Abstract
Background Exposure to a pre-natal famine environment has been associated with a persistent decrease in DNA methylation of the IGF2 gene, although study findings on other loci have been highly variable. There have been no studies to date of the relation between pre-natal famine and overall global DNA methylation in adulthood.
Methods Our study population includes 350 births with pre-natal exposure to the Dutch famine of 1944–45 selected from three birth clinics, 290 births from these clinics born before or after the famine as unexposed time controls and 307 same-sex siblings of either birth group as unexposed family controls. All study subjects were interviewed and underwent a medical examination at a mean age of 58 years when blood samples were also collected. As measures of genomic DNA methylation, we analysed two repetitive elements, LINE-1 (long interspersed nucleotide element 1) and Sat2 (Satellite 2 DNA sequence) by pyrosequencing and MethyLight, respectively, and overall genomic DNA methylation using the Luminometric methylation assay (LUMA).
Results Mean DNA methylation by LUMA was 75.2% [standard deviation (SD) 4.7], by LINE-1 was 77.1% (SD 2.5) and by Sat2 was 122.2 (SD 56.2). Pre-natal famine exposure was associated with negligible changes in all three assays {LUMA: −0.16% [95% confidence interval (95% CI) −0.49 to 0.81], P = 0.63; LINE-1: −0.05 % (95% CI −0.33 to 0.22), P = 0.70; and Sat2: −0.51% (95% CI −7.38 to 6.36), P = 0.88} relative to unexposed controls, adjusting for age at examination and within family clustering.
Conclusion Our results show no relation between overall global DNA methylation in adults and pre-natal famine exposure. Further work should focus on selected regions in the genome that may be differentially methylated in response to changes in early life exposures and predict adult health outcomes.
Keywords: Pre-natal exposure delayed effects, Dutch famine, The Netherlands, World War II, DNA methylation, LINE-1, Sat2, LUMA
Introduction
Global DNA methylation has been associated with chromosomal instability and with a number of health outcomes. Although more data exist at the tissue level, a number of recent studies of global white blood cell (WBC) DNA methylation have shown a relation between lower methylation and cancers of the colon,1,2 bladder,3–5 stomach,6 breast7 and liver.8 Other studies have reported on associations with schizophrenia and myelodysplastic syndrome.9,10 Lower methylation levels have also been associated with benzene exposure, persistent organic pollutants, lead and air pollution.5,11–14 Although most data do not support an association between active cigarette smoking and global DNA methylation in WBC, we have found associations between pre-natal smoke exposure and WBC methylation measured in adulthood.15,16 There is ample suggestion, therefore, that global DNA methylation may be sensitive to environmental exposures at several periods during the life course.
Among men and women with pre-natal exposure to the Dutch famine of 1944–45 who underwent medical examinations at age ~58 years, we have previously documented persistent methylation changes on the imprinted IGF2 gene and other loci but global DNA methylation studies in this population have not yet been undertaken.17,18 We therefore use here various methods to examine potential changes in global DNA methylation in relation to pre-natal famine.
We employ the Luminometric methylation assay (LUMA)19 that targets all CCGG sequences in the genome using methylation-sensitive restriction enzymes to discriminate methylated and unmethylated DNA and also assays of genomic methylation of retrotransposable elements and repeat sequences as surrogate markers, using pyrosequencing of the long interspersed nucleotide element-1 (LINE-1) and quantitative real-time PCR (qRT-PCR) with Taqman probes (Methylight) of satellite repeat-2 (Sat2). We selected LUMA and LINE-1 because of their extensive use in epidemiological studies and Sat2 because of our previous results suggesting pre-natal exposure to smoking was associated with decreased Sat2 methylation in WBC.16
Methods
Study population
As described elsewhere,20 we identified a birth cohort of 3307 live-born singleton births at three institutions in famine-exposed cities in The Western Netherlands. We selected all 2417 births between 1 February 1945 and 31 March 1946 (infants whose mothers were exposed to the famine during or immediately preceding that pregnancy). We sampled 890 births from 1943 and 1947 (infants whose mothers were not exposed to famine during their pregnancy) to serve as comparison groups.
