Abstract
It is well known that natural products are a rich source of compounds for applications in medicine, pharmacy, and biology. However, the exact molecular mechanisms of natural agents in human health have not been clearly defined. Here, we demonstrate for the first time that the polyphenolic phytoalexin resveratrol promotes expression and activity of Argonaute2 (Ago2), a central RNA interference (RNAi) component, which thereby inhibits breast cancer stem-like cell characteristics by increasing the expression of a number of tumour-suppressive miRNAs, including miR-16, -141, -143, and -200c. Most importantly, resveratrol-induced Ago2 resulted in a long-term gene silencing response. We also found that pterostilbene, which is a natural dimethylated resveratrol analogue, is capable of mediating Ago2-dependent anti-cancer activity in a manner mechanistically similar to that of resveratrol. These findings suggest that the dietary intake of natural products contributes to the prevention and treatment of diseases by regulating the RNAi pathway.
Natural products are a rich source of valuable medicinal agents. More than half of the currently available drugs are natural or related compounds. In the case of cancer, the percentage of natural compounds exceeds 60%. Research on natural products as potential anti-cancer agents dates back to at least the Egyptian Ebers Papyrus of 1550 B.C. However, more recent scientific investigations began with the studies of Hartwell and co-workers on the application of podophyllotoxin and its derivatives as anti-cancer agents1. A large number of plant, marine, and microbial sources have been tested, and hundreds of active compounds have been isolated. Despite these advances, the underlying mechanisms of natural products in human health are not fully understood.
Resveratrol, which is a multi-functional polyphenolic compound, is a phytoalexin present in a wide variety of plant species, including grapes, mulberries, and peanuts2. Since its discovery, resveratrol has been shown to exhibit a plethora of physiological properties that may be useful in human medicine. More interest was focused on resveratrol at the beginning of the 1990s when it was first shown to be present in red wine3. Experimental studies have shown that resveratrol inhibits the growth of various cancer cells and induces apoptotic cell death4,5. Recently, a phase I/II clinical trial in patients with colon cancer was conducted to examine the effects of resveratrol treatment on colon cancer progression and colonic mucosa in patients with colon cancer and its effects in modulating the Wnt signalling pathway2. Although these data provide evidence of multiple anti-tumour effects induced by resveratrol, the exact mechanism is not clearly understood.
MicroRNAs (miRNAs) have emerged as key post-transcriptional regulators of gene expression that are involved in diverse physiological and pathological processes6. The inhibition of the miRNA biogenesis pathway results in severe developmental defects and lethality in many organisms7. It has been suggested that a considerable number of miRNAs have roles in cancer cells. Indeed, an increasing number of experimental studies have shown that the knock-down or the re-expression of specific miRNAs could induce drug sensitivity, inhibit the proliferation of cancer cells, and suppress cancer cell invasion and metastasis8,9,10. Recent studies have shown that natural products, including curcumin, isoflavone, I3C, DIM, and EGCG, could alter the expression of specific miRNAs, which may lead to the increased sensitivity of cancer cells to conventional anti-cancer agents and, therefore, tumour growth inhibition11,12,13,14. However, the exact molecular mechanism of miRNA induction and the biological significance of resveratrol-induced miRNAs have not been reported.
Diet is one of the most important modifiable cancer risk determinants15. Dietary components have been implicated in many pathways involved in carcinogenesis. In addition, carcinogenic processes are associated with the altered expression of several miRNAs. Recent studies have reported that a widespread down-regulation of miRNAs is commonly observed during human cancer-cell initiation and progression16,17. In this study, we hypothesised that the dietary intake of natural products maintains tumour-suppressive miRNA expression in cancer cells, leading to the prevention of carcinogenesis. We demonstrated that resveratrol suppresses cancer cell malignancy in vitro and in vivo through the transcriptional activation of tumour-suppressive miRNAs and Argonaute2 (Ago2). Furthermore, we provided evidence that Ago2 over-expression enhances the RNA interference (RNAi) activity. These findings suggest that the dietary intake of natural products safely reduces a wide range of negative consequences with an overall improvement in human health and survival by modulating miRNA biogenesis.
Results
Resveratrol reduces the cancer stem-like cells population by up-regulating miR-141 and miR-200c
To identify the potential anti-cancer activity of resveratrol, we investigated the effects of this compound on tumour formation in vivo. We orthotopically inoculated female SCID hairless outbred mice with MDA-MB-231-luc-D3H2LN cells (200 cells), which were then treated with resveratrol (25 mg/kg/day) or ethanol (control) via intraperitoneal injection every day for one week. Tumour growth was then monitored using an IVIS imaging system. The weight of the mice did not significantly change between the groups during the course of the experiment, suggesting that resveratrol did not have notable adverse effects on mice (Supplementary Fig. 1a). The results demonstrated that the resveratrol administration into the mice significantly suppressed tumour formation, while obvious tumours were observed in vehicle-treated mice, indicating that resveratrol is capable of inhibiting the survival and growth of cancer cells in vivo (Fig. 1a). A recent report has shown that solid tumours contain a distinct population of cells with the ability to form tumours in mice; these cells are known as tumour-initiating or cancer stem-like cells (CSCs) and display increased drug resistance and metastatic ability because they consistently form tumours, whereas other cancer cell populations were depleted of cells capable of tumour formation18,19. To identify the effects of resveratrol on the CSC phenotype, breast cancer cells were examined for changes in the CSC population, which is a highly tumourigenic CD44+/CD24− subpopulation with stem cell-like self-renewal properties and the ability to produce differentiated progeny after resveratrol treatment18. Compared to vehicle-treated control cells, cells treated with 50 μM resveratrol demonstrated a significant 6-fold decrease in the CD44+/CD24− population in MDA-MB-231-luc-D3H2LN cells (Fig. 1b). In addition, mammosphere formation, which has been widely used for breast CSC enrichment, of the CD44+/CD24− fraction from MDA-MB-231-luc-D3H2LN cells was suppressed after resveratrol treatment (Supplementary Fig. 1b). We also assessed apoptosis using TUNEL staining and a caspase assay and found that resveratrol did not induce apoptosis (Supplementary Figs. 1c and 1d). Human breast cancers are driven by a CSC component that may contribute to tumour metastasis and therapeutic resistance20. Indeed, we found that the combination of resveratrol with low therapeutic doses of docetaxel elicits significantly greater cancer cell growth inhibition in vitro and in vivo (Supplementary Figs. 1e–g). These findings strongly suggest that resveratrol demonstrates multiple anti-cancer effects through the reduction of the CSC population.
