Abstract
Objective
To determine the association of normal end row loops (ERL) at diagnosis of juvenile dermatomyositis (JDM) with clinical findings in untreated children and identify predictors of the development of decreased ERL.
Methods
Clinical and laboratory data of 80 untreated children with JDM were collected. ERL scores were recorded at time of diagnosis, and at 24 months and 36 months thereafter. Twelve children with normal ERL at diagnosis were compared with the remaining 68 children. Outcomes included: duration of untreated disease, time on immunosuppresive medications, family medical history, disease activity score (DAS), and levels of creatine phosphokinase (CPK), aldolase, absolute CD3−CD56+/16+ NK cells, and von Willebrand factor antigen (vWF:Ag). Cross-sectional and longitudinal analyses were performed.
Results
At diagnosis, children with normal ERL had a shorter duration of untreated disease (p=0.03) and a lower skin DAS (p=0.045). Over time, an increased likelihood for having abnormal ERL was associated with a longer duration of untreated disease and with higher skin DAS.
Conclusions
The presence of a normal number of ERL in JDM appears to be associated with a shorter duration of symptoms and may be a useful indicator of disease chronicity in the newly diagnosed child. Normal ERL is also associated with lower skin DAS. The lack of association between normal ERL and other variables indicates that normal NFC should not be used as a justification to delay immunosuppressive therapy in children with typical JDM symptoms.
Juvenile dermatomyositis (JDM) is a systemic vasculopathy characterized by skin and muscle involvement. Diagnostic criteria include muscle weakness and rash, electromyographic evidence of myopathy, muscle biopsy demonstrating inflammatory infiltrate with or without atrophy, and elevated muscle enzymes, although muscle enzymes may be normal (1). The etiology of JDM has not been established, but evidence suggests that environmental triggers such as an antecedent infection may play a role (2). Many children with JDM have a TNFα–308A allele which is associated with vascular occlusion(3) and increased TNF-α production (4). In addition, JDM disease activity has been associated with decreased absolute numbers of CD3−CD56+/16+ NK cells and elevated von Willebrand factor antigen (vWF:Ag) levels (5). Genes involved in immune responses, including those related to dendritic cell maturation and vasculature remodeling, are expressed at higher levels in muscle biopsies from children with greater duration of untreated disease (6).
One particular manifestation of JDM is the presence of abnormal nailfold capillaries, evidenced by capillary dropout, capillary dilatation, and bushy loops (7). Nailfold capillaroscopy (NFC) is a noninvasive, reproducible technique that provides information about abnormalities in periungual microvasculature. It takes into account quantitative measurements of capillary density or end-row loop (ERL) loss as well as the presence of avascularity and abnormal capillaries represented by “bushy” or “bizarre” loops (7,8). ERL scores between 7.0 and 11.5 have been observed in healthy children without JDM or other recognized autoimmune diseases (9). Serial nailfold capillary observations have demonstrated progression of nailfold changes over time in children with JDM (6), 10- 12) and their degree of morphologic changes may correlate with the clinical course of the disease (10). While abnormal NFC are not part of the Bohan and Peter criteria for dermatomyositis, they may reflect systemic vasculopathy, and the data can be used to evaluate patients with JDM (13).
ERL regeneration is associated with a shorter duration of untreated disease, a unicyclic disease course resulting in discontinuation of all immunosuppressive therapy before 36 months (versus non-unicyclic in which continuous or repeated immunosuppressive therapy is required), and a lower skin disease activity score (DAS), (11), (12). Lower numbers of ERL in children with JDM are also associated with decreased bioavailability of oral prednisolone compared with intravenous methylprednisolone, suggesting a relationship between systemic vasculopathy and decreased absorption of oral prednisolone (14). Another recent study also showed that six months after diagnosis, the presence of abnormal nailfold capillaries with Gottron’s papules predicted a longer time to remission (15).
Given the potential uses of NFC in providing valuable clinical information with regard to JDM, we studied untreated children fulfilling criteria for definite/probable JDM for the following: 1) the association of normal ERL at the time of diagnosis with clinical and laboratory data, and 2) predictors of decreased number of ERL over time.
