Abstract
Primary cilia project from the surface of most vertebrate cells, and function in sensation and signaling during both development and adult tissue homeostasis. Mounting evidence links ciliary defects with a wide variety of diseases, underscoring the importance of understanding how these dynamic organelles are assembled and maintained. However, despite their physiological and clinical relevance, the logic and machinery that regulate ciliogenesis remain largely enigmatic. Here, we summarize emerging data that connect the assembly and disassembly of the primary cilium to cell cycle progression and we examine how determinants of cell architecture, including the planar cell polarity pathway, may regulate ciliogenesis. Additionally, identification of the genes underlying diverse ciliopathies in human patients is shedding light on the regulation of the formation of this complex organelle.
Keywords: Centriole, centrosome, von Hippel-Lindau, VHL, CP110, Cep97, AuroraA, HEF1, IFT27, MKS1, Meckelin, MKS3
Introduction
One fundamental problem confronting every cell is how to collect information about its environment. Extending from the surface of most vertebrate cells is a single, sophisticated microtubule-based projection called the primary cilium. Although many biologists once considered this organelle to be “vestigial”, it is now becoming clear that primary cilia play important roles in receiving information (Singla and Reiter, 2006). For example, photoreceptors and odorant receptors function on modified cilia, and cilia are essential for sound reception. Therefore, it is not much of an exaggeration to say that we see, smell and hear through cilia. Moreover, it is now clear that cells also use primary cilia to communicate with each other (Eggenschwiler and Anderson, 2007). For instance, cilia play roles in establishing proper left-right patterning, regulating intracellular calcium levels, and interpreting several intercellular signals, including Hedgehog (Hh), PDGF and Wnt (Ross et al., 2005; Schneider et al., 2005; Hirokawa et al., 2006; Tabin, 2006; Corbit et al., 2007; Eggenschwiler and Anderson, 2007; Yoder, 2007).
Given the diverse cellular functions of cilia during both development and adult tissue homeostasis, it is not surprising that a growing variety of diseases are attributed to ciliary disfunction (Bisgrove and Yost, 2006). Whereas defects in ciliary motility lead to primary cilia dyskinesia (PCD) and altered left-right axis patterning, defects in immotile primary cilia function are frequently associated with kidney cysts, retinal degeneration, polydactyly, obesity and/or neural tube defects (here, referred to collectively as ciliopathies). The pleiotropic nature of these disorders may reflect the many roles cilia play in mechanosensation and signal transduction, highlighting the clinical importance of understanding how these organelles are assembled and maintained.
The formation of the primary cilium requires dynamic intracellular remodeling events, initially involving the migration and docking of the mother centriole to the plasma membrane (Pazour and Witman, 2003). The nine doublet microtubules of the ciliary axoneme then extend from the nine triplet microtubules of the basal body, a centriole-derived microtubule-organizing center. Elongation of the axoneme at the distal tip relies on intraflagellar transport (IFT), the bi-directional transit system that carries cargo within the cilium (Rosenbaum and Witman, 2002). IFT particles, comprised of at least sixteen components including IFT88 and IFT27, are transported in the anterograde direction by the Kinesin-2 motor, and in the retrograde direction by a Dynein motor (Rosenbaum and Witman, 2002). Disruption of either the IFT motors themselves or the basal body proteins essential for their function leads to impaired cilia assembly (Rosenbaum and Witman, 2002; Pazour and Witman, 2003)
Many mammalian cells that have the ability to form cilia are not always ciliated. For example, endocardial cells can be ciliated, but the majority are not (Iomini et al., 2004). Although the regulatory influences that trigger a cell to begin the process of ciliary assembly or disassembly remain largely enigmatic, the continuing identification and characterization of ciliary components has begun to shed some light on the nature of some of these influences. For example, ciliogenesis is exquisitely coordinated with the cell cycle, and recent studies have identified centrosomal proteins involved in this coordination. Moreover, the identification of the genes underlying human ciliopathies has suggested many additional candidates that may participate in the regulation of ciliogenesis. Here, we review recent data from both areas.
Coordination of ciliogenesis and the cell cycle
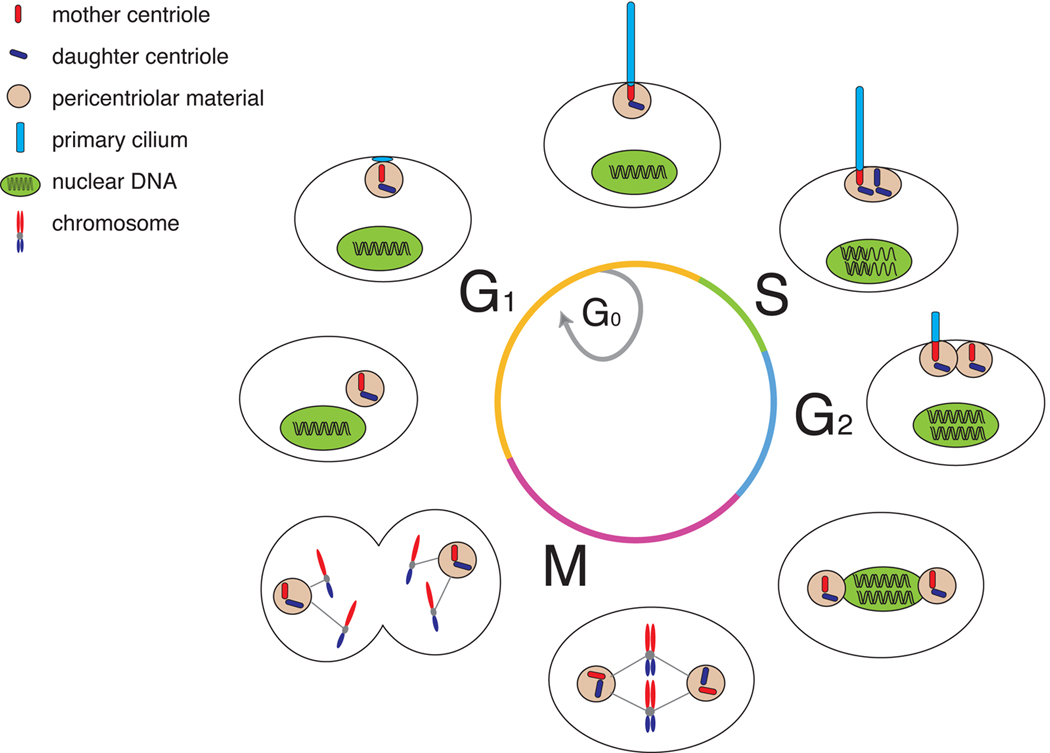
Centrosome dynamics are precisely synchronized with, and essential for the fidelity of, the cell cycle (Vorobjev and Chentsov Yu, 1982; Pan et al., 2004; Tsou and Stearns, 2006). During cytokinesis, each daughter cell inherits a single centrosome associated with one spindle pole (Figure 1). This centrosome is duplicated at the G1/S transition under the control of CDK2 (Hinchcliffe et al., 1999; Lacey et al., 1999). During G2, centrioles lengthen and centrosomes separate in preparation for forming spindle pole bodies during mitosis. Thus, much like chromosomes, centrosomes duplicate once and only once per cell cycle. Failure to obey this rule can result in disastrous consequences including multipolar mitotic spindles and chromosomal missegregation (Boveri, 1914; Boveri, 1929). Indeed, centrosome amplification is thought to be a significant cause of aneuploidy in cancer cells (Lingle et al., 1998; Pihan et al., 1998).