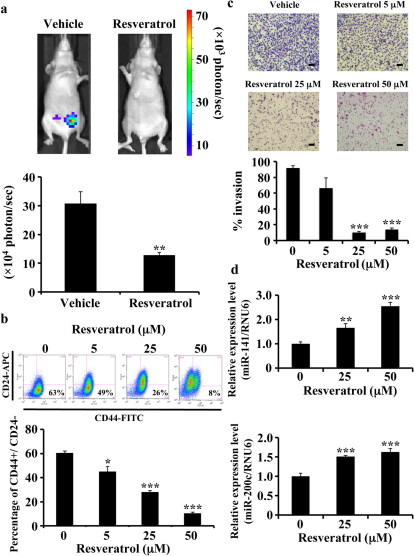
Figure 1. Multiple anti-cancer effects of resveratrol through the activation of miR-141 and miR-200c.
(a) MDA-MB231-luc-D3H2LN cells (200 cells) were injected into the mammary fat pad of six-week-old female SCID hairless outbred mice (n = 5). They were then treated with resveratrol (25 mg/kg/day) by intraperitoneal injection every day for 8 days. Tumour growth was monitored by injecting luciferin in the mice followed by measuring bioluminescence using an IVIS imaging system. Representative mouse images at day 8 (upper panel) and quantified bioluminescence images at day 8 (lower panel) are shown. (b) MDA-MB231-luc-D3H2LN cells were treated with resveratrol or DMSO (control) at the specified doses for 3 days. The percentage of CD44+/CD24− cells after compound treatment in independent experiments with MDA-MB231-luc-D3H2LN cell populations is shown. The CD44+/CD24− denoting the CSC-enriched fraction. (c) MDA-MB231-luc-D3H2LN cells were grown, treated with resveratrol or DMSO (control) for 1 day, and then subjected to an invasion assay. Representative photographs (upper panel) and quantification (lower panel) are shown. Scale bar: 100 μm. (d) The miR-141 and miR-200c expression levels in MDA-MB231-luc-D3H2LN cells. The expression levels of the indicated miRNAs were examined in MDA-MB231-luc-D3H2LN cells after 48 hour resveratrol treatment (all data are shown as the mean ± s.e.m., *P<0.05, **P<0.01, ***P<0.001).
To examine whether resveratrol could influence the breast cancer cell metastasis ability, the highly invasive breast cancer cell line MDA-MB-231-luc-D3H2LN was used in in vitro invasion assays. As shown in Fig. 1c, the invasion of MDA-MB-231-luc-D3H2LN cells was suppressed by resveratrol treatment. Previous studies have documented aberrant miRNA expression in cancer, and our observations prompted us to hypothesise that the anti-cancer resveratrol effects were mediated by miRNAs, particularly by a group of tumour-suppressive miRNAs21. A recent study has demonstrated that miR-141 and miR-200c strongly inhibit breast cancer invasion ability22. We found that resveratrol exposure increases miR-141 and miR-200c expression in MDA-MB-231-luc-D3H2LN cells (Fig. 1d). These findings suggest that resveratrol exhibits multiple anti-cancer effects through the inhibition of CSC phenotypes by activating miR-141 and miR-200c. In addition, to determine whether the up-regulation of miR-141 and miR-200c is mediated at the transcriptional level, we measured the expression levels of the primary miRNAs of miR-141 and miR-200c and found that these miRNAs are up-regulated at the primary transcript level (Supplementary Fig. 1h). Taken together, these results indicate that resveratrol increases the expression of tumour-suppressive miRNAs via the induction of miRNA transcription. Similar results were obtained in two other human breast cancer cell lines (MCF7 and MCF7-ADR) and MCF10A, an immortalised, non-transformed epithelial cell line (Supplementary Figs. 2–4).
Resveratrol up-regulates the expression of tumour-suppressive miRNAs
We demonstrated that resveratrol specifically reduced the CSC fraction (Fig. 1b). In addition, we also observed that miR-141 and miR-200c, which are known to suppress the CSC phenotype, are both induced by resveratrol treatment (Fig. 1d). These observations suggest that a part of the anti-cancer effects of resveratrol is mediated by miRNAs, particularly tumour-suppressive miRNAs. Indeed, a morphological change is observed after resveratrol treatment (Fig. 2a), suggesting that resveratrol induces a variety of miRNAs in cancer cells. To confirm whether miRNAs are globally up-regulated in the multiple anti-cancer effects induced by resveratrol in MDA-MB-231-luc-D3H2LN cells, we performed a comprehensive miRNA profiling of untreated MDA-MB-231-luc-D3H2LN cells and compared the results to those obtained in resveratrol-treated cells. As shown in Fig. 2b, we found that a subset of tumour-suppressive miRNAs is transcriptionally up-regulated by resveratrol (Table 1). To validate the microarray results, we performed qRT-PCR. A set of mature tumour-suppressive miRNAs, including miR-16 and miR-143, are significantly up-regulated in a variety of breast cancer cell lines, including MDA-MB-231-luc-D3H2LN, MCF7, MCF7-ADR, and MCF10A (Supplementary Figs. 2–5). These results indicated that resveratrol globally up-regulates tumour-suppressive miRNAs in human breast normal epithelial and cancer cells.
