Abstract
Objective
To determine the impact of stenting ureteroenteric anastomoses on postoperative stricture rate and gastrointestinal recovery in continent and noncontinent urinary diversions (UDs). Patients and Methods: We retrospectively reviewed the clinical and pathologic data on 192 consecutive patients who underwent a radical cystectomy and UD. Patients received either a continent or noncontinent UD with or without stent placement through the ureteroenteric anastomoses. Stricture rate, gastrointestinal recovery, length of hospital stay, and stricture characteristics were analyzed. Study endpoints were compared between four groups – stented and nonstented continent and stented and nonstented noncontinent UDs.
Results
36% of patients were stented and 64% were nonstented at the time of UD. Total ureteral stricture rate was 9.9%. There was no statistically significant difference in stricture rate (p = 0.11) or length of hospital stay (p = 0.081) in stented compared to nonstented patients. There was a significantly (p = 0.014) greater rate of ileus in patients who were nonstented in both continent and noncontinent UDs.
Conclusion
Stenting of ureteroenteric anastomoses in both continent and noncontinent UD has no effect on postoperative stricture rate, but is associated with lower rates of postoperative ileus.
Key Words: Urinary diversion, Stricture, Stent, Ileus, Radical cystectomy
Introduction
Bladder cancer has the fifth highest incidence of any cancer, with an estimated 70,530 new cases and 14,680 deaths in the US in 2010 [1]. Approximately 70% of urothelial carcinomas are non-muscle-invasive at diagnosis [2]. For those patients that progress to muscle-invasive disease, radical cystoprostatectomy in males and anterior exenteration in females remains the standard of care. A variety of continent and noncontinent urinary reconstructions utilizing both small and large bowel have been described as bladder substitutes at the time of urinary diversion (UD).
Several varieties of ureteroenteric anastomoses have been described, which can generally be characterized as refluxing or nonrefluxing. Nonrefluxing anastomoses are designed to prevent infected urine from reaching the renal pelvis, which is thought to lead to pyelonephritis and subsequent renal deterioration. However, nonrefluxing anastomoses are more prone to stricture, likely secondary to mechanical compression of the distal ureter. Ureteral stenting is commonplace for nonrefluxing anastomoses, but the need for ureteral stents in refluxing anastomoses is less clear. The rationale for using stents includes ensuring accurate alignment and providing mechanical support, which may ultimately lead to the prevention of urine leaks and ureteral strictures.
The role of stents in urinary tract reconstruction remains controversial. Regan and Barrett found that the use of single-J stents in ileal conduits helped prevent urine leaks and ureteral strictures [3]. However, other studies have suggested that ureteral stents may increase the risk of stricture formation by serving as a nidus for infection [4,5].
In order to assess the efficacy of ureteral stents in the prevention of ureteroenteric anastomotic strictures, we retrospectively analyzed a single surgeon's experience with 192 consecutive patients who underwent radical cystectomy (RC) and UD. The primary objectives of this study are to assess the impact of ureteral stents on ureteroenteric anastomotic stricture rates, postoperative gastrointestinal recovery, and length of hospital stay. Stricture characteristics and management strategies are described.
Patients and Methods
From 2003 to 2007, 192 consecutive patients underwent RC and bilateral pelvic lymphadenectomy for the treatment of bladder cancer by a single surgeon (M.P.S.). The clinical indication for RC in all patients was either muscle-invasive bladder cancer or high-grade, non-muscle-invasive bladder cancer refractory to intravesical therapy. Clinical and pathological information was obtained from the medical record. Institutional Review Board approval was obtained for this study.
All RC specimens were examined by a dedicated genitourinary pathologist. The 1997 AJCC/TNM classification system was used for pathologic staging, and the WHO classification was used for pathologic grading. A distal urethral margin was sent for frozen section analysis when neobladder diversion was being considered. Neobladder diversion was performed only if the margin was free of malignancy.