Figure 1. Dual use of the centrioles during cell cycle and primary cilium formation.
In most cells, primary cilium formation first occurs during G1 following centrosomal docking to the membrane. IFT and accessory proteins build the ciliary axoneme, which extends directly from the mother centriole’s triplet microtubules. During this stage of the cell cycle, as well as in G0, the cilium functions as a cellular antenna, interpreting extracellular signals such as Hedgehog and PDGF. Upon entry into S phase, the cell’s centrioles and the DNA begin to replicate. The centrioles reach maturity during late G2, at which point the cilium is disassembled so that the engaged centrioles can be liberated for mitotic spindle formation. Once cell division is complete, the centrioles can proceed to ciliary re-assembly in G1.
However, as with all rules, there are exceptions. For example, multi-ciliated cells such as tracheal and ependymal cells must synthesize at least as many centrioles as there are vertebrate cilia. Multi-ciliated cells may avoid the difficulties of dividing with greater than two centrosomes by terminally differentiating and permanently exiting the cell cycle (Spassky et al., 2005; Rawlins et al., 2007; Vladar and Stearns, 2007).
Given the intimate connection between cilia and centrosomes, it is perhaps not surprising that ciliogenesis is also coordinated with the cell cycle. Cilia are assembled during G1, most abundant in G0, and retracted in many cells at the entry into mitosis (Figure 1) (Rieder et al., 1979; Tucker et al., 1979). However, in some cell types, disassembly of the cilium can occur at other parts of the cell cycle phase, including prior to S-phase (Rieder et al., 1979; Tucker et al., 1979; Quarmby and Parker, 2005).
Ciliary disassembly at some point prior to entry into mitosis would seem to be essential, as the centrioles that template the microtubules of the ciliary axoneme during interphase are the same centrioles that form the spindle pole bodies during mitosis. However, centrioles are not required for cell cycle progression or mitotic spindle organization in either plants or Drosophila (Basto et al., 2006). Similarly, some mammalian cell types, such as HeLa, RPE1 and mammary epithelial cells, progress through the cell cycle despite the loss of centrioles (Habedanck et al., 2005; Uetake et al., 2007).
However, in support of the idea that centrosomes are more important for mitosis and cell cycle progression, reduction of certain centrosomal components such as Pericentrin and Centriolin leads to a p53-dependent arrest of RPE1 cells in G1 (Srsen et al., 2006; Graser et al., 2007; Mikule et al., 2007). Thus, the way in which the centrosome cycle is coordinated with the cell cycle may differ depending on cell type and whether the cell line is transformed or not.
Many of the centrosomal proteins essential for cell cycle progression are also required for primary cilium formation (Graser et al., 2007; Mikule et al., 2007). Indeed, study of the centrosomal proteins CP110 and Cep97 have begun to reveal some of the ways in which ciliogenesis is controlled. CP110 is phosphorylated by CDK2 (Chen et al., 2002) and recruited to the centrosome by Cep97 (Figure 2a,b) (Spektor et al., 2007). Depletion of either CP110 or Cep97 in U2OS cells leads to defective cytokinesis and abnormal mitotic spindles, as well as the production of cilia-like structures (Spektor et al., 2007). These cilia-like structures emanate from the distal ends of centrioles but contain nonciliary proteins such as Centrin and γ-Tubulin, suggesting that their composition is not identical to that of normal cilia.