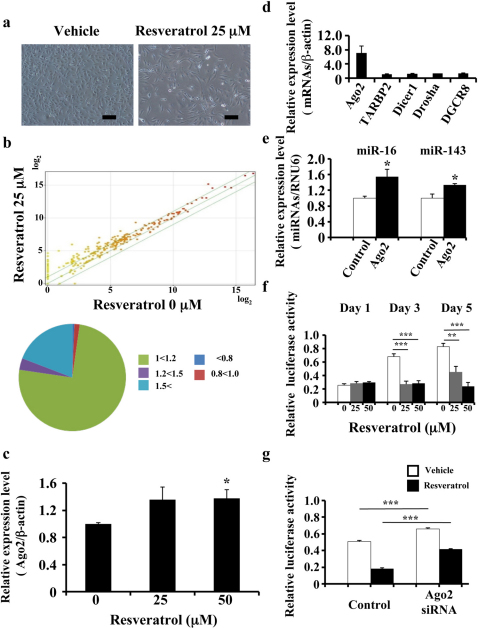
Figure 2. Association between resveratrol and Ago2.
(a) Characteristic microscopic images of MDA-MB231-luc-D3H2LN cells in the presence of DMSO (control) or resveratrol. Scale bar: 100 μm. (b) The effects of resveratrol treatment on miRNA expression in MDA-MB231-luc-D3H2LN cells by miRNA microarray analysis. The proportions of miRNAs at different fold change levels are shown in the lower panel. (c) MDA-MB231-luc-D3H2LN cells were treated with resveratrol or DMSO (control). After 2 days of culture, the cell extract was subjected to real-time mRNA qRT-PCR. (d), (e) MDA-MB231-luc-D3H2LN cells were grown and transiently transfected with Ago2 or EGFP-IRES vector (control). After 2 days of culture, the cell extract was subjected to real-time mRNA (d) and miRNA (e) qRT-PCR. The values on the y-axis are depicted relative to the expression level of the EGFP-IRES control vector, which is defined as 1. (f) MDA-MB231-luc-D3H2LN cells were grown and transiently transfected with luciferase siRNA or AllStars negative control siRNA (0.1 nM) under resveratrol treatment. After 1, 3, or 5 days of culture, the cells were subjected to a luciferase reporter assay. The values on the y-axis are depicted relative to the luciferase activity of the AllStars Negative Control siRNA, which is defined as 1. (g) MDA-MB231-luc-D3H2LN cells were grown and transiently transfected with luciferase siRNA or AllStars Negative Control siRNA and Ago2 siRNA or AllStars Negative Control siRNA. After 3 days of culture, the cells were subjected to a luciferase reporter assay. The values on the y-axis are depicted relative to the luciferase activity of the negative control siRNA, which is defined as 1 (all data are shown as the mean ± s.e.m., *P<0.05, **P<0.01, ***P<0.001).
Table 1. A list of miRNAs which were up-regulated more than 2.0-fold by resveratrol in MDA-MB-231-luc-D3H2LN cells compared with control.
| miRNA | Fold change |
|---|---|
| Tumor-suppressive miRNA | |
| hsa-miR-141 | 4.48 |
| hsa-miR-26a | 2.33 |
| hsa-miR-195 | 3.38 |
| hsa-miR-126 | 2.41 |
| hsa-miR-185 | 2.75 |
| hsa-miR-340 | 11.07 |
| hsa-miR-128 | 2.13 |
| hsa-miR-34a | 2.65 |
| hsa-miR-193b | 2.58 |
| hsa-miR-335 | 2.42 |
| hsa-miR-200c | 3.47 |
| hsa-miR-196a | 2.67 |
| hsa-miR-497 | 4.60 |
| hsa-miR-125a-3p | 3.00 |
| Onco- miRNA | |
| hsa-miR-378* | 4.81 |
| hsa-miR-10b | 5.11 |
| hsa-miR-132 | 7.23 |
| hsa-miR-222 | 2.40 |
Resveratrol enhances the Ago2 RNAi potency
Although our data provide evidence that resveratrol globally up-regulates tumour-suppressive miRNAs and one of the mechanisms that is mediated by primary miRNA up-regulation, we also hypothesised that changes at other levels of the RNAi pathway may play a role in enhancing the resveratrol-mediated miRNA activity in cells in addition to transcriptional alterations. It is known that miRNA generation occurs in a multi-step process23,24. If one of the components associated with the miRNA pathway is under-expressed or qualitatively impaired, the pathway as a whole is destabilised. To examine the effect of resveratrol on the miRNA machinery, we measured the expression levels of a selected group of miRNA machinery-related genes, including Dicer1, Drosha, TARBP2, DGCR8, and Ago2, after the resveratrol treatment of MDA-MB-231-luc-D3H2LN cells. We found that resveratrol exposure significantly increased Ago2 expression in MDA-MB-231-luc-D3H2LN cells (Fig. 2c and Supplementary Fig. 6a). To elucidate the resveratrol-mediated Ago2 up-regulation mechanism, we assessed the Ago2 promoter activity and the Ago2 mRNA and protein half-lives after resveratrol treatment. As shown in supplementary Fig. 6b, the Ago2 protein half-lives were unchanged after resveratrol treatment. In contrast, the Ago2 mRNA was slightly increased after resveratrol treatment (Supplementary Fig. 6c). In addition, resveratrol induced the luciferase activity of a plasmid containing the Ago2 promoter upstream of the luciferase gene, suggesting that resveratrol transcriptionally induced the expression of Ago2 (Supplementary Fig. 6d). The Ago2 protein is a key regulator of miRNA homeostasis and, upon recognition, it can either cleave or remain tethered to an mRNA to repress its translation and/or regulate its stability25. To reveal the relationship between Ago2 and miRNAs, we first quantified the miRNA expression in MDA-MB-231-luc-D3H2LN cells transfected with the Ago2 expression vector. The induction of Ago2 expression by the Ago2 expression vector was confirmed by qRT-PCR (Fig. 2d). After transfection of the Ago2 expression vector, a subset of miRNAs including miR-16, miR-141, miR-143, and miR-200c was higher than in the control cells (Fig. 2e and Supplementary Fig. 6e). To further study the relationship between resveratrol-induced Ago2 and RNAi activity, MDA-MB-231-luc-D3H2LN cells were