Abstract
Huntingtin peptides with elongated polyglutamine domains, the root causes of Huntington's disease, hinder histone acetylation, which leads to transcriptional dysregulation. However, the range of acetyltransferases interacting with mutant Huntingtin has not been systematically evaluated. We used genetic interaction tests in Drosophila to determine whether specific acetyltransferases belonging to distinct protein families influence polyglutamine pathology. We found that flies expressing a mutant form of the Huntingtin protein (Httex1pQ93) exhibit reduced viability, which is further decreased by partial loss of Pcaf or nejire, while the tested MYST family acetyltransferases did not affect pathology. Reduced levels of Pcaf also led to the increased degeneration of photoreceptor neurons in the retina. Overexpression of Pcaf, however, was not sufficient to ameliorate these phenotypes, and the level of soluble Pcaf is unchanged in Httex1pQ93-expressing flies. Thus, our results indicate that while Pcaf has a significant impact on Huntington's disease pathology, therapeutic strategies aimed at elevating its levels are likely to be ineffective in ameliorating Huntington's disease pathology; however, strategies that aim to increase the specific activity of Pcaf remain to be tested.
Key Words: Polyglutamine, Huntington's disease, Histone acetyltransferase, Pcaf, Nejire, Drosophila
Introduction
Huntington's disease (HD) belongs to a group of neurodegenerative diseases caused by the expansion of a polyglutamine (polyQ) domain in the affected protein. Transcriptional dysregulation is one of the molecular mechanisms underlying polyQ pathology [1]. The effect on transcription can be partially explained by the fact that mutant Huntingtin (Htt) binds to several transcription factors [1]. One group of affected factors is the histone acetyltransferases (HATs) [2]. Mutant Htt binds to CBP, p300 and Pcaf and inhibits their activities leading to a decreased level of histone acetylation [3]. Furthermore, mutant Htt depletes CBP by targeting it for degradation [4], and depletion of CBP is associated with cell death in HD models [5]. The importance of altered protein acetylation in HD is underscored by experiments showing that inhibition of histone deacetylase enzymes ameliorates HD phenotypes in several animal models [3,6,7]. Although the HATs belong to different protein families, for example the GNAT (e.g. Pcaf), the MYST and the CBP/p300 families [8], most studies to date have focused only on the role of CBP in disease pathogenesis leaving the role of other HATs unknown. Here we describe a genetic interaction study designed to determine whether HATs other than CBP are involved in polyQ pathogenesis.
To investigate the consequences of reduced HAT activity on polyQ pathology, we used a Drosophila model of HD, which was previously shown to be sensitive to acetylation levels [3,6]. We compared the phenotypes of flies that express Httex1pQ93 in the nervous system with their siblings that, in addition, carry a HAT mutation as well. We found that partial loss of Pcaf (the single fly homolog of human Pcaf and Gcn5) or nejire/dCBP (nej, homolog of human CBP) by the PcafE333St or nej3 mutations, respectively, significantly reduced the viability of Htt-expressing flies, while reducing the MYST family acetyltransferases enok, mof or CG1894 did not have a significant effect on pathology (table 1).
Table 1.
Results of genetic interaction crosses involving HAT mutants and Htt
| HAT | Affected | Mutation | Relative | p value | n | HAT mutation; | Htt | HAT mutation; | No |
|---|---|---|---|---|---|---|---|---|---|
| family | gene | eclosion, % | Htt exp. | exp. | no Htt | Htt | |||
| CBP/p300 | nej | nej3 | 67.3 | <0.01 | 1,268 | 150 | 209 | 469 | 440 |
| MYST | enok | enok2 | 92.5 | 0.47 | 2,341 | 202 | 208 | 989 | 942 |
| mof | mof2 | 100.5 | 0.96 | 1,437 | 232 | 206 | 528 | 471 | |
| CG1894 | Df(3R)Ex6259 | 83.7 | 0.12 | 1,432 | 197 | 215 | 533 | 487 | |
| GNAT | Pcaf | PcafE333St | 49.4 | <0.001 | 3,419 | 114 | 215 | 1,600 | 1,490 |
| Df(3L)iro-2 | 54 | <0.001 | 4,163 | 340 | 612 | 1,629 | 1,582 | ||
| UAS-Pcaf1 | 102.2 | 0.89 | 1,002 | 99 | 88 | 427 | 388 | ||
| UAS-Pcaf3B | 118.4 | 0.3 | 785 | 109 | 93 | 290 | 293 | ||
| UAS-Pcaf5L | 135.5 | 0.08 | 867 | 89 | 74 | 331 | 373 | ||
| UAS-Pcaf7 | 126.2 | 0.32 | 390 | 51 | 46 | 137 | 156 | ||
The relative eclosion rates, statistical significance, number of all scored progenies (n) and the number of offspring in the four genotype categories are shown for loss of function HAT mutants, HAT deletions and UAS-Pcaf overexpressor lines.