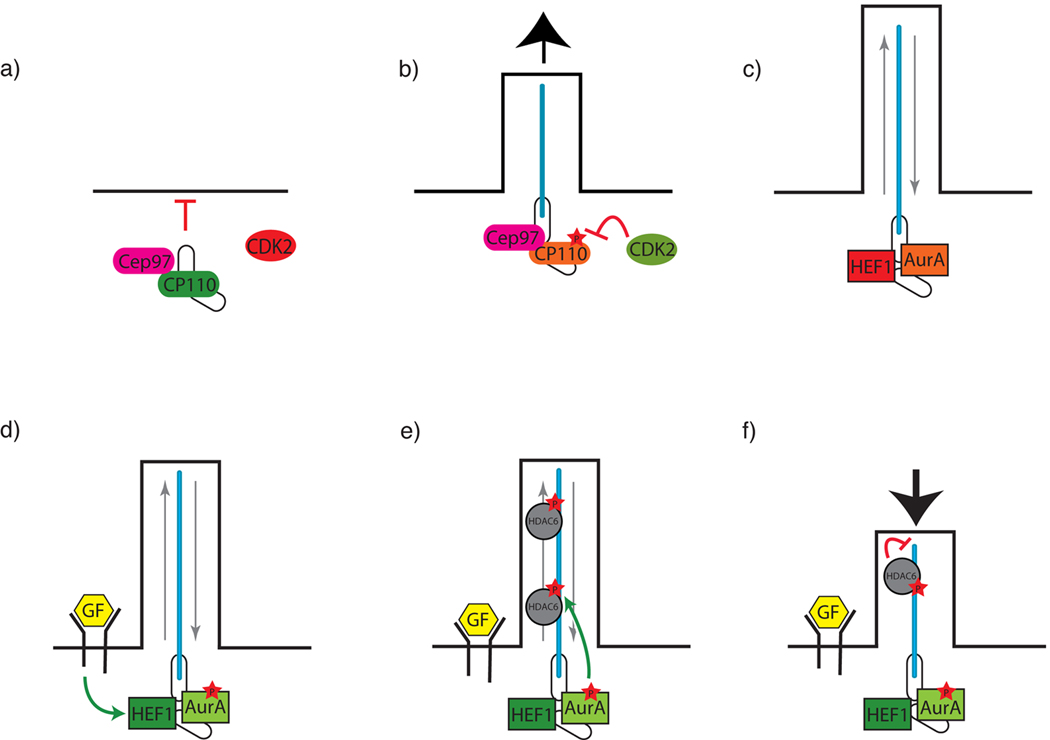
Figure 2. A model of CP110 and AuroraA-mediated coordination of ciliogenesis with the cell cycle.
CP110 is recruited to the centrosome by Cep97, where it inhibits ciliogenesis (a). Activation of CDK2 leads to CP110 phosphorylation, allowing ciliogenesis to occur (b). Two other proteins, HEF1 and AuroraA (AurA) localize to the ciliary basal body and participate in ciliary deassembly prior to mitosis (c). Upon growth factor (GF) stimulation, HEF1 activates AuroraA (d), which subsequently phosphorylates HDAC6 (e). Activated HDAC6 promotes ciliary disassembly by de-acetylating axonemal tubulin (f).
In cells that can form cilia such as RPE-1 and NIH-3T3 cells, inhibition of CP110 or Cep97 increases the proportion of cells displaying cilia (Spektor et al., 2007). Conversely, ectopic expression of CP110 in non-proliferating cells results in the suppression of cilia formation. Together, these results suggest that one of the centrosomal functions of CP110 and Cep97 is to suppress ciliogenesis. Given that CP110 is phosphorylated by CDKs, one attractive model is that active CDK2 phosphorylation of centrosomal CP110 in G1 inhibits the repressive functions of CP110, allowing ciliogenesis to occur (Figure 2a,b).
If repression of CP110 function leads to the induction of ciliogenesis in G1, what accounts for the dismantling of cilia prior to mitosis? Recent work implicates a known regulator of mitosis, AuroraA, and an interacting protein, HEF1, in the control of cilia disassembly (Pugacheva and Golemis, 2005; Pugacheva et al., 2007). AuroraA is a member of the Ipl family of kinases, and is modestly related to CALK, a kinase involved in Chlamydomonas flagellar retraction (Bischoff et al., 1998; Pan et al., 2004; Marumoto et al., 2005). Over-activity of AuroraA and HEF1 is associated with supernumerary centrosomes and multipolar spindles (Pugacheva and Golemis, 2005). Both of these proteins localize to the centrosome during G2 and M phases, and just prior to cilia disassembly, both are activated at the basal bodies of hTERT-RPE cells (Figure 2c,d) (Pugacheva et al., 2007). Pharmacological or siRNA inhibition of AuroraA blocks ciliary disassembly, whereas injection of active AuroraA initiates loss of cilia. Furthermore, siRNA against HEF1 leads to reduced levels of AuroraA activation. Taken together, these results suggest that HEF1 may stabilize and activate AuroraA, which initiates ciliary disassembly.
HDAC6, an α-tubulin deacetylase that promotes microtubule destabilization, may be a key player in AuroraA-mediated ciliary disassembly (Pugacheva et al., 2007). Activated AuroraA does not induce efficient ciliary disassembly in HDAC6 depleted cells, indicating that HDAC6 acts downstream of AuroraA (Pugacheva et al., 2007). Moreover, AuroraA can phosphorylate HDAC6 in vitro (Pugacheva et al., 2007). Thus, one possible mechanism by which growth factors may induce ciliary disassembly is through the induction of HEF1 expression, thereby activating AuroraA (Figure 2d). AuroraA subsequently phosphorylates HDAC6, which destabilizes the microtubules of the primary cilium by deacetylating axonemal tubulin, leading to rapid cilium resorption (Figure 2e,f). Thus, inhibition of CP110 may generate cilia in G1, whereas the activation of AuroraA may precipitate the disassembly of cilia in G2, accounting for one means of coordinating cilium formation with the cell cycle.
Another important class of regulatory proteins that may act in the coordination of ciliogenesis with the cell cycle is the NIMA-related kinase (Nek) family. Several Neks are essential for cell cycle progression and some of these localize to the centrosome (Tan and Lee, 2004) (Yin et al., 2003; Yissachar et al., 2006). Mutations in the gene encoding Nek8, a ciliary Nek, cause cystic kidney disease in both mice and humans, further underscoring the connection between Neks, cilia, and cell proliferation (Liu et al., 2002; Mahjoub et al., 2005; Otto et al., 2007; Otto et al., 2008). Although the functions of many of the Neks remain to be clearly elucidated, phylogenetic analysis suggests that the Nek family is expanded in organisms that have cilia on cells capable of replicating (i.e., that have not permanently exited the cell cycle), suggesting that one role for Neks may be in coordinating ciliary disassembly and the commitment to mitosis (Parker et al., 2007).