transfected with luciferase siRNA in the presence of resveratrol treatment and subjected to an in vitro firefly luciferase assay. If the induction of Ago2 expression leads to the enhancement of RNAi activity in cells, the luciferase siRNA silencing effect of the luciferase gene in MDA-MB-231-luc-D3H2LN cells may be enhanced after Ago2 over-expression even in the presence of a low siRNA dose and a prolonged period after siRNA transfection. As shown in Fig. 2f, the resveratrol-induced Ago2 resulted in a long-term gene-silencing response in MDA-MB-231-luc-D3H2LN cells. In addition, Ago2 over-expression in HEK293 cells demonstrated a long-term gene-silencing response that was similar to resveratrol-treated MDA-MB-231-luc-D3H2LN cells (Supplementary Fig. 6f). Moreover, we performed an RNAi experiment to target Ago2 after resveratrol treatment and then assessed the RNAi activity demonstrated by the luciferase siRNA directed against the luciferase gene. The reduction in Ago2 expression by Ago2 siRNA was confirmed by qRT-PCR (Supplementary Fig. 6g). As shown in Fig. 2g, Ago2 siRNA-mediated silencing inhibited the RNAi activity in MDA-MB-231-luc-D3H2LN cells. Taken together, these results indicate that the resveratrol anti-cancer activities were mediated by not only tumour suppressive miRNA upregulation but also by the enhancement of the RNAi activity regulated by Ago2.
Resveratrol-induced miRNA exert an anti-cancer effect
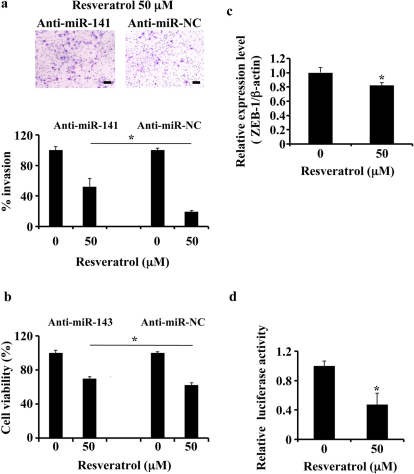
It has been reported that miR-141 inhibits the epithelial-mesenchymal transition and cancer cell migration in breast cancer cells26. In addition, we found that resveratrol induced the expression of miR-141 and miR-200c in MDA-MB-231-luc-D3H2LN cells (Fig. 1d). In contrast, the CSC population was decreased (Fig. 1b). To show direct evidence of whether multiple phenotypes induced by resveratrol were regulated by tumour-suppressive miRNAs, MDA-MB-231-luc-D3H2LN cells were transfected with an antisense oligonucleotide targeting miR-141 (i.e., a miR-141 inhibitor) in the presence of resveratrol treatment. MiR-141 repression by the miR-141 inhibitor was confirmed by qRT-PCR (supplementary Fig. 7a). As shown in Fig. 3a, the miR-141-induced inhibition of invasion was abrogated by the addition of the miR-141 inhibitor, and the MDA-MB-231-luc-D3H2LN cell invasiveness was increased. To confirm the link between resveratrol and miRNA expression, we investigated the growth of breast cancer cells in the presence or absence of a miR-143 inhibitor27. In the presence of resveratrol, miR-143-induced inhibition significantly increased the survival of MDA-MB-231-luc-D3H2LN cells relative to the control (Fig. 3b). It has been shown that miR-200c up-regulation in breast cancer cells inhibits Zeb1 expression, resulting in E-cadherin induction in breast cancer cell lines28. As shown in Fig. 1d, we found miR-200c up-regulation after resveratrol treatment, suggesting that resveratrol treatment activates this pathway and demonstrating its anti-cancer activity. Indeed, resveratrol addition significantly suppressed Zeb1 expression in the breast cancer cell lines (Fig. 3c) and induced E-cadherin expression in those cells (supplementary Fig. 7b). Furthermore, to show the direct effects of resveratrol on the miRNA machinery, we performed a Zeb1 3′UTR assay and demonstrated that resveratrol treatment significantly down-regulated the luciferase activity of a plasmid containing the Zeb1 3′UTR (Fig. 3d). Taken together, these results suggested that resveratrol plays an important role in breast cancer prevention by up-regulating tumour-suppressive miRNAs.
Figure 3. Multiple anti-cancer effects of tumour-suppressive miRNAs induced by resveratrol.
(a) MDA-MB231-luc-D3H2LN cells were grown and transiently transfected with anti-miR-141 or anti-miR-NC (control). After 4 hours, the cells were treated with resveratrol or DMSO (control) for 1 day and subjected to an invasion assay. Representative photographs (upper panel) and quantification (lower panel) are shown. Scale bar: 100 μm. (b) MDA-MB231-luc-D3H2LN cells were cultured and transiently transfected with anti-miR-143 or anti-miR-NC (control). After 4 hours, the cells were treated with resveratrol or DMSO (control) for 72 hours, and the cell viability was measured by the MTS assay. (c) MDA-MB231 cells were treated with resveratrol or DMSO (control). After 2 days of culture, the cell extract was subjected to real-time mRNA qRT-PCR. (d) MDA-MB231 cells were grown and transiently transfected with a ZEB-1 3′UTR or psiCheck2 vector (control) under resveratrol treatment. After 1 day of culture, the cells were subjected to a luciferase reporter assay. The values on the y-axis are depicted relative to the luciferase activity of cells treated with DMSO, which is defined as 1 (all data are shown as the mean ± s.e.m., *P<0.05).