Patients and Methods
Patients
Eighty children diagnosed with probable/definite JDM at the Rheumatology clinic of Children’s Memorial Medical Center in Chicago from 1993 to 2007 were included in this study, approved by the Children’s Institutional Review Board (IRB #10778), after age- appropriate informed consent was obtained from the patient and their legal guardians. Patients were excluded from the study if they had already started any medical treatment for JDM, including glucocorticosteroids. Histories, physical findings, laboratory data, and NFC observations were entered into a database. Twelve children with normal number of ERL at diagnosis were compared with 68 untreated children with definite/probable JDM who had abnormal ERL. The following clinical data were collected: family medical history, DAS, serum levels of creatine phosphokinase (CPK), aldolase, vWF:Ag, absolute CD3−CD56+/16+ NK cells, duration of untreated disease (determined by the historical onset of rash or weakness obtained from parents/guardians), and time on immunosuppressive medications.
Nailfold capillaroscopy and Disease Activity Scores
All children had NFC at the time of diagnosis consisting of freeze-frame video microscopy images of each of eight fingers, excluding thumbs. The total number of ERL per millimeter over the eight digits were divided to yield the mean ERL for each patient. An ERL score of less than 7.00 was considered abnormal. All NFC studies were performed by one investigator at the time of the clinic visit, and DAS scoring was determined by another independent investigator.
DAS is comprised of subscores: skin DAS and muscle DAS. Skin DAS is determined by the presence of dermatologic findings including the extent and severity of the rash, Gottron’s papules and/or telangiectasias on the nailfolds, palate, and/or eyelids. Skin DAS ranges from 0-9; higher numbers correlate with severity and extent. The range for muscle DAS is 0-11, and higher scores correlate with weakness and loss of function.
Statistical analysis
Demographic characteristics such as age, gender distribution, and race were compared between the two study groups. Outcomes analyzed at the time of diagnosis include the following: family medical history (autoimmune disease, cancer, infant deaths, lipid abnormalities and cardiovascular disease), DAS for muscle and skin, serum levels of CPK, aldolase, vWF:Ag, and absolute CD3−CD56+/16+ NK. Based on the normal values in our laboratory, levels of CPK, aldolase, and vWF:Ag were categorized as normal or abnormal values, while absolute number of CD3−CD56+/16+ NK cells and DAS were analyzed as continuous data.
Time on immunosuppressive medications was compared between the two groups using logrank test. Chi-square, Fisher’s Exact and Wilcoxon Rank tests were used for the cross-sectional analyses at diagnosis.
ERL findings were classified as a dichotomous variable (normal or abnormal) for the cross-sectional analysis at baseline, as well as for the longitudinal analysis examining covariates potentially associated with ERL normality in a multiple logistic regression model, using a generalized estimating equation approach. ERL was also analyzed as a continuous variable comparing the two groups of children longitudinally at diagnosis and 24 months and 36 months thereafter, using a linear mixed model, with time as a random effect. Results from this model are reported as adjusted means with corresponding standard error (SE).
The logistic regression modeling was used to look for potential predictors of abnormal ERL at baseline, 24 months, and 36 months. All analyses were conducted using SAS, and statistical significance was set at 0.05.
Results
Distribution of gender, age and race are listed in Table 1. There was no significant difference in age, gender, or race between the two groups. Clinical characteristics of the children with normal nailfolds are listed in Table 2. At the time of diagnosis, a shorter duration of untreated disease was observed in children with normal ERL (median 3.1 months, range 1.0-13, versus 6.5 months, range 0.7-111, p=0.03). Children with normal ERL also had significantly lower DAS Skin (median 5.0 range 4 – 7, vs. median 6, range 0 – 9, p=0.045). There was no significant difference in the other outcomes at diagnosis, including family history of autoimmune disease (p=0.53) and time on medications (p=0.60).
Table 1.