As proteins like Neks are associated both with cell cycle control and the regulation of ciliogenesis, the question arises as to whether the cilium itself reciprocally regulates any aspect of cell cycle progression. Depletion of some centrosomal proteins, including many required for cilium formation, does not result in G1 arrest in hTERT-RPE1 cells, indicating that cilia are not essential for cell cycle progression in all cell types (Graser et al., 2007). Additionally, mouse embryonic carcinoma cells that cannot form primary cilia due to loss of the centrosomal protein Odf2 progress normally through the cell cycle (Ishikawa et al., 2005).
However, other proteins with essential functions in ciliogenesis, but with no known roles in centrosome duplication, also function in the cell cycle. Inhibition of Chlamydomonas IFT27, a small G-protein similar to mammalian Rabl4, results in the expected loss of flagella and also the unexpected inhibition of cytokinesis (Qin et al., 2007). In vertebrates, IFT88, an essential component of the IFT machinery, also serves as a centrosomal protein that can inhibit cell cycle progression of HeLa cells (Robert et al., 2007). IFT88 may interact with the S-phase regulator Che1 to relieve its repression of the tumor suppressor Rb, providing some insight as to how IFT88 may restrain cell cycle progression (Robert et al., 2007). Thus, inhibiting the function of ciliogenic proteins can promote cycle progression (e.g., IFT88), disrupt the cell cycle (e.g., IFT27), or have no effect (e.g., Odf2).
These contrasting findings may reflect cilium-independent functions for these various proteins. However, it should be noted that each of these proteins has been studied in different systems, and transformed cells such as HeLa and embryonic carcinoma cells may differ from untransformed cell lines in terms of the influence that the cilium has on the cell cycle. Similarly, the cell cycles of different organisms or cell types within an organism may be regulated differently by cilia.
Even if there is no strict ciliary control over the cell cycle, the cilium may yet influence the cell cycle through its signaling functions. One of the most well-established functions for mammalian cilia is in the interpretation of Hedgehog (Hh) signals (Eggenschwiler and Anderson, 2007). Mutations that disrupt cilia also disrupt Hh signal transduction (Huangfu et al., 2003; Huangfu and Anderson, 2005; Liu et al., 2005; May et al., 2005) and many components of the Hh signal transduction pathway localize to cilia (Corbit et al., 2005; Haycraft et al., 2005; Rohatgi et al., 2007).
Although not as well understood as their roles in tissue patterning, Hh signals also regulate cell proliferation. Hh signals turn on the expression of Cyclin D and Cyclin E and the proto-oncogene N-myc, promoting progress through the G1-S transition and inducing cell proliferation (Kenney and Rowitch, 2000; Duman-Scheel et al., 2002; Kenney et al., 2003). Hh signals also block the function of tumor suppressors such as p21 and Rb and inhibit apoptosis by downregulating Fas expression (Fan and Khavari, 1999; Kenney and Rowitch, 2000). Similarly, the cilium can transmit PDGF signals which can promote cell cycle progression through the Mek1/2-Erk1/2 pathways (Schneider et al., 2005), and cilium-associated proteins can restrain canonical Wnt signaling (Simons et al., 2005; Corbit et al., 2007; Gerdes et al., 2007). Thus, one possible mechanism by which the cilium can influence the cell cycle is through the transduction of important mitogenic signals. Highly transformed cells that have become independent of these mitogenic cues may therefore be less dependent on ciliary functions, and may therefore better tolerate mutations that abrogate ciliogenesis.
Taken together, these studies strongly suggest that primary cilium formation and cell cycle progression may reciprocally influence one another, although the exact mechanisms and the universality of these mechanisms remain to be fully elucidated. Perhaps centriole sharing by the cilium and the centrosome, as well as the dual use of key players such as AuroraA, CP110, Neks and IFT88, facilitates the communication between the cilium and the cell cycle.
The influence of growth factors on ciliogenesis
Many cell types produce cilia upon serum starvation (Tucker et al., 1979; Vorobjev and Chentsov Yu, 1982). As discussed above, one reason for this phenomenon can be attributed to the ability of serum starvation to promote exit from the cell cycle, increasing the proportion of cells in G0. However, serum starvation may promote ciliogenesis through other means, such as the removal of secreted factors that directly inhibit ciliogenesis. In support of this possibility, serum stimulation of primary mouse embryonic fibroblasts lacking Vhlh, the mouse homolog of the gene underlying von Hippel-Lindau (VHL) syndrome, causes loss of the cilium (Thoma et al., 2007; Lolkema et al., 2008). This inhibition of ciliogenesis is dependent on intact phosphoinositide signaling, implicating PI(3)K in mediating an anti-ciliogenic cue normally counteracted by VHL activity.
von Hippel-Lindau syndrome is a rare autosomal dominant cancer syndrome. The VHL gene acts as a classic tumor suppressor; germline inheritance of a single loss-of-function mutant allele of VHL combined with somatic inactivation of the remaining wild-type allele can lead to hemangioblastomas, pheochromocytomas, and clear cell renal carcinomas (CCRCs) (Kuehn et al., 2007). Although VHL can perform diverse cellular functions, perhaps its most widely appreciated role is as a hypoxia sensor. VHL is a component of an E3 ubiquitin ligase complex that targets Hypoxia-Inducible Factor (HIF) for destruction in the presence of oxygen. Also relevant to its functions as a tumor suppressor, VHL can associate with and increase the activity of p53 (Roe and Youn, 2006).
One of the hallmarks of von Hippel-Lindau syndrome is the development of cysts in the kidney or pancreas, similar to those caused by defective ciliary function. Indeed, the renal cysts of von Hippel-Lindau patients lack primary cilia (Esteban et al., 2006) and some, but not all, VHL− CCRC cell lines lack cilia (Esteban et al., 2006; Thoma et al., 2007). Importantly, lentivirally-mediated re-expression of wild-type VHL in unciliated VHL− CCRC cells restores ciliogenesis (Esteban et al., 2006). Thus, VHL can promote ciliogenesis in at least some cell types.