Since the role of CBP is well established in HD pathogenesis, we sought to investigate in detail the effect of Pcaf, which was not previously characterized. First, we tested whether Df(3L)iro-2, an independent deletion that removes the Pcaf gene but does not share the same genetic background as the Pcaf E333St null allele, has the same effect. We found that Htt-expressing flies heterozygous for Df(3L)iro-2 also exhibited significantly reduced viability by 46% (table 1). Next we asked whether reduced Pcaf levels lead to neuronal toxicity. We compared Htt-expressing control flies with siblings expressing Htt and also carrying the PcafE333St allele, and found that the average number of rhabdomeres (light gathering structures of photoreceptor neurons) per ommatidium decreased from 4.66 ± 0.08 to 4.32 ± 0.11 (n = 10, p = 0.021), as measured by the pseudopupil assay. We obtained a similar result with the Df(3L)iro-2 deletion, where the average number of rhabdomeres decreased from 4.78 ± 0.11 to 4.42 ± 0.03 (n = 7, p = 0.028), indicating that reduced Pcaf levels enhance neurodegeneration.
Since reducing Pcaf is deleterious, we next asked whether Htt pathology could be ameliorated by overexpressing Pcaf. We generated transgenic flies expressing a full-length Pcaf cDNA under UAS control and tested four independent UAS-Pcaf transgenic strains. We found that the eclosion rates of flies expressing both Pcaf and Htt simultaneously in the nervous system were slightly, albeit not significantly, higher than those of mutant Htt-expressing control siblings (table 1). Similarly, we found no significant difference in neurodegeneration between flies coexpressing Htt and Pcaf, compared to siblings expressing only Htt, as measured by the pseudopupil assay (data not shown).
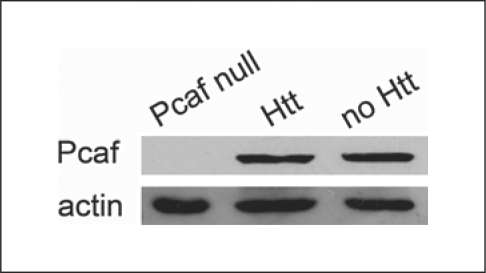
In addition, we sought to determine whether the quantity of soluble Pcaf protein is altered in Httex1pQ93-expressing flies and found that mutant Htt expression does not result in the reduction of the level of Pcaf (fig. 1).
Fig. 1.
Mutant Huntingtin does not reduce the level of soluble Pcaf. Immunoblots of head extracts of Httex1pQ93-expressing flies (Htt) are shown next to wild-type control (no Htt), as well as larval extracts of homozygous PcafE333St (Pcaf null).
Taken together, we found that partial loss of Pcaf significantly enhanced polyglutamine pathology, but the level of Pcaf was not reduced by mutant Htt, and we could not rescue HD phenotypes by the overexpression of Pcaf, indicating that the interaction of mutant Htt and Pcaf does not involve the depletion of soluble Pcaf by either degradation or sequestration to insoluble aggregates. This result, however, does not exclude the possibility that a soluble toxic form of Htt might inhibit the function of either Pcaf itself or of Pcaf-containing complexes. Since Pcaf acts as a catalytic subunit in large multiprotein complexes in metazoans [9], we speculate that its interaction with a polyQ peptide might cripple entire complexes, which cannot be rescued by overexpression of Pcaf alone. We conclude that although Pcaf has a significant impact on HD pathology, therapeutic strategies aimed at elevating the levels of Pcaf protein are unlikely to be effective in ameliorating HD pathology. The question, however, remains open whether strategies that aim to increase the specific activity of Pcaf might be useful. Interestingly, of the three HAT families of proteins tested, only the GNAT and the CBP/p300 families exhibit a strong influence over HD pathology, while the MYST family members have decidedly less impact.