The stilbene family regulates miRNA biogenesis
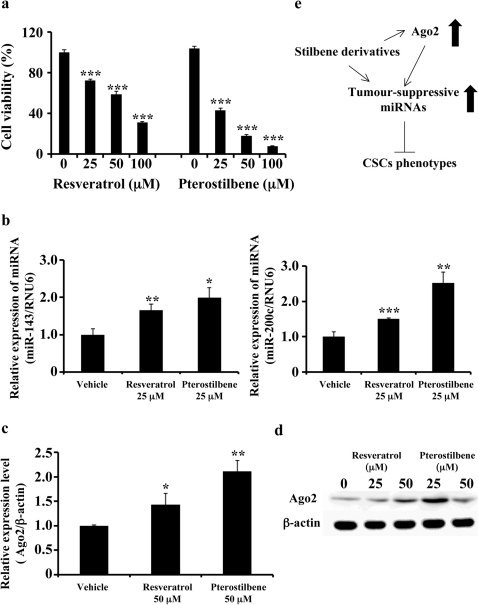
The naturally occurring dimethylether resveratrol analogue pterostilbene is a stilbene family member that is generated by plants. Pterostilbene has also been reported to possess chemopreventive activity in cancer and other resveratrol-like health benefits29,30. To determine whether pterostilbene induced the expression of tumour-suppressive miRNAs in a similar manner as resveratrol, we assessed the effect of pterostilbene on miRNA expression. As shown in Fig. 4a, pterostilbene treatment suppressed cell growth more significantly than resveratrol treatment in MDA-MB-231-luc-D3H2LN cells. In addition, the expression of tumour suppressive miRNAs (i.e., miR-143 and miR-200c) and Ago2 was significantly higher in pterostilbene-treated MDA-MB-231-luc-D3H2LN cells than in resveratrol-treated cells (Figs. 4b-d and Supplementary Fig. 8). Taken together, these results suggest that resveratrol-induced tumour-suppressive miRNA expression and its anti-cancer activity are conserved among stilbene family members (Fig. 4e).
Figure 4. Effects of pterostilbene on human breast cancer cells.
(a) MDA-MB231-luc-D3H2LN cells were cultured in the presence or absence of resveratrol or pterostilbene at the indicated concentrations for 72 hours. Cell viability was measured using the MTS assay. The control wells were treated with DMSO. (b), (c) Expression levels of miR-143, miR-200c (b), and Ago2 (c) in MDA-MB231-luc-D3H2LN cells. The expression levels of the indicated miRNAs were examined in MDA-MB231-luc-D3H2LN cells 48 hours after treatment with pterostilbene. (d) MDA-MB231-luc-D3H2LN cells were treated with stilbenes for 72 hours, and Ago2 expression was detected by immunoblotting. Actin was used as a loading control. (e) Model of the regulation of tumour-suppressive miRNAs and Ago2 expression in the stilbene family (all data are shown as the mean ± s.e.m., *P<0.05, **P<0.01, ***P<0.001).
Discussion
Resveratrol exhibits strong anti-oxidant activity and is capable of inducing apoptosis in cancer cells. Therefore, resveratrol is believed to be efficacious at multiple carcinogenesis stages4. However, the underlying molecular mechanism of its anti-tumour activity has yet to be defined. In this study, we demonstrated that resveratrol up-regulated tumour-suppressive miRNAs, resulting in the induction of an anti-cancer effect against the CSC phenotype in cancer cells. We also demonstrated that resveratrol inhibited the invasiveness of breast cancer cells as one of the CSC phenotypes by activating miR-141 and miR-200c. However, the reason why resveratrol reduces the CSC population remains elusive. Recent studies have provided evidence that miR-200c strongly inhibits the ability of breast CSCs to form tumours in vivo31. These findings suggest that resveratrol shows multiple anti-cancer effects by reducing the CSC population through miR-200c activation.
Argonaute proteins are widely expressed and are involved in post-transcriptional gene silencing. Using microarrays to compare control and Ago2−/− cells, recent studies have demonstrated that Ago2 loss results in the global reduction of mature miRNAs in erythroblasts, fibroblasts, and hepatocytes32. However, it has not been determined whether Ago2 alterations can contribute to miRNA expression and the RNAi response. In this study, we show that Ago2 up-regulation by resveratrol leads to an increase in tumour-suppressive miRNAs and the enhancement of RNAi activity.
Pterostilbene has anti-diabetic properties and has been shown to be cytotoxic to a number of cancer cell lines in vitro29,30. Although pterostilbene and resveratrol have similar pharmacological properties, pterostilbene contains two methoxy groups and one hydroxyl group, while resveratrol has three hydroxyl groups (Supplementary Fig. 9). A recent study demonstrated that pterostilbene shows 95% bioavailability when orally administered, while resveratrol only has 20% bioavailability33. Furthermore, pterostilbene is a more powerful chemopreventive agent than resveratrol in colon cancer34, showing that pterostilbene has several key advantages over resveratrol. In this study, we demonstrate that pterostilbene is more reliable than resveratrol in mediating the anti-cancer effect by inducing tumour-suppressive miRNAs and Ago2 expression. The reason for the difference in the anti-cancer activity of pterostilbene and resveratrol in cancer cells may be due to the expressed miRNAs.