In contrast to some CCRC cells, however, VHL appears to be dispensable for cilia formation in primary cells. As noted above, deletion of Vhlh and shRNA against VHL in embryonic fibroblasts and human kidney epithelial cells, respectively, only inhibits ciliogenesis if the phosphoinositide pathway is active (Thoma et al., 2007). How might PI(3)K activity restrict ciliogenesis? One consequence of phosphoinositide signaling is the inactivation of GSK3β through Akt-mediated phosphorylation of serine 9 (Cross et al., 2001). GSK3β is a ubiquitous protein kinase that regulates microtubule dynamics and is required for ciliogenesis in Chlamydomonas (Wilson and Lefebvre, 2004). GSK3β is closely related to another kinase, GSK3α, collectively referred to as GSK3. Although inhibition of GSK3 alone does not affect ciliogenesis, when combined with loss of VHL, GSK3 inhibition abrogates ciliogenesis in both fibroblasts and kidney epithelial cells (Thoma et al., 2007). It is possible that the anti-ciliogenic factor(s) found in serum induces PI(3)K activation of Akt, and the subsequent inactivation of GSK3. These experiments suggest that GSK3 and VHL may have parallel or overlapping functions in promoting ciliogenesis.
How VHL and GSK3 collaborate to promote ciliogenesis remains enigmatic, although several independent studies have provided some insight. It is not yet clear whether VHL inhibition of HIF function contributes to its ciliogenic functions. Esteban et al. found that siRNA-mediated knockdown of HIF-1α significantly restores ciliogenesis in VHL− CCRC lines, whereas activation of HIF-1α in VHL+ CCRC cells abolishes cilia formation, suggesting that the constitutive activation of HIF may underlie the absence of cilia (Esteban et al., 2006) (Figure 3, model 1). However, hypoxia itself does not sensitize CCRC or kidney epithelial cells to ciliary disassembly, implying that some of the ciliogenic activity of VHL may be HIF-independent (Lutz and Burk, 2006; Thoma et al., 2007).
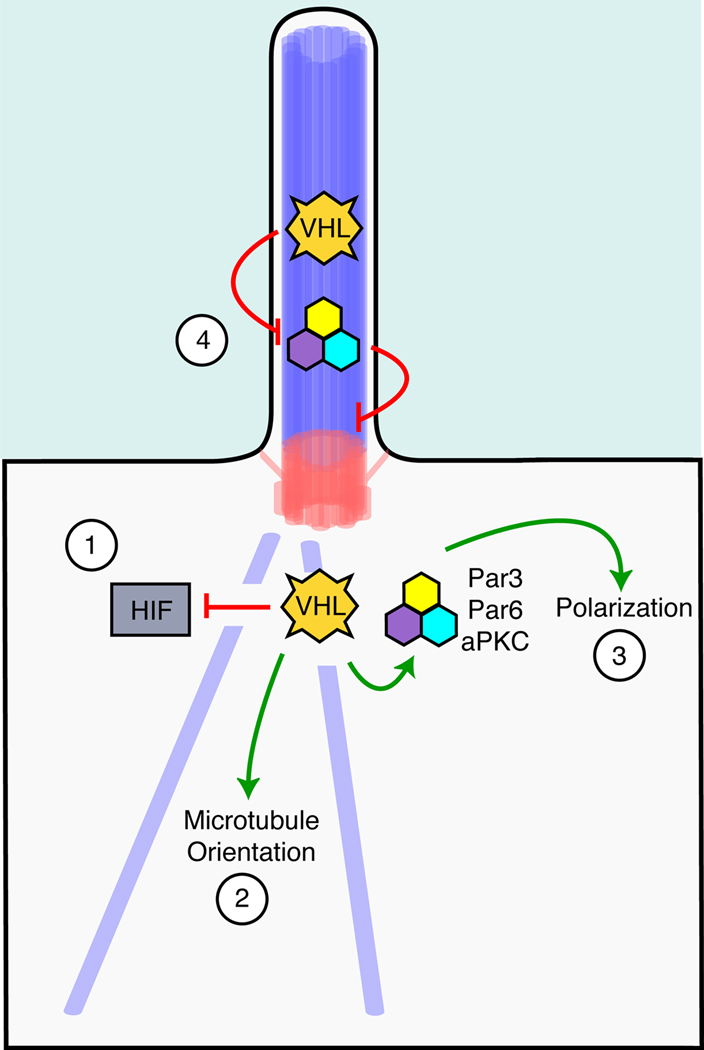
Figure 3. Possible mechanisms by which VHL promotes ciliogenesis.
1) VHL serves as an E3 ubiquitin ligase that targets HIF for destruction. HIF may inhibit ciliogenesis itself. 2) VHL binds and promotes the oriented growth of microtubules. As microtubules form the core of the ciliary axoneme, VHL-mediated stabilization of these microtubules may be necessary for ciliary maintenance. 3) VHL binds to the Par3-Par6-aPKC complex, which is necessary for apicobasal polarization. This polarization may be necessary for correct docking of the mother centriole to the apical plasma membrane, an early step in ciliogenesis. 4) VHL may ubiquitinate cilium-associated aPKC, inhibiting the ability of aPKC to dissociate microtubules from the basal body.
A second possible explanation for the role of VHL in regulating ciliogenesis arises from the observation that VHL regulates microtubule stability and orients microtubule growth (Hergovich et al., 2003; Lolkema et al., 2004; Schermer et al., 2006). VHL associates with and protects microtubules from nocodazole-induced depolymerization (Schermer et al., 2006). Interestingly, VHL− cells have uncoordinated microtubule movement, as assessed by live imaging of the plus-end microtubule-binding protein EB1. Specifically, in wild-type cells, newly formed microtubules migrate toward the outer plasma membrane, whereas migration in VHL− cells is randomized. Thus, VHL may participate in ciliogenesis through its ability to stabilize and orient microtubular growth (Figure 3, model 2).
A third explanation for how VHL may promote ciliogenesis stems from the finding that VHL interacts with the Par3–Par6-aPKC proteins, a trimeric complex involved in establishing cell polarity (Schermer et al., 2006). Perhaps unsurprisingly given its roles in apicobasal polarization of epithelial cells and centrosomal positioning in migrating cells, aPKC is essential for ciliogenesis in MDCK cells (Fan et al., 2004). Like the Par3–Par6-aPKC complex, VHL may promote ciliogenesis by simply promoting apicobasal polarization and correct docking of the centrosome at the apical membrane (Figure 3, model 3). In support of this possibility, VHL increases CCRC cell polarization (Lutz and Burk, 2006).