Demographics of 80 children with JDM
| n (%) | Age at First Visit, Mean Years (SD) |
Duration of Untreated Disease, Mean Months (SD) |
|
|---|---|---|---|
| NORMAL ERL 1st VISIT | 12 (15) | 6.6 (2.3) | 4.4 (4.2) |
| Male Gender | 4 (33) | 5.4 (1.5) | 5.6 (5.4) |
| Female Gender | 8 (67) | 7.2 (2.4) | 3.9 (3.7) |
| White Ethnicity | 11 (92) | 6.7 (2.4) | 4.7 (4.3) |
| Non White Ethnicity | 1 (8) | 6.1 | 2.1 |
| ABNORMAL ERL 1st VISIT | 68 (85) | 6.7 (3.8) | 12.1 (19.1) |
| Male Gender | 14 (21) | 6.1 (4.6) | 9.2 (6.2) |
| Female Gender | 54 (79) | 6.9 (3.6) | 12.9 (21.1) |
| White Ethnicity | 48 (71) | 6.8 (3.9) | 10.1 (16.0) |
| Non White Ethnicity | 20 (29) | 6.5 (3.7) | 16.9 (24.8) |
| TOTAL | 80 | 6.7 (3.6) | 11.0 (17.8) |
Table 2.
Clinical characteristics of individual children with JDM and normal ERL
| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Abnormal ERL n=68 median (range)/ number tested |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAS Skin (0-9) | 7 | 4 | 5 | 5 | 4 | 6 | 6 | 5 | 5 | 4 | 5 | 4 | 6 (0, 9)/64 |
| DAS Muscle (0-11) | 9 | 5 | 8 | 2 | 8 | 4 | 1 | 2 | 9 | 9 | 6 | 0 | 4 (0, 10)/64 |
|
MRI T2
Findings for Myositis |
Yes | Yes | Yes | * | Yes | Yes | ** | No | Yes | * | Yes | Yes | 44 Positive/65 |
|
Positive
Muscle Biopsy |
Yes | * | Yes | * | Yes | Yes | Yes | * | Yes | Yes | Yes | No | 52 Positive/56 |
| CPK (IU/L) | 1246 | 71 | 859 | 322 | 83 | 177 | 109 | * | 2222 | 167 | 281 | 1747 | 138.5 (44, 17884) /62 |
| Aldolase (U/L) | 21.2 | 8.4 | 18.7 | 10.3 | 9.5 | 9.7 | 5.5 | * | 18.9 | 12.9 | 24 | 24.2 | 9.2 (2.8, 204)/61 |
| SGOT (IU/L) | 175 | 109 | 182 | 44 | 51 | 41 | 28 | * | 213 | 67 | 379 | * | 46 (18, 782)/65 |
| LDH (U/L) | 432 | 344 | 512 | 310 | 253 | 265 | 198 | * | 434 | 535 | 546 | 662 | 316 (166, 1970)/63 |
| Elevated vWF Ag | No | * | Yes | No | Yes | No | No | * | Yes | Yes | Yes | * | 14/60 |
| Absolute NK cells | 141 | 111 | 14 | 376 | 61 | 294 | 186 | 200 | 64 | 23 | 21 | 161 | 128 (25, 684)/64 |
| ERL/mm | 7.0 | 8.1 | 8.7 | 8.5 | 9.0 | 8.2 | 9.2 | 7.1 | 10.3 | 8.1 | 7.1 | 7.5 | 4.6 (2.3, 6.7)/68 |
Not performed
Increased signal, unusual pattern for JDM
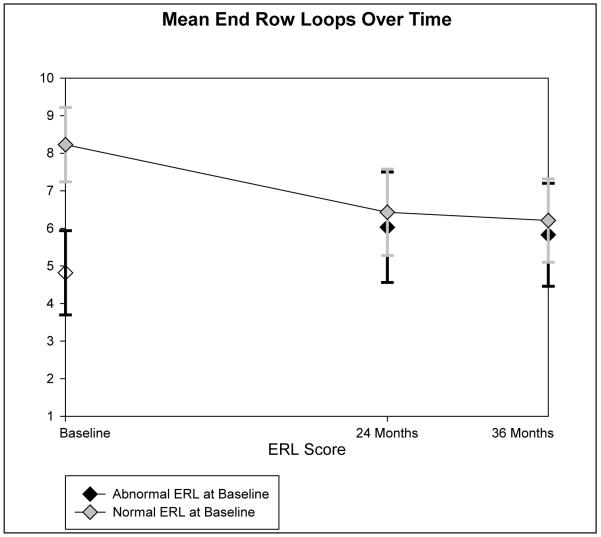
Trends of mean ERL in the two groups of children are plotted in Figure 1. Seven of the 12 children with normal nailfolds at the time of diagnosis developed abnormal ERL by 24 months. One child did not have NFC at 24 months, but a capillaroscopy exam at 47 months was abnormal. One child did not have any follow up NFC. Three children continued to have normal nailfolds at 24 months, and two of these children developed abnormal nailfolds at 36 months.