It has been demonstrated that most tumours are characterised by globally diminished miRNA expression16,17,35. Thus, the delivery of tumour suppressive miRNAs may allow for the therapeutic restitution of physiological regulation programs lost in cancer and other disease states. However, miRNA therapy shares many of the disadvantages of other treatment approaches including delivery limitations and instability. Therefore, novel methods are required to resolve these issues. Based on this study, we hypothesise that the down-regulation of miRNAs in cancer cells is compensated by resveratrol, which induces the derepression of tumour suppressive miRNAs. Down-regulation of oncogenic miRNAs and up-regulation of tumour-suppressive miRNAs by resveratrol in prostate cancer cells has been reported36; however, the connection between resveratrol and the miRNA biogenesis machinery has not been investigated in detail. In this report, we demonstrated that resveratrol leads to a reduction in malignancy by not only activating tumour-suppressive miRNA transcription (Figs. 1d and 2b) but also enhancing the RNAi activity mediated by Ago2 induction (Figs. 2d, e, f and g). Our demonstration that resveratrol potently suppresses even a severe and multifocal carcinogenesis model in the absence of measurable toxicity provides proof of the principle that miRNA replacement by resveratrol may be a clinically viable anti-cancer therapeutic strategy.
In conclusion, this study shows that an orally available small molecule can safely reduce many of the negative consequences at doses acceptable in humans with an overall improvement in health and survival. Our results raise the possibility that the regulation of tumour-suppressive miRNAs by natural agents could be a novel strategy in the design of combinational approaches using conventional therapies for tumour recurrence prevention and in achieving successful treatment outcomes in patients with cancer.
Methods
Reagents
Trans-resveratrol (98% purity) was purchased from Cayman Chemical, pterostilbene (98% purity) from Tokyo Chemical Industry, cycloheximide solution and 5, 6-dichlorobenzimidazole riboside from sigma, and docetaxel from Sanofi-Aventis. The antibiotic solution (containing 10,000 U/mL penicillin and 10 mg/mL streptomycin), the trypsin-EDTA mixture (containing 0.05% trypsin and EDTA), and FBS (fetal bovine serum) were obtained from Invitrogen. The FITC-conjugated anti-CD44 (clone L178) antibody was obtained from Becton Dickinson, and the APC- conjugated anti-CD24 (clone ML5) antibody, from Biolegend. The duplexes of each small interfering RNA (siRNA), targeting human Ago2 mRNA (siAgo2-1, GCACGGAAGUCCAUCUGAAUU, UUCAGAUGGACUUCCGUGCUU; siAgo2-2, GCAGGACAAAGAUGUAUUAUU, UAAUACAUCUUUGUCCUGCUU; siAgo2-3, GGGUCUGUGGUGAUAAAUAUU, UAUUUAUCACCACAGACCCUU; siAgo2-4, GUAUGAGAACCCAAUGUCAUU, UGACAUUGGGUUCUCAUACUU) and negative control 1 were purchased from Applied Biosystems37.
Plasmids
The primary-miR-143 expression vector was purchased from TaKaRa BIO. The full-length human Ago2 cDNA was cloned into pIRES2-EGFP vector (Clontech). We amplified the upstream of human Ago2 gene (−1,770/−1 relative to the TSS) by PCR using human genomic DNA as template, and we cloned it into the pGL3-Basic vector. For the 3′UTR reporter plasmids, the nucleotides +3,399 to +3,953 of human ZEB1 cDNA were amplified and cloned downstream of the luciferase gene in the psiCHECK2 vector (Promega). For cloning the following primers were used for PCR: Ago2 promoter: 5′-ACGCGTATAGGGGATATGTGAAGGAGACA-3′ (forward) and 5′-CTCGAGATA CGCGCGCGCCACGGGCCCCG-3′ (reverse); ZEB1 3′UTR Fragment: 5′-ATAATACGCGTTAAAGGAAGCTGATTAATTAGATATGC-3′ (forward) and 5′-ATAATAAGCTTTTTGTAGTGCAGAAGTTCTCACATTTT-3′ (reverse)22.
Cell culture
HEK293 cells (American Type Culture Collection) were cultured in Dulbecco's Modified Eagle's Medium containing 10% heat-inactivated FBS and an antibiotic-antimycotic (Invitrogen) at 37°C in 5% CO2. MDA-MB-231 cells (American Type Culture Collection) and MDA-MB-231-luc-D3H2LN cells (Xenogen) were cultured in RPMI containing 10% heat-inactivated FBS and antibiotic-antimycotic at 37°C in 5% CO2. Human mammary carcinoma cell lines, MCF7 cells and multidrug-resistant MCF7-ADR cells were provided by Shien-Lab, Medical Oncology, National Cancer Center Hospital of Japan. These cells were maintained in RPMI supplemented with 10% heat-inactivated FBS and antibiotic-antimycotic at 37°C in 5% CO2. MCF10A cells, which were a spontaneously immortalized nontumorigenic epithelial cell line, (American Type Culture Collection) were maintained in an MEBM medium with 1% GA-1000, 50 μg/ml hydrocortisone, 1 μg/ml hEGF, 500 μg/ml insulin, and 4% BPE (Lonza) at 37°C in 5% CO2.
Cell proliferation assay (MTS assay)
Five thousand cells per well were seeded in 96-well plates. The following day, the cells were treated with resveratrol. After 3 days of culture, cell viability was measured using the Tetra Color One assay kit (Seikagaku Kohgyo) according to the instructions of the manufacturer. The absorbance at 450 nm was measured using Envision (Wallac).
Transwell invasion assay
Breast cancer cell invasion was assayed in 24-well Biocoat Matrigel invasion chambers (8 μm; Becton Dickinson) according to the manufacturer's protocol. Briefly, the cells were treated with resveratrol, and on the following day, 20,000 cells were plated in the upper chamber. The upper chamber contained resveratrol and the bottom chamber contained 10% FBS as a chemoattractant. Twenty-two hours later, the non-invasive cells were removed with a cotton swab. The cells that migrated through the membrane and stuck to the lower surface of the membrane were fixed with methanol and stained with Diff Quick staining. For quantification, the cells were counted under a microscope in four random fields. All assays were performed in triplicate. The data are expressed as the invasion percentage through the Matrigel matrix and membrane relative to migration through the control membrane according to the manufacturer's instructions.