Patients underwent RC and either continent (orthotopic ileal neobladder, Indiana pouch with continent catheterizable stoma) or noncontinent (ileal conduit) UD. All ureteroenteric anastomoses were end-to-side, refluxing anastomoses. The decision to stent the ureteroenteric anastomosis was made at the time of surgery by the attending surgeon. Absolute indications for stent placement were a single renal unit and previous radiation therapy to the abdomen and/or pelvis. For those patients in whom a ureteral stent was placed, a 5-Fr pediatric feeding tube was utilized. A closed suction drain was placed at the time of surgery in all patients. For patients undergoing a continent UD, ureteral stents were removed along with the urinary catheter at 3 weeks postoperatively. Patients undergoing a noncontinent UD typically had their ureteral stents removed 2 weeks postoperatively.
Patients were followed according to our institution's protocol with post-operative visits initially at 2 weeks and then at 3-month intervals for the first year. Patients were then followed on a biannual basis. Patients were monitored with a physical exam, laboratory studies, chest X-ray, and urine cytology at each visit. Upper tract monitoring was performed with renal ultrasonography every 6 months. Abdominal and pelvic cross sectional imaging was performed on an annual basis. Abnormal surveillance imaging or a positive cytology prompted further work up as indicated.
Study endpoints were ureteroenteric anastomotic stricture rate, rate of post-operative ileus, and length of postoperative hospital stay. Strictures were suspected if patients were symptomatic, had acute renal failure (ARF), or had moderate or severe hydronephrosis on surveillance imaging studies. Moderate-to-severe hydronephrosis was defined as renal pelvic dilation with moderate-to-severe caliceal dilation. All strictures were documented with appropriate imaging studies.
All patients’ medical records were queried for a clinical diagnosis of Ileus, defined as prolonged return of bowel function leading to nausea, vomiting, and abdominal distention. Abdominal distention was defined as a subjective increase of the patient's abdominal girth on serial physical examinations with accompanied abdominal pain. Length of post-operative hospital stay was determined from the patient's medical record. Stricture rates, post-operative ileus rates, and average length of hospital stay were calculated for each group. Study endpoints were compared by χ2 analysis between four groups – stented and nonstented continent and stented and nonstented noncontinent diversions. Ureteroenteric strictures characteristics and management strategies are described. Statistically significance was defined as p < 0.05. Statistical analysis was performed using STATA version 10.0 (STATA Corporation, College Station, Texas).
Results
A total of 192 patients received RC with UD. The median age was 66.5 years (range 42–85), 83% were male and 87% were of Caucasian ethnicity. A total of 4.7% (9 patients) had a history of prior abdominal or pelvic radiation therapy, and 15.1% (29 patients) received neoadjuvant chemotherapy. Pathologic data from the RC specimens are presented in table 1. Overall, 36% (69/192) of patients were stented and 64% (123/192) were nonstented at the time of UD. Of the stented patients, 33% had continent and 67% had noncontinent UD. Of the nonstented patients, 49% had continent and 51% had noncontinent UD.
Table 1.
Clinicopathologic data for 192 patients who underwent radical cystectomy and UD
| Age, median (range) | 66.5 (42–85) |
| Sex | |
| Male | 160 (83%) |
| Female | 32 (17%) |
| Race | |
| Caucasian | 167 (87%) |
| African-American | 13 (6.8%) |
| Other | 12 (6.2%) |
| Continent diversion | |
| Neobladder | 77 (40.1%) |
| Catheterizable resevoir | 6 (3.1%) |
| Noncontinent diversion | |
| Ileal conduit | 109 (56.8%) |
| Pathologic stage | |
| p0 | 13 (6.8%) |
| pCIS | 33 (17.2%) |
| pT1 | 36 (18.8%) |
| pT2 | 34 (17.8%) |
| pT3 | 56 (29.2%) |
| pT4 | 20 (10.4%) |
| Node status | |
| Positive | 43 (22.4%) |
| Negative | 149 (77.6%) |
| Radiation status | |
| Prior therapy | 9 (4.7%) |
| Adjunctive | 9 (4.7%) |
| Chemotherapy | |
| Neoadjunctive | 29 (15.1%) |
| Adjunctive | 42 (21.9%) |
The mean follow-up was 25 months (range 1–156 months). The mean follow up was 25.8 months (range 1–102 months) in the stent group and 24.5 months (range 1–156 months) in the nonstent group (p = 0.70). The total stricture rate for the cohort was 9.9%. The stricture rate was 14.5 and 7.4% in the stented and nonstented groups, respectively (p = 0.11) (table 2). The stricture rate was 9.6 and 10.1% in patients with continent and noncontinent UD, respectively (p = 0.44).