Figure 1.
Sequential studies at diagnosis, 24, and 36 months of the number of End Row Loops (ERL).
In the longitudinal model examining ERL counts as a continuous variable over time, children with normal ERL at baseline continued to have significantly higher ERL at 24 and 36 months, but the difference between normal and abnormal groups decreased significantly over time (p=0.03). At diagnosis, the mean difference was 3.2 (SE=0.32). The mean difference between ERL scores of the two groups at 24 and 36 months was 2.5 (SE=0.17) and 2.1 (SE=0.24). This was adjusted for duration of untreated disease.
In a multiple logistic regression model looking for potential predictors of abnormal ERL over the 36 month period, children with longer duration of untreated disease were more likely to have abnormal ERL (p=0.02), and children with higher skin DAS had a higher likelihood of having abnormal ERL, (p=0.004). The model was adjusted for age which was not found to be statistically significant. DAS muscle, CD3−CD56+/16+ NK cells, family medical history, and abnormal levels of CPK were not found to be associated with NFC over the 36 months
Discussion
This investigation studied children with JDM who have normal nailfolds at diagnosis as a group. We have found that the presence of a normal number of ERL at the time of diagnosis in JDM is related to a shorter duration of untreated illness and a lower skin DAS. These observations support earlier reports regarding decreased ERL, skin DAS, and duration of untreated disease (11, 12).
Persistent NFC abnormalities together with Gottron’s papules at six months may be associated with a longer time to remission (15). Additionally, ERL regeneration has been associated with a less persistent disease course (12), and ERL loss with decreased bioavailability of oral prednisolone (14). However, we did not find significantly shorter time requirements of immunosuppressive treatment in children with normal ERL. Variability between individual treatment regimens were not analyzed in our study. Time to discontinuation of immunosuppressive medications simply indicates the presence of one or more immunosuppressive drug, including methotrexate, hydroxychloroquine, oral prednisone, intravenous methylprednisolone, and intravenous immunoglobulin. The absence of a significant difference in time required for immunosuppressive treatment between the two groups certainly suggests that children with JDM and normal ERL should not be treated less aggressively for their disease. It is worthwhile to note that even the few children who had persistently normal number of ERL at 24 and 36 months (3 children at 24 months) did not require a shorter duration of medications, although a meaningful observation is not possible due to this limited population.
JDM disease activity has been associated with decreased absolute numbers of CD3−CD56+/16+ NK cells and elevated vWF:Ag levels (Reviewed in 5). The absence of a significant difference between the levels of vWF:Ag at baseline and the absolute number of NK cells in the two groups of our study might suggest that the immunological activity reflected by these markers either do not interact with ERL or may take time to have an effect.
We also observed that a longer duration of untreated disease and higher skin DAS at diagnosis were each associated with the presence or development of abnormal ERL over time. In a previous report, a longer duration of untreated disease was associated with reduced patient height, weight, and with pathologic calcifications (1). Since nailfold capillaries are a useful indicator of underlying vasculopathy, these findings emphasize the importance of timely diagnosis and effective treatment in a child suspected of having JDM.
A limitation of our study is the small number of children who have normal ERL at diagnosis, with even fewer children who have persistently normal ERL over time. Larger studies are needed to confirm our findings, though the low incidence of JDM remains a challenge to acquiring larger pools of data. Another limitation relates to the data on duration of immunosuppressive medications. Regimens varied between individual patients and over time, making it difficult to detect confounding factors in the treatments themselves.
The presence of a normal number of ERL in children who fulfill the diagnostic criteria for definite/probable JDM appears to be associated with a shorter duration of symptoms and may be a useful indicator of disease chronicity in the newly diagnosed child. In addition, a longer duration of untreated disease and higher skin DAS at diagnosis were each associated with a higher likelihood of having or developing abnormal ERL over time. This is valuable information, since a longer duration of untreated symptoms has been found to be an important factor in the impact of JDM on growth and clinical presentation (1). However, the lack of association between normal NFC and other clinical variables indicate that normal NFC results should not be used as a justification to delay or modify immunosuppressive therapy in children with JDM who meet diagnostic criteria.
Acknowledgments
Supported in part by: NIH/NIAMS grant R01-AR48289, Cure JM and Macy’s Miracle Foundation. (to LMP)
References
- 1.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatrics. 2006;148:247–53. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: Results from the national institute of arthritis and musculoskeletal and skin diseases research registry. Arthritis Rheum. 2005;15:166–72. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 3.Lutz J, Huwiler KG, Fedczyna T, Lechman TS, Crawford S, Kinsella TR, et al. Increased plasma thrombospondin-1 (TSP-1) levels are associated with the TNF alpha-308A allele in children with juvenile dermatomyositis. Clinical Immunol. 2002;103:260–3. doi: 10.1006/clim.2001.5212. [DOI] [PubMed] [Google Scholar]
- 4.Pachman LM, Liotta-Davis M, Hong D, Kinsella TR, Mendez E, Kinder J, Chen EH. TNFα-308A allele in juvenile dermatomyositis-association with increased TNFα production, disease duration, and pathological calcifications. Arthritis Rheum. 2000;43:2368–2377. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Feldman BM, Reed AM, Rider LG, Pachman LM. Juvenile dermatomyositis & other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–2212. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen YW, Shi R, Geraci N, Shrestha S, Gordish-Dressman H, Pachman LM. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunology. 2008;9:43. doi: 10.1186/1471-2172-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussbaum AI, Silver RM, Maricq HR. Serial changes in nailfold capillary morphology in childhood dermatomyositis. Arthritis Rheum. 1983;26:1169–72. doi: 10.1002/art.1780260919. [DOI] [PubMed] [Google Scholar]
- 8.Dolezalova P, Young SP, Bacon PA, Southwood TR. Nailfold capillary microscopy in healthy children and in childhood rheumatic diseases: A prospective single blind observational study. Ann Rheum Dis. 2003;62:444–9. doi: 10.1136/ard.62.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pachman LM, Sundberg J, Kinder J, Maduzia L, Daugherty L. Nailfold capillary studies in children with pediatric connective tissue diseases: systemic lupus erythematosus (SLE), juvenile dermatomyositis (JDMS), Raynaud’s phenomenon (RP)--comparison with data from normal children. Arthritis Rheum. 1996;39:R14. (Abstract) [Google Scholar]
- 10.Silver RM, Maricq HR. Childhood dermatomyositis: serial microvascular studies. Pediatrics. 1989;83:278–282. [PubMed] [Google Scholar]
- 11.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis associate with loss of end row nailfold capillary loops. J Rheumatol. 2004;31:1644–9. [PubMed] [Google Scholar]
- 12.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfold capillaroscopy changes and skin involvement over thirty-six months with duration of untreated disease in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58:571–6. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown VE, Pilkinton CA, Feldman BM, Davidson JE. An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM) Rheumatology. 2006;45:990–3. doi: 10.1093/rheumatology/kel025. [DOI] [PubMed] [Google Scholar]
- 14.Rouster-Stevens KA, Gursahankey A, Ngal K, Daru JA, Pachman LM. Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Care Res. 2008;59:222–6. doi: 10.1002/art.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stringer E, Singh-Grewal D, Feldman BM. Predicting the course of juvenile dermatomysositis: significance of early clinical and laboratory features. Arthritis Rheum. 2008;58:3585–92. doi: 10.1002/art.23960. [DOI] [PubMed] [Google Scholar]