We provided the names and addresses at birth of all 3307 infants to the Population Register in the municipality of birth. Of these, 308 persons (9%) were reported to have died and 275 (8%) to have emigrated. For 294 persons (8.9%), a current address could not be located and the Population Registry in Rotterdam declined to trace 130 individuals born out of wedlock. A current address was obtained for 2300 individuals (70% of the birth cohort).
All 2300 traced persons were invited by mail to participate. Initially, our study design called for the recruitment of same-sex sibling pairs and the lack of an available sibling was a reason for ineligibility. We received a reply from 1767 persons, of whom 347 expressed willingness to participate together with a sibling. Of those who declined (1420), 67% (951) reported not having a same-sex sibling available for study and 381 of these later agreed to participate. This resulted in a total of 1075 individuals who were interviewed by telephone, and 971 of this group also underwent a medical examination.
All study protocols were approved by the Human Subjects (medical ethics) committees of the participating institutions. Study participants provided verbal consent at the start of the telephone interview and written informed consent at the start of the clinical examination.
Data collection
Telephone interviews
A 1-h telephone interview included questions on socio-demographic characteristics, including age, gender, education and smoking and drinking habits. Following the telephone interview, we scheduled a clinic visit to the Leiden University Medical Center.
Clinical examinations and measures
Following registration and the obtaining of informed consent, we measured height to the nearest 1 mm with a portable stadiometer (SECA, Hamburg, Germany) and weight to the nearest 100 g with the participant standing in underclothing without shoes on a portable digital scale (SECA). Further details on the obtained anthropometric and other measurements are provided elsewhere.21
DNA extraction
Drawn blood samples were spun, processed and stored into labelled vials at −70°C according to standard protocols. Subsequent DNA extraction was done from WBCs using Qiagen Blood Maxi kits (QI-51194) eluting 2 times with 1 ml. The DNA solution was collected in 1.5 ml micro tubes with screw lids labelled with study numbers assigned to study participants that had no relation to exposure status. DNA concentrations were measured using the Picogreen Quant-it kit preceded by a standard A260 spectrophotometer measurement for the initial concentration estimation that is required for the picogreen assay. The first elution containing 90% of the DNA was split in two batches and kept in microtubes. One batch was previously used for the epigenetic analysis of IGF2 methylation with the Leiden University Medical Center. For this study, we are using DNA from the second batch. Working sets were prepared of a 50 ng/µl in 10 mM Tris/0.01 mM EDTA buffer, pH8 (10 µg) in ABgene 96-wells 0.8 ml storage plates (ab-0765 96) and covered with ABgene adhesive sealing foil (ab-0626).
DNA methylation assays
The LUMA assay as developed by Karimi et al.19 is based on the ability of two isoschizomers to differentially digest sequences depending on the methylation status of the CpG site within the sequence. Isoschizomers MspI and HpaII target the same sequence 5′-CCGG-3′; however, MspI will digest all CCGG sites and HpaII digestions will be blocked by the presence of a 5 m-C in the second position. An additional enzyme, EcoRI, is used to digest the DNA and create an overhang that has a sequence that does not contain C and G nucleotides and serve as a reference for DNA amount present in the reaction mixture. Enzymatic digests are set up as double digest by either EcoRI and HpaII or EcoRI and MspI. Pyrosequencing with a PyroMark Q24 system is used to sequence the overhangs left by each double digest. We ran a modified version of the assay.22 The percentage of DNA methylation was expressed as [1-(HpaII/EcoRI ΣG/ΣT)/ (MspI/ EcoRI ΣG/ΣT)]*100. The coefficient of variation for this assay was 4.9% for samples run on the same day, and 6.2% for samples run on separate days.
For the analysis of LINE-1 and Sat2 methylation, DNA was bisulphite treated using EZ DNA Methylation Kit (Zymo Research Corporation, Irvine, CA, USA) following the manufacturer's recommendations. Pyrosequencing for LINE-1 was carried out as described previously by others.12 The biotinylated PCR products were purified and pyrosequencing was run on a PyroMark Q24. We used non-CpG cytosine residues as internal controls to verify efficient sodium bisulphite DNA conversion, and ran universal unmethylated and methylated DNAs as controls. Methylation quantification was performed using the PyroMark Q24 1.010 software. The degree of methylation was expressed for each DNA locus as percentage of the methylated cytosine over the sum of methylated and unmethylated cytosine. The average of three CpG sites was used in the analysis. The coefficient of variation for this assay was 3.6% for samples run on the same day and 1.2% for samples run on different days.