Table 2.
Stricture and ileus rates in patients with UD after RC
| Stent | No stent | RR (95% CI) | p value | |
|---|---|---|---|---|
| Stricture rate | 14.5% | 7.4% | 1.98 (0.846, 4.63) | 0.11 |
| Ileus rate | 5.8% | 18.7% | 0.31 (0.112, 0.859) | 0.014 |
Overall, 14% of patients had an ileus in the immediate postoperative period. Ileus occurred in 5.8% of the stented group and 18.7% of the nonstented group. This finding was statistically significant (p = 0.014) (table 2). The rate of ileus was 14.5% in all continent UD and 13.8% in all noncontinent UD (p = 0.47).
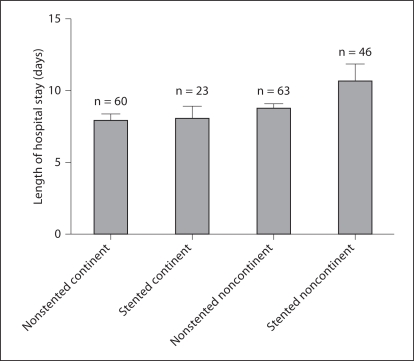
The length of postoperative hospital stay was 9.78 days in stented patients and 8.32 in nonstented patients (p = 0.81). In patients with noncontinent UD, the length of hospital stay was 10.60 and 8.68 days for patients with stented and nonstented anastomoses, respectively (p = 0.122). In patients with continent UD, the length of hospital stay was 7.95 and 8.08 days for patients with stented and nonstented anastomoses, respectively (p = 0.886) (fig. 1).
Fig. 1.
Length of stay in patients with stented and nonstented, continent and noncontinent UDs after radical cystectomy.
A total of 19 ureteroenteric strictures were diagnosed at a mean of 10.5 months. Data on stricture length was available in 21% of patients. Presenting symptoms included ARF, asymptomatic hydronephrosis, and obstructive pyelonephritis. A total of three strictures (15.8%) were malignant. Malignant strictures were diagnosed with endoscopic evaluation and biopsy. Endoscopic management (balloon dilation or holmium laser incision) was attempted in 8 patients (42.1%) and was successful in 1 patient (12.5%) who underwent antegrade dilation of a focal, left-sided stricture. A total of 7 patients (36.8%) underwent open revision of their ureteroeneteric anastomosis with an 85.7% success rate. Two patients presented with asymptomatic hydronephrosis on surveillance imaging and were found to have a nonfunctioning kidney on nuclear renography. Both patients were managed conservatively with regular imaging and laboratory surveillance of the contralateral kidney. Patients managed with ureteral stents or nephrostomy tubes were either medically unfit for surgical revision or unwilling to undergo further therapeutic interventions.
Discussion
The results of the present study suggest that stenting the ureteroenteric anastomosis has no effect on stricture rates in either continent or noncontinent UD. However, patients with stented anastomoses have a statistically significant lower rate of postoperative ileus. This is presumably due to decrease urinary leak and urinary ascites. No statistically significant difference was noted in length of hospital stay regardless of stent status or type of UD.
The decision to stent ureteroenteric anastomoses in UD has been a controversial issue in the Urologic literature. It is generally accepted that nonrefluxing anastomoses are stented to prevent mechanical obstruction from post-operative tissue edema. Until recently, there has been a paucity of evidence suggesting a benefit of stenting refluxing anastomoses. In early experiences with UD, ureteral stents were not routinely used due to their unproven benefit in preventing urine leak [6]. In fact, the risk of infection associated with indwelling stents led to the hypothesis that ureteral stents could contribute to the formation of strictures [4,5].