Materials and Methods
Stocks carrying the mutations PcafE333St, Df(3L)iro-2, Df(3R)Exel6259, enok2, nej3, and the pan-neuronal elav-GAL4 driver w P{w+mW.hs=GawB}elavC155 were from the Bloomington Drosophila Stock Center. The mof2 allele was kindly provided by John C. Lucchesi (Emory University, Atlanta, Ga., USA). The w; P{UAS-Httex1p-Q93}4F1 transgenic line expressing the first exon of human Htt with a 93-residue-long polyQ repeat under UAS control was generated in our laboratory previously [3].
UAS-Pcaf transgenic lines were produced by cloning the full-length Pcaf cDNA from the GH11602 clone (from the Drosophila Genomics Resource Center, Bloomington, Indiana University) to the EcoRI site of pUAST, and generating transgenics by standard P element-mediated transformation.
Viability tests were done by crossing elav>Gal4/Y HAT mutant/Marker males to Httex1pQ93/Httex1pQ93 females and scoring the number of offspring in the four genotype categories. Relative eclosion rates were calculated as the ratio of HAT mutant to HAT wild-type Htt-expressing siblings normalized by the ratio of HAT mutant to HAT wild-type siblings not expressing Htt. The statistical significance of differences in eclosion was determined by a χ2 probe. Pseudopupil analysis was performed on 6-day-old females as described previously [3]; statistical significance was evaluated by unpaired t test.
Immunoblotting was performed on head extracts from 1-day-old flies using polyclonal antibodies against Pcaf/dGcn5 [10] and actin (Sigma A5060) with goat-anti-rabbit-HRP secondary antibody (Dako P0448); detection was done using Immobilon Western Chemiluminescent HRP substrate (Millipore).
Acknowledgements
The authors wish to thank John C. Lucchesi (Emory University, Atlanta, Ga., USA) and the Bloomington Drosophila Stock Center for providing fly stocks, the Drosophila Genomics Resource Center for cDNA clones and Sofia G. Georgieva (Institute of Gene Biology, Moscow, Russia) for the dGcn5 antibody. This work was supported by the National Institutes of Health (NS045283 to J.L.M., NS52789 to L.M.T.), the Hereditary Disease Foundation (HDF-24085), and the Huntington's Disease Society of America (35326).
References
- 1.Riley BE, Orr HT. Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev. 2006;20:2183–2192. doi: 10.1101/gad.1436506. [DOI] [PubMed] [Google Scholar]
- 2.Bodai L, Pallos J, Thompson LM, Marsh JL. Altered protein acetylation in polyglutamine diseases. Curr Med Chem. 2003;10:2577–2587. doi: 10.2174/0929867033456530. [DOI] [PubMed] [Google Scholar]
- 3.Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Zhu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- 4.Cong S, Pepers BA, Evert BO, Rubinsztein DC, Roos RAC, van Ommen GB, Dorsman JC. Mutant huntingtin represses CBP, but not p300, by binding and protein degradation. Mol Cell Neurosci. 2005;30:560–571. [PubMed] [Google Scholar]
- 5.Jiang H, Poirier MA, Liang Y, Pei Z, Weiskittel CE, Smith WW, DeFranco DB, Ross CA. Depletion of CBP is directly linked with cellular toxicity caused by mutant huntingtin. Neurobiol Dis. 2006;23:543–551. doi: 10.1016/j.nbd.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington's disease. Hum Mol Genet. 2008;17:3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PAS, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc Natl Acad Sci USA. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy Z, Tora L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene. 2007;26:5341–5357. doi: 10.1038/sj.onc.1210604. [DOI] [PubMed] [Google Scholar]
- 10.Lebedeva LA, Nabirochkina EN, Kurshakova MM, Robert F, Krasnov AN, Evgenev MB, Kadonaga JT, Georgieva SG, Tora L. Occupancy of the Drosophila hsp70 promoter by a subset of basal transcription factors diminishes upon transcriptional activation. Proc Natl Acad Sci USA. 2005;102:18087–18092. doi: 10.1073/pnas.0509063102. [DOI] [PMC free article] [PubMed] [Google Scholar]