin resistance. Int J Mol Med. 2010;26:311–316. [PMC free article] [PubMed] [Google Scholar]
- 40.Shakir KM, Amin RM. Endocrine crises. Hypoglycemia. Crit Care Clin. 1991;7:75–87. [PubMed] [Google Scholar]
- 41.Fischer KF, Lees JA, Newman JH. Hypoglycemia in hospitalized patients. Causes and outcomes. N Engl J Med. 1986;315:1245–1250. doi: 10.1056/NEJM198611133152002. [DOI] [PubMed] [Google Scholar]