The relationship between urinary ascites and stented ureteroenteric anastomoses has been addressed by several studies. Regan and Barrett [3] retrospectively studied a cohort of 362 patients receiving ileal conduit UD with Bricker (refluxing, end-to-side) ureteroileal anastomoses. In their series, they noted a nonsignificant decrease in the urinary leak rate in patients with stented anastomoses as compared to those with nonstented anastomoses (p = 0.095). In fact, they quote a 0% urinary leak rate in patients with a stented anastomosis. Beddoe et al. [7] obtained similar results in their cohort of patients undergoing colon conduits after pelvic radiation for gynecologic malignancies. The authors report a significant decrease in urinary leak in patients with stented anastomoses (p < 0.05). Both studies are retrospective studies and do not specifically define how urinary leaks are diagnosed.
More recently, Mattei et al. [8] performed a prospective, randomized trial evaluating postoperative outcomes in patients receiving stented and nonstented anastomoses during UD after RC. Diversions included both ileal conduits and ileal neobladders. Their analysis did not demonstrate a significant difference in the incidence of urinary ascites (p = 0.32). Interestingly, the authors did find a significant difference in both return of bowel function (flatus) and incidence of nausea between the stented and nonstented groups. Delay in return of bowel function (ileus) after UD is often attributed to urinary ascites resulting from an anastomotic urine leak.
The results of the present study are in agreement with previously published data. We document a statistically significant decrease in the rate of ileus in patients with stented anastomoses. Although urinary ascites is not strictly documented with an elevated drain creatinine in all patients, it is postulated that post-operative urine leak contributes to the development of ileus.
Ureteroenteric anastomotic stricture rate is a commonly studied outcome after UD. Surgical series of both refluxing and nonrefluxing anastomoses have reported stricture rates less than 10% when ureteral stents are placed [3,7,9,10,11,12]. Regan and Barrett [3] report a 0% stricture rate in patients undergoing stented, Bricker style ureteroileal anastomoses. This is compared to the 4.6% stricture rate in the nonstented group (p = 0.06). Beddoe et al. [7] documented a nonsignificant decrease in the stricture rate in patients receiving stented ureterocolonic anastomoses. The present study adds to the evidence that stenting a refluxing, ureteroenteric anastomosis does not lead to a significant decrease in the rate of anastomotic stricture. This study is the first to document no statistically significant difference in stricture rates in stented and nonstented ureteroenteric anastomoses in the setting of a continent UD. These data suggest that placement of ureteroenteric stents does not influence stricture rates in continent and noncontinent UD.
In the present study, strictures were almost universally recalcitrant to endoscopic management with a success rate of 12.5%. Our success rate with endoscopic stricture management is lower than previous reports. Laven et al. [13] reported success rates of 57% with endoureterotomy applying the holmium laser. Milhoua et al. [14] reported a 27% success rate using a variety of endoscopic strategies including balloon dilation, electrosurgical incision, and incision with the holmium laser. Lower success rates in the present series could be related to several factors. All strictures treated endoscopically were either bilateral strictures or left-sided strictures which are often more resistant to endoscopic management [15]. In contrast to the studies by Laven et al. [13], 67.5% of strictures in the present study were treated with balloon dilation instead of incision. Possible differences in efficacy between techniques could explain the lower success rate in the present study.
Conversely, we report an 87.5% success rate with open revision of the ureteroenteric anastomosis. This is consistent with previous series by Laven et al. [15] and Milhoua et al. [14] who reported success rates of 80 and 87.5%, respectively. These data suggest that open revision remains the gold standard for treatment of ureteroeneteric anastomotic strictures.
The present analysis has several weaknesses. It is a nonrandomized, retrospective analysis. The decision to stent the ureteroenteric anastomosis was determined by the attending surgeon intra-operatively. Urinary ascites was not directly documented by sending drain fluid for analysis of creatinine. The differences in ileus rates between stented and nonstented anastomoses cannot be directly attributed to urinary leak. Rather, we can only hypothesize that this is a major contributing factor. Despite these limitations, this retrospective study confirms previous findings of improved return of bowel function with stented ureteroenteric anastomoses. Most importantly, it is the first study to document no difference in stricture rates in stented and nonstented ureteroenteric anatomoses in continent UD.
Conclusions
Ureteral stent placement during UD has no effect on the postoperative stricture rate. This is true in continent and noncontinent UD. However, stented anastomoses are associated with significantly lower rates of postoperative ileus, presumably due to lower rates of urinary ascites. Ureteroenteric anastomoses should be stented at the time of UD.