As it is not possible to design a pyrosequencing assay for the short Sat2 repeat, the MethyLight assay was carried out using the sequences of probes and forward and reverse primers for the Sat2M1 consensus sequence described in Weisenburger et al.23 Standard curves for the AluC4 repeat control reaction were generated from 1:25 serial dilutions of bisulphite-converted, CpGenome universal methylated and unmethylated DNAs (Zymo Research Corporation, Irvine, CA, USA). Assays were run on an ABI Prism 7900 Sequence Detection System (Perkin-Elmer, Foster City, CA, USA). Universal methylated DNA served as a methylated reference, and AluC4 was used to measure the levels of input DNA to normalize the signal for each methylation reaction. MethyLight data were expressed as a percent of methylated reference (PMR) values. PMR = 100%*2exp − [ΔCt (Sat2 in sample – AluC4 in sample) − ΔCt (100% methylated Sat2 in reference sample − AluC4 in reference sample)]. Each MethyLight reaction was performed in duplicate, and the PMR values represent the mean. The coefficient of variation for the Sat2 measurement was 25.2% for samples run on the same day and 28.5% for samples run on different days.
Exposure to famine
We used the date of last menstrual period (LMP) as noted in the hospital records to define the start of gestation unless it was missing or implausible (12%). In those cases, we inferred the LMP date from relevant annotations on the birth record and estimated gestational age from birthweight and date of birth, using cut-points from tables of gender-, parity- and birthweight-specific gestational ages from the combined birth records of the Amsterdam midwives school (1948–57) and the University of Amsterdam Obstetrics Department (1931–65).20,24
We characterized exposure to famine during gestation by determining the gestational ages (in weeks after the LMP) during which the mother was exposed to an official ration of <900 kcal/day. We considered the mother exposed in gestational Weeks 1–10, 11–20, 21–30 or 31 to delivery if these gestational time windows were entirely exposed. Thus, pregnancies with LMP between November 26, 1944 and March 4, 1945 were considered exposed in Weeks 1–10; between September 18, 1944 and December 24, 1944 in Weeks 11–20; between July 10, 1944 and October 15, 1944, in Weeks 21–30; and between May 2, 1944 and August 24, 1944, in Weeks 31 through delivery. By these definitions, any participant could have been exposed to famine during at most two adjacent 10-week periods. Individuals exposed in at least one of the 10-week periods were considered to have had some pre-natal famine exposure. We also compared the exposure windows as previously used in the study of IGF2-DMR methylation in this population.17
Statistical methods
We computed means and SDs, and categorical frequencies for selected exposure groups as appropriate and assessed differences among exposure groups with ANOVA or chi-square tests. We used linear regression analyses to estimate the association between methylation percentage of the three selected DNA measures in relation to famine exposure in one or more of the four 10-week gestation periods. These were either combined into one group (‘any’ exposure) or analysed separately as four distinct exposure groups each defined by an indicator variable. Testing for the significance of the four 10-week exposure periods combined was carried out with a four degrees of freedom Wald's test.
We carried out gender-specific analyses and evaluated possible significant interactions between exposure and gender. In the absence of such interactions (all with P-values between 0.30 and 0.90), we only present pooled models with adjustment for gender. We included age at examination (with linear and quadratic terms) in all regressions. Models without adjustment for age and gender gave essentially the same results as adjusted models and are not further reported.
We first analysed the entire study population of 947 individuals (famine exposed, hospital controls and family controls), and then repeated all analyses on the subset of 296 sibling pairs, ignoring the 355 individuals without a same-sex sibling control. The subset analyses did not materially change any of the findings and are not reported separately. All analyses of SAT2 were repeated on the log-transformed variable to normalize distributions. As the findings did not materially change, these analyses are not reported separately. Statistical analyses were conducted with SAS v.9.2 software (SAS Institute, Cary, NC, USA) using hierarchical linear regression models with a random intercept (PROC MIXED) to account for within family clustering where indicated. The findings were confirmed with STATA v.11 software (StataCorp, College Station, TX, USA), using the xtreg command.