Finally, biochemical analyses show that Kinesin-2, the anterograde IFT motor, interacts with both VHL and the Par3–Par6-aPKC protein complex (Fan et al., 2004; Lolkema et al., 2007). This association with Kinesin-2 raises the possibility that VHL may function directly in regulating ciliary axoneme growth or stability. Consistent with this hypothesis, both the Par3–Par6-aPKC complex and VHL localize to primary cilia of kidney epithelial cells (Fan et al., 2004; Schermer et al., 2006; Thoma et al., 2007).
Moreover, like VHL, the Par3–Par6-aPKC complex can regulate microtubule dynamics. During Drosophila cellularization, aPKC has a critical role in dissociating microtubules from centrosomes (Harris and Peifer, 2005). VHL can ubiquitinate aPKC (Okuda et al., 2001), raising the intriguing possibility that VHL controls the ability of the Par3–Par6-aPKC complex to sever microtubule-centrosome associations. Perhaps VHL-mediated ubiquitination of aPKC keeps this activity in check during ciliogenesis, and the absence of VHL allows aPKC to release axonemal microtubules from the basal body leading to ciliary resorption or shedding (Figure 3, model 4).
Like VHL, GSK3 can also interact functionally with Par3 and aPKC to affect microtubule orientation and centrosome positioning (Xu et al., 2007), perhaps explaining the functional overlap between VHL and GSK3 in promoting ciliogenesis. It should be noted that none of these four models of how VHL may participate in ciliary dynamics are mutually exclusive, and more than one mechanism may be operating in cells.
PCP pathway and ciliogenesis
Studies of wing bristle formation and eye development in Drosophila have identified many genes required for the coordinate orientation of cells along an axis orthogonal to the apicobasal axis. This planar cell polarity (PCP) pathway depends on many components known to be critical for Wnt signal transduction, including Frizzled and Dishevelled (Dvl). Other components of the PCP pathway, like Inturned and Fuzzy, are not shared with the Wnt pathway and act downstream of Dvl in Drosophila bristle orientation. Morpholino knockdown of Inturned and Fuzzy in Xenopus embryos disrupts convergent extension, a coordinated morphogenetic cell movement known to depend on the vertebrate PCP pathway (Park et al., 2006).
Interestingly, inhibition of many components of the PCP pathway also abrogates ciliogenesis. For example, inhibition of Xenopus Inturned and Fuzzy inhibits formation of epidermal motile cilia (Park et al., 2006). Inhibition of Inturned and Fuzzy also disrupts Xenopus Hh signaling suggesting that primary cilium formation may also be affected (Park et al., 2006). Similarly, inhibition of Frizzled-2, a Frizzled-2 target called duboraya, or Dvl abrogates cilium formation in the zebrafish Kuppfer’s vesicle (Oishi et al., 2006).
How might PCP pathway components participate in ciliogenesis? Knockdown of these genes disrupts the organization of the apical actin network (Oishi et al., 2006; Park et al., 2006). Although neither Inturned nor Fuzzy have well-defined biochemical activities, they appear to affect microtubule orientation in addition to the actin network. Disorganization of the actin and microtubule cytoskeletons might compromise ciliogenesis through the mispositioning of basal bodies. A similar mechanism underlies the failure of ciliogenesis in Foxj1 mutant mice (Pan et al., 2007). Thus, while one manifestation of PCP pathway-mediated coordination of the actin and microtubular cytoskeletons is planar polarization, a perhaps functionally distinct manifestation may be the apical docking of the mother centriole essential for subsequent production of a cilium.
Reciprocally, do cilia regulate any aspect of PCP signaling? Mutation of several BBS genes, which encode ciliary, centrosomal and basal body proteins, cause phenotypes similar to those caused by PCP defects (Ross et al., 2005). Inversin, a component of primary cilia (Otto et al., 2003; Watanabe et al., 2003) can act as a molecular switch between the β-catenin-dependent and PCP signaling pathways (Simons et al., 2005), and the core PCP protein Vangl2 can localize to the basal body and the cilium (Simons et al., 2005; Jones et al., 2008).
Polarized localization of a non-ciliary pool of Vangl2 precedes asymmetric kinocilium positioning in hair cells (Jones et al., 2008). Whereas loss of IFT88 in the cochlea does not alter Vangl2 localization, it does cause misorientation of basal bodies and disruption of cochlear convergent extension. These data suggests that IFT88, and perhaps the cilium itself, are important for basal body polarization and subsequent interpretation of cues from the core PCP pathway (Jones et al., 2008).
Human diseases may provide clues to the regulation of ciliogenesis
In addition to its role in cystic kidney diseases, defective cilia formation and function are implicated in inherited pleiotropic diseases such as Bardet-Biedl Syndrome, Alstrom Syndrome, Jeune Syndrome, and Meckel-Gruber Syndrome (MKS). These diseases share many overlapping clinical manifestations that point to malfunctioning cilia as the shared underlying cause. Whereas some of these human mutations are believed to affect basal body dynamics (Kim et al., 2004; Kim et al., 2005), ciliary structure (Beales et al., 2007) or function (Li et al., 2007), others may compromise the regulation of ciliogenesis itself. Indeed, studies of MKS have begun to identify novel effectors of ciliogenesis.
MKS is a perinatally lethal autosomal recessive disease, whose clinical manifestations can include occipital encephalocele, cystic kidneys, and polydactyly. MKS can be caused by mutations in multiple genes and to date four underlying genes have been identified: MKS1, Meckelin, CEP290 and RPGRIP1L, all of which encode cilia- and centrosome-related proteins. (Kyttala et al., 2006; Smith et al., 2006; Dawe et al., 2007; Delous et al., 2007; Frank et al., 2007).