Acknowledgements
The project described was supported by Award Number T32DK007552 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ro JY, Staerkel GA, Ayala AG. Cytologic and histologic features of superficial bladder cancer. Urol Clin North Am. 1992;19:435–453. [PubMed] [Google Scholar]
- 3.Regan JB, Barrett DM. Stented versus nonstented ureteroileal anastomoses: Is there a difference with regard to leak and stricture? J Urol. 1985;134:1101–1103. doi: 10.1016/s0022-5347(17)47644-0. [DOI] [PubMed] [Google Scholar]
- 4.Faulknier B, Chaksupa D, Malas A, Rosencrance JG. Persistent candiduria complicating intraureteral stenting: a case report and review of literature. W V Med J. 2003;99:25–27. [PubMed] [Google Scholar]
- 5.Keane PF, Bonner MC, Johnston SR, Zafar A, Gorman SP. Characterization of biofilm and encrustation on ureteric stents in vivo. Br J Urol. 1994;73:687–691. doi: 10.1111/j.1464-410x.1994.tb07557.x. [DOI] [PubMed] [Google Scholar]
- 6.Richie JP, Skinner DG. Complications of urinary conduit diversion. In: Smith RB, Skinner DG, editors. Complications of Urologic Surgery: Prevention and Management. Philadelphia: Saunders; 1976. [Google Scholar]
- 7.Beddoe AM, Boyce JG, Remy JC, Fruchter RG, Nelson JH., Jr Stented versus nonstented transverse colon conduits: a comparative report. Gynecol Oncol. 1987;27:305–315. doi: 10.1016/0090-8258(87)90250-2. [DOI] [PubMed] [Google Scholar]
- 8.Mattei A, Birkhaeuser FD, Baermann C, Warncke SH, Studer UE. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol. 2008;179:582–586. doi: 10.1016/j.juro.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 9.De Carli P, Micali S, O'Sullivan D, Mainiero G, Cusumano G, Fattahi H, Cancrini A. Ureteral anastomosis in the orthotopic ileal neobladder: comparison of 2 techniques. J Urol. 1997;157:469–471. doi: 10.1097/00005392-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Hassan AA, Elgamal SA, Sabaa MA, Salem KA, Elmateet MS. Evaluation of direct versus non-refluxing technique and functional results in orthotopic y-ileal neobladder after 12 years of follow up. Int J Urol. 2007;14:300–304. doi: 10.1111/j.1442-2042.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- 11.Hautmann RE, de Petriconi R, Gottfried HW, Kleinschmidt K, Mattes R, Paiss T. The ileal neobladder: Complications and functional results in 363 patients after 11 years of follow-up. J Urol. 1999;161:422–427. doi: 10.1016/s0022-5347(01)61909-8. discussion 427–428. [DOI] [PubMed] [Google Scholar]
- 12.Kouba E, Sands M, Lentz A, Wallen E, Pruthi RS. A comparison of the bricker versus wallace ureteroileal anastomosis in patients undergoing urinary diversion for bladder cancer. J Urol. 2007;178:945–948. doi: 10.1016/j.juro.2007.05.030. discussion 948–949. [DOI] [PubMed] [Google Scholar]
- 13.Laven BA, O'Connor RC, Steinberg GD, Gerber GS. Long-term results of antegrade endoureterotomy using the holmium laser in patients with ureterointestinal strictures. Urology. 2001;58:924–929. doi: 10.1016/s0090-4295(01)01396-6. [DOI] [PubMed] [Google Scholar]
- 14.Milhoua PM, Miller NL, Cookson MS, Chang SS, Smith JA, Herrell SD. Primary endoscopic management versus open revision of ureteroenteric anastomotic strictures after urinary diversion – single institution contemporary series. J Endourol. 2009;23:551–555. doi: 10.1089/end.2008.0230. [DOI] [PubMed] [Google Scholar]
- 15.Laven BA, O'Connor RC, Gerber GS, Steinberg GD. Long-term results of endoureterotomy and open surgical revision for the management of ureteroenteric strictures after urinary diversion. J Urol. 2003;170:1226–1230. doi: 10.1097/01.ju.0000086701.68756.8f. [DOI] [PubMed] [Google Scholar]