Results
LUMA measures were missing for seven individuals, Sat2 for six and all three methylation measures for four individuals. As the number of missing samples was small, we limited our analysis to the 947 participants with available DNA measures from all (LUMA, LINE-1 and Sat2) assays. This study group includes 350 hospital births with pre-natal famine exposure, 290 hospital births without famine exposure (time controls born before or after the famine) and 307 same-sex sibling controls without famine exposure.
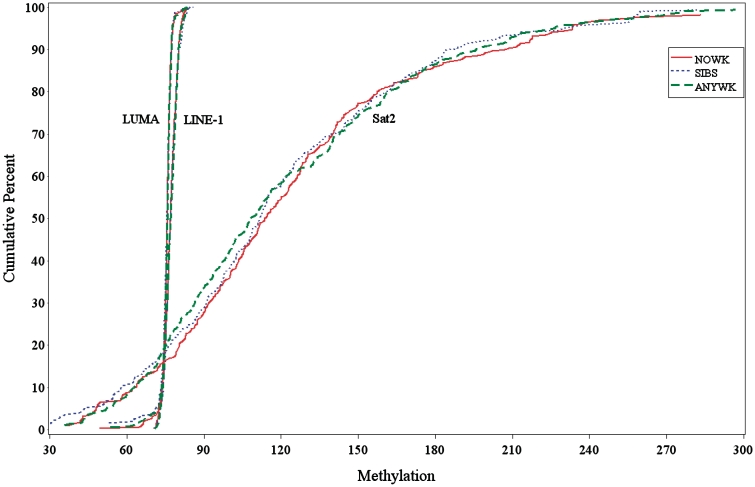
The siblings in our study were somewhat younger than the hospital birth series and had a mean age of 57.3 years (range: 36–76 years). The age range of the famine exposed participants was 57–60 years and of hospital controls was 55–62 years. The other selected demographic and behavioural characteristics of the exposure groups did not differ (Table 1). The cumulative distributions for LUMA, LINE-1 and Sat2 for each exposure group are shown in Figure 1. Although the range for the Sat2 distribution is much wider than for the other two measures, a visual inspection shows no differences in distributions of any of the measures in any of the exposure categories. This is confirmed by comparing the mean values of all DNA methylation measures in men and women by exposure category (Table 2).
Table 1.
Demographic and lifestyle characteristics of 947 adults examined between 2003 and 2005 at age ~58 years, by pre-natal famine exposure category
| Hospital controlsa (n = 290) | Sibling controls (n = 307) | Famine exposed (n = 350) | P-valueb | |
|---|---|---|---|---|
| Male | 47 | 43 | 46 | 0.54 |
| Age (years) | 58.8 (1.6) | 57.3 (6.3) | 58.9 (0.5) | <0.001 |
| Education exceeding primary or lower vocational schooling | 73 | 69 | 66 | 0.15 |
| Current smoker | 24 | 22 | 26 | 0.61 |
| Alcohol: one drink a week or more | 76 | 74 | 79 | 0.27 |
Values are represented as mean (SD) or % unless otherwise specified.
aBorn in the same institutions as exposed, but before the famine or after the famine and not exposed to famine during gestation.
bComparing the three exposure categories by analysis of variance or chi-square test, as appropriate.
Figure 1.
Cumulative frequency distributions of selected DNA genomic methylation percent measures for 947 adults examined between 2003 and 2005 at age ~58 years, by pre-natal famine exposure category. Nowk: Hospital controls, not exposed in pregnancy. Sibs: Sibling controls, not exposed in pregnancy. Anywk: Famine exposed, any week in pregnancy
Table 2.