MKS1 encodes a B9 domain-containing protein originally suggested to have a ciliary role by comparative genomics (Li et al., 2004). Consistent with its involvement in ciliary function, the Chlamydomonas ortholog of MKS1 encodes a core centrosomal component, and the C. elegans ortholog is regulated by an Rfx transcription factor (Efimenko et al., 2005; Keller et al., 2005). Rfx transcription factors bind to an evolutionarily conserved X-box motif found in the regulatory regions of many genes encoding components of the cilium (Efimenko et al., 2005). The promoter for the gene encoding the multipass transmembrane protein Meckelin also contains an X-box motif, implicating Meckelin in ciliary biology (Smith et al., 2006).
In mice, Mks1 and Meckelin are both broadly expressed, notably in tissues affected by the disease, including the brain, lung, liver, kidney and digits (Dawe et al., 2007). MKS1, CEP290 and RPGRIP1L localize to the basal body of ciliated cells, whereas Meckelin, a transmembrane protein, localizes to the cilium itself (Kyttala et al., 2006; Dawe et al., 2007; Delous et al., 2007; Frank et al., 2007). Loss of cilia causes MKS-like phenotypes in developing mice, suggesting that MKS-associated genes may be essential for ciliogenesis. This hypothesis is borne out by knockdown studies indicating that MKS1 and Meckelin are essential for correct centrosomal positioning and ciliogenesis in IMCD3 cells (Dawe et al., 2007).
MKS shares substantial allelism with other less severe syndromes, including Joubert Syndrome, retinal degeneration syndromes such as Leber congenital amaurosis, nephronophthisis, and anosmia (Chang et al., 2006; Baala et al., 2007; Delous et al., 2007; Frank et al., 2007; McEwen et al., 2007), suggesting that varying degrees of ciliary dysfunction may underlie the pathogenesis of a wide variety of human syndromes. Perhaps anosmia or retinal degeneration result from minimal disruption of ciliary function, and Meckel syndrome results from more profound abrogation of ciliogenesis. One prediction made by this hypothesis is that disruption of any gene encoding an essential regulatory or structural component of cilia would result in an MKS-like phenotype in humans.
Several of the MKS loci encode interacting proteins. MKS1 and Meckelin coimmunoprecipitate (Dawe et al., 2007), and RPGRIP1L interacts with NPHP4, the product of a gene mutated in one form of nephronophthisis (Roepman et al., 2005). NPHP4 interacts in turn with Nephrocystin, another nephronophthisis-associated protein (Mollet et al., 2005). This biochemical evidence suggests that the MKS- and NPHP-associated proteins may form one or a few discrete biochemical complexes, and may be involved in the same biological processes, such as promoting proper apicobasal polarization or correct docking of the centrosome to the plasma membrane. Whether the other NPHP, Joubert syndrome, and Leber congenital amaurosis-associated proteins share these functions or operate in discrete processes remains to be elucidated.
Conclusions and perspectives
The primary cilium is clearly a dynamic organelle whose assembly and disassembly is just beginning to be understood. Here, we have examined emerging data regarding how ciliogenesis and the cell cycle are coordinated, and how signaling pathways and disease-associated proteins may modulate ciliogenesis. Less is known about other possible modulating influences, such as mechanical shear stress in the case of endothelial cilia, and even oxygen tension in the case of Tetrahymena flagella (Brown et al., 2003; van der Heiden et al., 2006). Additional research will shed light on the molecular mechanisms by which these influences intersect with the ciliary machinery and, undoubtedly, reveal still other ciliogenic influences. In particular, much remains to be uncovered about the molecular mechanisms by which the cell cycle and ciliogenesis are coordinated. Increased understanding of this regulation may provide novel insights into the etiology and therapy of diverse ciliopathies.
In addition to providing important insights into pathogenesis, research into the regulation of ciliogenesis may lead to increased understanding of the mechanisms by which cells can alter their responsiveness to a range of environmental influences. Given that this sensory organelle transduces information about the extracellular space, regulating ciliary assembly and disassembly may be one way that a cell can control its receptivity to this information. Thus, by modulating fundamental aspects of cytoarchitecture, including polarity, centriole association with the membrane, and microtubule stability, the cell might regulate ciliogenesis and, consequently, the repertoire of environmental and intercellular influences it can sense. These fascinating studies into ciliogenesis link the regulation of cell architecture to the regulation of signal transduction, and suggest that the cell itself is an active and dynamic participant in shaping both.
Acknowledgments
We thank members of the Reiter lab for helpful discussions. This work was supported by funding from the NSF (N.S.), the Sandler Family Supporting Foundation, the Burroughs Wellcome Fund, and the NIH (R01AR054396).