Cell growth inhibition by cytotoxic agents and resveratrol
Breast cancer cells were plated as described above and allowed to attach overnight. The cultures were replenished with fresh medium containing 25 μM resveratrol for 24 hours and then exposed to 2.5 nM of the chemotherapeutic agent docetaxel for an additional 48 hours. Thus, for a single-agent treatment, the cells were exposed to resveratrol or docetaxel for 72 hours. The effect of resveratrol pretreatment on cell viability was examined by the MTS assay method.
Cell sorting and flow cytometric analysis
MDA-MB-231-luc-D3H2LN cells were treated with resveratrol. After culturing for 3 days, MDA-MB-231-luc-D3H2LN cells were suspended in their culture medium and subjected to a JSAN cell sorter (Bay Bioscience). At least one million cells were pelleted by centrifugation at 180 x g for 5 minutes at 4°C, resuspended in a 5-μL mixture of a monoclonal mouse anti-human CD44-FITC antibody (Becton Dickinson, clone L178) and a monoclonal mouse anti-human CD24-APC antibody (Biolegend, clone ML5), and incubated for 30 minutes at 4°C. Three independent experiments were performed.
Mammosphere assay
The CD44+/CD24− fraction from MDA-MB-231-luc-D3H2LN cells were resuspended in 1∶1 DMEM/F12 (Invitrogen) basal medium freshly supplemented with 20 ng/mL human basic fibroblast growth factor (Invitrogen), 20 ng/mL epidermal growth factor (Invitrogen), 10 μg/mL heparin (Sigma-Aldrich), and 1∶50 B27 supplement without vitamin A (Sigma-Aldrich) and seeded in 10-cm Ultra-Low Attachment Surface plates (Corning) at a density of 5000 cells. Ten days later, the plates were analysed for mammosphere formation.
Tumourigenicity assays in SCID hairless outbred mice
Six-week-old female SCID hairless outbred (SHO) mice were subcutaneously injected with 200 MDA-MB231-luc-D3H2LN cells in 25 μL of PBS and 25 μL of matrigel (n = 5). The mice were then treated with resveratrol (25 mg/kg/day) or ethanol (control) by intraperitoneal injection every day for 8 days. The tumour growth was monitored by injecting luciferin in the mice followed by measuring bioluminescence using an IVIS imaging system. The data were analysed using the LIVINGIMAGE 2.50 software (Xenogen). Six-week-old female SCID Hairless Outbred (SHO) mice were subcutaneously injected with 2000 MDA-MB231-luc-D3H2LN cells in 25 μL of PBS and 25 μL of Matrigel (n = 5). The mice were then treated with resveratrol (25 mg/kg) by intraperitoneal injection (IP) every day for 2 weeks and then with docetaxel (20 mg/kg) by intraperitoneal injection (IP) once per week for 2 weeks. The normalised fold changes (day 22 or day 29/day 15) of bioluminescence emitted from the whole body of the mice are shown. All experimental protocols involving animals were approved by the the Institute for Laboratory Animal Research, National Cancer Center Research Institute.
Isolation of microRNAs
Total RNAs were extracted from cultured cells using the QIAzol and miRNeasy Mini Kit (Qiagen) according to the manufacturer's protocol.
Quantitative Real-Time PCR (qRT-PCR)
The qRT-PCR method has been previously described38. PCR was performed in 96-well plates using the 7300 Real-Time PCR System (Applied Biosystems). All reactions were performed in triplicate. All of the TaqMan microRNA assays were purchased from Applied Biosystems. hRNU6 was used as an invariant control. SYBR Green I qRT-PCR was performed, and the β-actin housekeeping gene was used to normalise the variation in the cDNA levels. The following pairs of primers were used for gene amplification: for pri-miR-16, 5′-GCAATTACAGTATTTTAAGAGATGAT-3′ (forward) and 5′- CATACTCTACAGTTGTGTTTTAATGT-3′ (reverse); for pri-miR-141-200c, 5′- TGAGCTTGGGACTGCAGAG-3′ (forward) and 5′-CTGAGCCACCTTCCCCTAC-3′ (reverse); for pri-miR-143, 5′-CAAGGTTTGGTCCTGGGTGCTCAAA-3′ (forward) and 5′-TGGTGGCCTGTGGCGGGACTCCAA-3′ (reverse); for ZEB1, 5′-AAGAATTCACAGTGGAGAGAAGCCA-3′ (forward) and 5′-CGTTTCTTGCAGTTTGGGCATT-3′ (reverse); for E-cadherin, 5′-GTCCTGGGCAGACTGAATTT-3′ (forward) and 5′-GACCAAGAAATGGATCTGTGG-3′ (reverse); and for β-actin, 5′-GGCACCACCATGTACCCTG-3′ (forward) and 5′-CACGGAGTACTTGCGCTCAG-3′ (reverse)39.
Quantification of the Ago2 mRNA half-life
MDA-MB231-luc-D3H2LN cells were incubated with 5,6-dichlorobenzimidazole riboside (50 μM), which is an inhibitor of mRNA synthesis. The cells were then treated with 50 μM resveratrol and harvested at the indicated time points. Total cellular RNA was isolated using the RNeasy Mini kit (Qiagen). qRT-PCR analysis of Ago2 mRNA at each time point was performed as described above. The fold-change in the Ago2 mRNA abundance at each time point was determined by the following equation: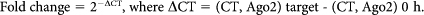
Transient transfection assays
The plasmid transfections were performed using Lipofectamine LTX (Invitrogen). The cell numbers and amount of plasmids for each transfection were determined according to the manufacturer's protocol. The transfection of siRNA and miRNA inhibitors was accomplished using the DharmaFECT transfection reagent (Thermo Scientific) according to the manufacturer's protocol.
Luciferase reporter assay
Cells were seeded in 96-well plates at 3000 cells per well the day before transfection. A total of 500 ng of Ago2 vector, 10 nM siRNA against luciferase and the AllStars negative control were added to each well. The cells were collected 1, 3, or 5 days after transfection and analysed using the Bright-Glo Luciferase Reporter Assay System (Promega).