Selected DNA genomic methylation measures for 947 adults examined between 2003 and 2005 at age ~58 years, by pre-natal famine exposure category
| Hospital controls (n = 290) | Sibling controls(n = 307) | Famine exposed(n = 350) | P-valuea | |
|---|---|---|---|---|
| LUMA | ||||
| Men | 75.5 (2.4) | 74.8 (6.3) | 75.1 (4.1) | 0.41 |
| Women | 75.5 (2.7) | 74.6 (7.4) | 75.4 (3.1) | 0.18 |
| All | 75.5 (2.6) | 74.7 (7.0) | 75.3 (3.6) | 0.10 |
| LINE-1 | ||||
| Men | 77.4 (2.4) | 77.4 (2.4) | 77.5 (2.4) | 0.93 |
| Women | 76.8 (2.4) | 77.1 (2.6) | 76.8 (2.5) | 0.51 |
| All | 77.1 (2.4) | 77.2 (2.5) | 77.1 (2.5) | 0.74 |
| Sat2 | ||||
| Men | 128.2 (57.6) | 119.9 (50.2) | 117.4 (50.4) | 0.24 |
| Women | 121.7 (58.8) | 120.9 (56.5) | 125.2 (61.9) | 0.75 |
| All | 124.7 (58.1) | 120.5 (53.8) | 121.6 (56.9) | 0.58 |
Values are expressed as mean (SD) or % unless otherwise specified.
aP-value for exposure contrasts in two-way ANOVA with interaction term.
When analysing the change in DNA methylation by the three measures in famine-exposed participants relative to controls, we observed no interaction of exposure and sex (data not shown). We did not see any significant changes in DNA methylation by any of the measures in relation to pre-natal famine exposure either defined by exposure in any of the four 10-week periods combined or considered separately (Table 3). The same lack of association was seen when the analysis was limited to the 296 sibling pairs in the study and when we analysed potential differences in DNA methylation using the exposure windows from our study of methylation of the IGF2-gene in this population17 (data not shown).
Table 3.
Change in selected DNA genomic methylation measures (percent units) for selected pre-natal famine exposure categories relative to unexposed controls; study population includes 947 adults examined between 2003 and 2005 at age ~58 years
| Exposure during any week of gestation (n = 350) | Exposure during weeks 1–10 of gestation (n = 73) | Exposure during weeks 11–20 of gestation (n = 125) | Exposure during weeks 21–30 of gestation (n = 143) | Exposure during weeks 31–40 of gestation (n = 129) | Wald test, P-valuea | |
|---|---|---|---|---|---|---|
| LUMA | ||||||
| βb | 0.16 | 0.02 | 0.48 | −0.64 | 0.28 | |
| 95%CI | −0.49 to 0.81 | −1.16 to 1.19 | −0.51 to 1.46 | −1.58 to 0.29 | −0.66 to 1.21 | |
| P-valuea | 0.63 | 0.69 | ||||
| LINE-1 | ||||||
| βb | −0.05 | −0.36 | 0.32 | −0.12 | 0.02 | |
| 95% CI | −0.33 to 0.22 | −0.89 to 0.17 | −0.11 to 0.75 | −0.53 to 0.29 | −0.40 to 0.44 | |
| P-valuea | 0.70 | 0.50 | ||||
| Sat2 | ||||||
| βb | −0.51 | −5.56 | 0.47 | 1.48 | −3.48 | |
| 95%CI | −7.38 to 6.36 | −18.6 to 7.49 | −10.2 to 11.2 | −8.74 to 11.7 | −13.8 to 6.86 | |
| P-valuea | 0.88 | 0.88 |
aP-values either for exposure during any week of gestation, or for exposures during Weeks 1–10, 11–20, 21–30 or 31–40 of gestation considered as a set by four degrees of freedom Wald's test.
bAdjusted for age, age2 and gender, as well as clustering within siblings.
With regard to the ranked inter-correlation of the methylation measures, there was no correlation between the LUMA measure and LINE-1 (Spearman's ρ = 0.04; P = 0.22) or Sat2 (ρ = −0.03; P = 0.42) but the correlation between LINE-1 and Sat2 was −0.29 (95% CI −0.35 to −0.23; P < 0.0001).
Discussion
In this study of 947 men and women examined at age ~58 years, we found no relation between any of three measures of genomic DNA methylation (LUMA, LINE-1 and Sat2) and pre-natal famine exposure. The absence of an association was confirmed when we limited the analysis to the 296 sibling pairs in the study. We therefore rule out potential confounding at the family level having any significant effect on the nature of this association. As some sibling controls were much younger than either participants with pre-natal famine exposure or hospital controls, we adjusted for age at examination in all analyses although this did not affect any regression estimates.
It is a recognized challenge in large epidemiological studies of DNA methylation to select an assay that is both cost efficient and valid. This is particularly important for measures of genomic DNA methylation that may exhibit some bias unless they are whole genome based. To minimize these potential limitations, we used three different assays as measures of genomic DNA methylation. The selected methods each have their strengths and limitations, but none suggests that there is any association between pre-natal famine exposure and DNA methylation at age ~58 years in this cohort.
LUMA provides a measure of 5 mC at CpG island and non-CpG island regions, which is more informative of overall genomic DNA methylation levels. However, the CCGG sequence accounts for only 8% of CpG sites of the genome.25 Contrasting uniform LUMA methylation measures of men and women with and without pre-natal famine exposure is still valid and highly relevant, however, for study questions pertaining to potential long-term changes in selected areas of the genome.
In this study, we also determined repetitive element methylation targeting two different sequences, by two different methods. LINE-1 are the only human retrotransposable elements and account for 17–20% of the genome. However, only 80–100 of these elements are still active and have transcriptional capacity due to loss of the 5′-UTR, mutations and rearrangements. Most of the transcriptionally active remaining elements are polymorphic, a fact that limits our ability to accurately detect DNA methylation percentages at these genomic regions.26 LINE-1 was measured by pyrosequencing, a methodology that provides an accurate quantitative measure of DNA methylation.27 The main limitation of this assay is the bisulphite conversion step, which can be incomplete. However, internal controls provide a measure of the quality of the conversion.
Sat2 elements belong to the satellite family of non-coding tandem repeats. These are located in heterochromatic regions and can interfere with transcription by inducing gene relocalization. Sat2 DNA methylation levels were measured by MethyLight, a high-throughput methodology that can be easily applied to large population studies. This methodology also required bisulphite conversion and does not provide a truly quantitative measure of DNA methylation. Unlike pyrosequencing that provides information on the average methylation level at each CpG site, MethyLight requires that all the CpG sites in the primers and Taqman probe are fully methylated for amplification to occur. An internal standard (Alu) accounts for the amount of template in the reaction. Since the number of copies of the elements may vary among individuals, accurate quantification is difficult. This may also account for the values >100%. The large variability of the assay is also a limitation. The PMR is calculated using four threshold cycle values each with their own variability. The lack of association of the two repetitive element measures with pre-natal famine exposure is again informative, however, as any differences could guide further targeted research along specific lines into biological mechanisms.
The inverse correlation between LINE-1 and Sat2 methylation observed was unexpected. We previously observed a positive correlation between LINE-1 and Sat2 methylation when methylation of both repetitive elements was measured by MethyLight.28 Quantification of fully methylated sequences by MethyLight clearly does not perfectly correlate with the average methylation levels obtained by pyrosequencing. This, in conjunction with the many samples with >100% methylation, suggests that the MethyLight may not be an appropriate assay for analysis of genomic DNA methylation.
In summary, our results using three different assays as a measure of genome-wide methylation do not support an effect of pre-natal famine on adult global DNA methylation levels in WBC. This is in contrast to our previous results on IGF2 methylation. Further work should focus on selected regions in the genome that may be differentially methylated in response to changes in early life exposures and predict adult health outcomes.
Funding
The National Institutes of Health [Grants RC-1 1HD063549 (ARRA Challenge Grant, partial); R01 HL-067914, to L.H.L. (PI), partial; P30ES009089 to R.M.S. (PI), partial].
KEY MESSAGES.
This study shows no relation between pre-natal famine exposure and three measures of global DNA methylation (LUMA, LINE-1, Sat2) at age ~58 years.
Earlier study in this population showed a persistent decrease in DNA methylation of the IGF2 gene after pre-natal famine early in pregnancy.
Further work should focus on possible methylation changes of other selected loci in the genome.
Acknowledgements
We thank the Vroedvrouwenscholen of Amsterdam and Rotterdam and the Obstetrics Department of the Leiden University Medical Center in Leiden for their help in accessing their archives, and the study participants for their cooperation. Clinical examinations and blood collections were carried out at the study centre of Gerontology and Geriatrics, Leiden University Medical Center, under the supervision of L. de Man. DNA extraction was carried out at the Department of Molecular Epidemiology, Leiden University Medical Center, Leiden, The Netherlands (B.T.Heijmans and P.E.Slagboom).
Conflict of Interest: None declared.
References
- 1.Lim U, Flood A, Choi S-W, et al. Genomic methylation of leukocyte DNA in relation to colorectal adenoma among asymptomatic women. Gastroenterology. 2008;134:47–55. doi: 10.1053/j.gastro.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pufulete M, Al-Ghnaniem R, Leather AJM, et al. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology. 2003;124:1240–48. doi: 10.1016/s0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 3.Cash HL, Tao L, Yuan J-M, et al. LINE-1 hypomethylation is associated with bladder cancer risk among non-smoking Chinese. Int J Cancer. 2011 doi: 10.1002/ijc.26098. doi:10.1002/ijc.26098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm CS, Kelsey KT, Butler R, et al. Implications of LINE1 methylation for bladder cancer risk in women. Clin Cancer Res. 2010;16:1682–89. doi: 10.1158/1078-0432.CCR-09-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547–52. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou L, Wang H, Sartori S, et al. Blood leukocyte DNA hypomethylation and gastric cancer risk in a high-risk Polish population. Int J Cancer. 2010;127:1866–74. doi: 10.1002/ijc.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J-Y, James SR, Link PA, et al. Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis. 2009;30:1889–97. doi: 10.1093/carcin/bgp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting Hsiung D, Marsit CJ, Houseman EA, et al. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidem Biomarkers Prev. 2007;16:108–14. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- 9.Shimabukuro M, Sasaki T, Imamura A, et al. Global hypomethylation of peripheral leukocyte DNA in male patients with schizophrenia: A potential link between epigenetics and schizophrenia. J Psychiatr Res. 2007;41:1042–46. doi: 10.1016/j.jpsychires.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Romermann D, Hasemeier B, Metzig K, et al. Global increase in DNA methylation in patients with myelodysplastic syndrome. Leukemia. 2008;22:1954–56. doi: 10.1038/leu.2008.76. [DOI] [PubMed] [Google Scholar]
- 11.Kim K-Y, Kim D-S, Lee S-K, et al. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010;118:370–74. doi: 10.1289/ehp.0901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollati V, Baccarelli A, Hou L, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–80. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 13.Xing C, Wang Q-f, Li B, et al. Methylation and expression analysis of tumor suppressor genes p15 and p16 in benzene poisoning. Chem-Biol Interact. 2010;184:306–9. doi: 10.1016/j.cbi.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–78. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terry MB, Ferris JS, Pilsner R, et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidem Biomarkers Prev. 2008;17:2306–10. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flom J, Ferris J, Gonzalez K, Santella R, Terry MB. Prenatal tobacco smoke exposure and genomewide methylation in adulthood. Cancer Epidem Biomarkers Prev. 2011;20:720. doi: 10.1158/1055-9965.EPI-11-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–49. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–53. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karimi M, Johansson S, Stach D, et al. LUMA (LUminometric Methylation Assay)–a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res. 2006;312:1989–95. doi: 10.1016/j.yexcr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Lumey LH, Stein AD, Kahn HS, et al. Cohort profile: the Dutch hunger winter families study. Int J Epidemiol. 2007;36:1196–204. doi: 10.1093/ije/dym126. [DOI] [PubMed] [Google Scholar]
- 21.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85:869–76. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 22.Bjornsson HT, Sigurdsson MI, Fallin MD, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877–83. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisenberger DJ, Campan M, Long TI, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–36. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloosterman GJ. On intrauterine growth. The significance of prenatal care. Int J Gynaecol Obstet. 1970;8:895–912. [Google Scholar]
- 25.Irizarry RA, Ladd-Acosta C, Carvalho B, et al. Comprehensive high-throughput arrays for relative methylation (CHARM) Genome Res. 2008;18:780–90. doi: 10.1101/gr.7301508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouha B, Schustak J, Badge RM, et al. Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A. 2003;100:5280–85. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tost J, Gut IG. DNA methylation analysis by pyrosequencing. Nat Protoc. 2007;2:2265–75. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- 28.Wu HC, Delgado-Cruzata L, Flom JD, et al. Global methylation profiles in DNA from different blood cell types. Epigenetics. 2011;6:76–85. doi: 10.4161/epi.6.1.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]