References
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, Munnich A, Lyonnet S, Salomon R, Encha-Razavi F, Gubler MC, Boddaert N, de Lonlay P, Johnson CA, Vekemans M, Antignac C, Attie-Bitach T. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80:186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–4143. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- Boveri BM. The origin of malignant tumors. Baltimore: Williams & Wilkins; 1929. p. 119. [Google Scholar]
- Boveri T. Zur Frage der Entstehung maligner Tumoren Jena. 1914:64. [Google Scholar]
- Brown JM, Fine NA, Pandiyan G, Thazhath R, Gaertig J. Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene. Mol Biol Cell. 2003;14:3192–3207. doi: 10.1091/mbc.E03-03-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O'Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell. 2002;3:339–350. doi: 10.1016/s1534-5807(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2007 doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T, Afford SC, Copp AJ, Kelly DA, Gull K, Johnson CA. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet. 2007;16:173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- Efimenko E, Bubb K, Mak HY, Holzman T, Leroux MR, Ruvkun G, Thomas JH, Swoboda P. Analysis of xbx genes in C. elegans. Development. 2005;132:1923–1934. doi: 10.1242/dev.01775. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban MA, Harten SK, Tran MG, Maxwell PH. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol. 2006;17:1801–1806. doi: 10.1681/ASN.2006020181. [DOI] [PubMed] [Google Scholar]
- Fan H, Khavari PA. Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol. 1999;147:71–76. doi: 10.1083/jcb.147.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Frank V, den Hollander AI, Bruchle NO, Zonneveld MN, Nurnberg G, Becker C, Bois GD, Kendziorra H, Roosing S, Senderek J, Nurnberg P, Cremers FP, Zerres K, Bergmann C. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum Mutat. 2007 doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7:517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Keller LC, Romijn EP, Zamora I, Yates JR, 3rd, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr Biol. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–9067. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- Kim JC, Ou YY, Badano JL, Esmail MA, Leitch CC, Fiedrich E, Beales PL, Archibald JM, Katsanis N, Rattner JB, Leroux MR. MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci. 2005;118:1007–1020. doi: 10.1242/jcs.01676. [DOI] [PubMed] [Google Scholar]
- Kuehn EW, Walz G, Benzing T. Von hippel-lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 2007;67:4537–4540. doi: 10.1158/0008-5472.CAN-07-0391. [DOI] [PubMed] [Google Scholar]
- Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestila M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- Lacey KR, Jackson PK, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci U S A. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Vega R, Nelms K, Gekakis N, Goodnow C, McNamara P, Wu H, Hong NA, Glynne R. A role for Alstrom syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet. 2007;3:e8. doi: 10.1371/journal.pgen.0030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci U S A. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- Lolkema MP, Mans DA, Snijckers CM, van Noort M, van Beest M, Voest EE, Giles RH. The von Hippel-Lindau tumour suppressor interacts with microtubules through kinesin-2. FEBS Lett. 2007;581:4571–4576. doi: 10.1016/j.febslet.2007.08.050. [DOI] [PubMed] [Google Scholar]
- Lolkema MP, Mans DA, Ulfman LH, Volpi S, Voest EE, Giles RH. Allele-specific regulation of primary cilia function by the von Hippel-Lindau tumor suppressor. Eur J Hum Genet. 2008;16:73–78. doi: 10.1038/sj.ejhg.5201930. [DOI] [PubMed] [Google Scholar]
- Lolkema MP, Mehra N, Jorna AS, van Beest M, Giles RH, Voest EE. The von Hippel-Lindau tumor suppressor protein influences microtubule dynamics at the cell periphery. Exp Cell Res. 2004;301:139–146. doi: 10.1016/j.yexcr.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Lutz MS, Burk RD. Primary cilium formation requires von hippel-lindau gene function in renal-derived cells. Cancer Res. 2006;66:6903–6907. doi: 10.1158/0008-5472.CAN-06-0501. [DOI] [PubMed] [Google Scholar]
- Mahjoub MR, Trapp ML, Quarmby LM. NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol. 2005;16:3485–3489. doi: 10.1681/ASN.2005080824. [DOI] [PubMed] [Google Scholar]
- Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, Martens JR. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, Baba M, Minato N, Ohno S, Shuin T. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Otto EA, Helou J, Allen SJ, O'Toole JF, Wise EL, Ashraf S, Attanasio M, Zhou W, Wolf MT, Hildebrandt F. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat. 2007 doi: 10.1002/humu.20669. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 Mutations Affect Ciliary and Centrosomal Localization and May Cause Nephronophthisis. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci. 2007;120:1868–1876. doi: 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Parker JD, Bradley BA, Mooers AO, Quarmby LM. Phylogenetic analysis of the neks reveals early diversification of ciliary-cell cycle kinases. PLoS ONE. 2007;2:e1076. doi: 10.1371/journal.pone.0001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, Doxsey SJ. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol. 2007;17:193–202. doi: 10.1016/j.cub.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–185. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- Robert A, Margall-Ducos G, Guidotti JE, Bregerie O, Celati C, Brechot C, Desdouets C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci. 2007;120:628–637. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- Roe JS, Youn HD. The positive regulation of p53 by the tumor suppressor VHL. Cell Cycle. 2006;5:2054–2056. doi: 10.4161/cc.5.18.3247. [DOI] [PubMed] [Google Scholar]
- Roepman R, Letteboer SJ, Arts HH, van Beersum SE, Lu X, Krieger E, Ferreira PA, Cremers FP. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci U S A. 2005;102:18520–18525. doi: 10.1073/pnas.0505774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, Hohne M, Kuhn W, Rapka M, Nitschke R, Zentgraf H, Fliegauf M, Omran H, Walz G, Benzing T. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol. 2006;175:547–554. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, Gattone VH, 2nd, Harris PC, Johnson CA. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Srsen V, Gnadt N, Dammermann A, Merdes A. Inhibition of centrosome protein assembly leads to p53-dependent exit from the cell cycle. J Cell Biol. 2006;174:625–630. doi: 10.1083/jcb.200606051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin CJ. The key to left-right asymmetry. Cell. 2006;127:27–32. doi: 10.1016/j.cell.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Tan BC, Lee SC. Nek9, a novel FACT-associated protein, modulates interphase progression. J Biol Chem. 2004;279:9321–9330. doi: 10.1074/jbc.M311477200. [DOI] [PubMed] [Google Scholar]
- Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol. 2007;9:588–595. doi: 10.1038/ncb1579. [DOI] [PubMed] [Google Scholar]
- Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heiden PL, Jedema I, Willemze R, Barge RM. Efficacy and toxicity of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Eur J Haematol. 2006;76:409–413. doi: 10.1111/j.1600-0609.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Stearns T. Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol. 2007;178:31–42. doi: 10.1083/jcb.200703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Chentsov Yu S. Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol. 1982;93:938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot Cell. 2004;3:1307–1319. doi: 10.1128/EC.3.5.1307-1319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR. Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci U S A. 2007;104:9296–9300. doi: 10.1073/pnas.0703153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem. 2003;278:52454–52460. doi: 10.1074/jbc.M308080200. [DOI] [PubMed] [Google Scholar]
- Yissachar N, Salem H, Tennenbaum T, Motro B. Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FEBS Lett. 2006;580:6489–6495. doi: 10.1016/j.febslet.2006.10.069. [DOI] [PubMed] [Google Scholar]
- Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]