Immunoblot analysis
SDS-PAGE gels were calibrated using Precision Plus protein standards (161-0375) (Bio-Rad), and anti-Ago2 (1∶200) and anti-actin (1∶1,000) were used as the primary antibodies. The dilution ratio of each antibody is indicated in parentheses. A peroxidase-labelled anti-mouse secondary antibody was used at a dilution of 1∶10,000. Bound antibodies were visualised by chemiluminescence using the ECL Plus Western blotting detection system (RPN2132) (GE HealthCare), and luminescent images were analysed using a LuminoImager (LAS-3000; Fuji Film Inc.).
Quantification of Ago2 protein half-life
MDA-MB-231-luc-D3H2LN cells at 80% confluency were treated with 30 μg/ml cycloheximide (Sigma-Aldrich). The cells were then treated with 50 μM resveratrol and harvested at the indicated time points. The effect of resveratrol on Ago2 stability was examined by immunoblotting as reported above.
Statistical analysis
The data presented in bar graphs are the means ± s.e.m. of at least three independent experiments. Statistical analyses were performed using the Student's t-test.
Author Contributions
TO supervised the project. KH performed a significant amount of the experimental work. TO, KH, NK, and YY wrote the manuscript and prepared the figures and tables. In vivo experiments were carried out by KH, NK, YY, RT, and FT.
Supplementary Material
Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity
Acknowledgments
This work was supported in part by a Grant-in-Aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control, a Grant-in-Aid for Scientific Research on Priority Areas Cancer from the Ministry of Education, Culture, Sports, Science and Technology, the National Cancer Center Research and Development Fund, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio), Project for Development of Innovative Research on Cancer Therapeutics, and the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)” initiated by the Council for Science and Technology Policy (CSTP). We thank Ayako Inoue for excellent technical assistance. We thank Dr.Izuho Hatada for providing the information about Ago2 promoter region.
References
- Hartwell J. L. & Schrecker A. W. Components of Podophyllin. V. The Constitution of Podophyllotoxin1. J Am Chem Soc 73, 2909–2916 (1951). [Google Scholar]
- Baur J. A. & Sinclair D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5, 493–506 (2006). [DOI] [PubMed] [Google Scholar]
- Renaud S. & de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339, 1523–1526 (1992). [DOI] [PubMed] [Google Scholar]
- Jang M. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 (1997). [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci 66, 663–673 (2000). [DOI] [PubMed] [Google Scholar]
- Hammell C. M., Lubin I., Boag P. R., Blackwell T. K. & Ambros V. nhl-2 Modulates microRNA activity in Caenorhabditis elegans. Cell 136, 926–938 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G. & Slack F. J. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9, 219–230 (2008). [DOI] [PubMed] [Google Scholar]
- Kong D. et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells 27, 1712–1721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. J. et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem 283, 31079–31086 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Takeshita F. et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther 18, 181–187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkamu T., Zhang X., Tan J., Zeng Y. & Kassie F. Alteration of microRNA expression in vinyl carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis 31, 252–258 (2010). [DOI] [PubMed] [Google Scholar]
- Tsang W. P. & Kwok T. T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem 21, 140–146 (2010). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res 69, 6704–6712 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M. et al. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol Cancer Ther 7, 464–473 (2008). [DOI] [PubMed] [Google Scholar]
- Lee H. P. et al. Dietary effects on breast-cancer risk in Singapore. Lancet 337, 1197–1200 (1991). [DOI] [PubMed] [Google Scholar]
- Croce C. M. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10, 704–714 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur A. et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res 67, 2456–2468 (2007). [DOI] [PubMed] [Google Scholar]
- Al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J. & Clarke M. F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100, 3983–3988 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M. Cancer stem cells and oncology therapeutics. Curr Opin Oncol 19, 61–64 (2007). [DOI] [PubMed] [Google Scholar]
- Al-Hajj M., Becker M. W., Wicha M., Weissman I. & Clarke M. F. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 14, 43–47 (2004). [DOI] [PubMed] [Google Scholar]
- Iorio M. V. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 65, 7065–7070 (2005). [DOI] [PubMed] [Google Scholar]
- Burk U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9, 582–589 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell M. A. & Hannon G. J. RNase III enzymes and the initiation of gene silencing. Nat Struct Mol Biol 11, 214–218 (2004). [DOI] [PubMed] [Google Scholar]
- Kim V. N. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6, 376–385 (2005). [DOI] [PubMed] [Google Scholar]
- Lingel A., Simon B., Izaurralde E. & Sattler M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature 426, 465–469 (2003). [DOI] [PubMed] [Google Scholar]
- Korpal M., Lee E. S., Hu G. & Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283, 14910–14914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B. et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem 350, 207–213 (2011). [DOI] [PubMed] [Google Scholar]
- Burk U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. Embo Reports 9, 582–589 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimando A. M. et al. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem 50, 3453–3457 (2002). [DOI] [PubMed] [Google Scholar]
- Stivala L. A. et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem 276, 22586–22594 (2001). [DOI] [PubMed] [Google Scholar]
- Shimono Y. et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138, 592–603 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll D. et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev 21, 1999–2004 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic I. M., Muzzio M., Huang Z., Thompson T. N. & McCormick D. L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou Y. S. et al. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem 59, 2725–2733 (2011). [DOI] [PubMed] [Google Scholar]
- Chang T. C. et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26, 745–752 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S., Hicks C. & Levenson A. S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol Nutr Food Res 55, 1219–1229 (2011). [DOI] [PubMed] [Google Scholar]
- Meister G. et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15, 185–197 (2004). [DOI] [PubMed] [Google Scholar]
- Mitchell P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105, 10513–10518 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. I. et al. Modulation of microRNA processing by p53. Nature 460, 529–533 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity