Abstract
Background
Functional electrical stimulation (FES) has been regularly used to offset several negative body composition and metabolic adaptations following spinal cord injury (SCI). However, the outcomes of many FES trials appear to be controversial and incoherent.
Objective
To document the potential consequences of several factors (e.g. pain, spasms, stress and lack of dietary control) that may have attenuated the effects on body composition and metabolic profile despite participation in 21 weeks of FES training.
Participant
A 29-year-old man with T6 complete SCI participated in 21 weeks of FES, 4 days per week.
Methods
Prior to and following training, the participant performed arm-crank-graded exercise testing to measure peak VO2. Tests conducted included anthropometrics and dual energy X-ray absorptiometry body composition assessments, resting energy expenditure, plasma lipid profiles and intravenous glucose tolerance tests.
Results
The participant frequently reported increasing pain, stress and poor eating habits. VO2 peak decreased by 2.4 ml/kg/minute, body mass increased by 8.5 kg, and body mass index increased from 25 to 28 kg/m2. Waist and abdominal circumferences increased by 2–4 cm, while %fat mass increased by 5.5%. Absolute increases in fat mass and fat-free mass of 8.4 and 1 kg, respectively, were reported. Fasting and peak plasma glucose increased by 12 and 14.5%, while lipid panel profiles were negatively impacted.
Conclusion
Failure to control for the listed negative emerging factors may obscure the expected body composition and metabolic profile adaptations anticipated from FES training.
Keywords: Spinal cord injuries, Paraplegia, Functional electrical stimulation, Rehabilitation, Disability, Ergometry, Exercise, Spasticity, Dietary control, Stress, Pain, Body composition, Carbohydrate intolerance, Lipid disorders
Introduction
Functional electrical stimulation (FES) has been regularly prescribed as an effective rehabilitation intervention after spinal cord injury (SCI) that evokes lower extremity muscle hypertrophy, decreases fat mass, and improves abnormal metabolic profiles.1–4 Several reports have documented improvement in the cardiovascular metabolic risk factors following FES-cycling.2,4,5 Griffin et al. documented that 10 weeks of FES cycling in individuals with SCI resulted in approximately 4% increase in lean mass with no changes in adipose tissue.4 The same study reported improvement in both plasma glucose and insulin; however, it did not show any improvement in plasma cholesterol levels or triglycerides as well as reduction in high-density lipoprotein-C.4 A review summarizing evidence from 22 studies contended that there is insufficient support to the hypothesis that exercise could improve carbohydrate and lipid disorders among adults with SCI.6 The factors that are responsible for these controversial results need to be well studied to ensure successful controlled interventions.
SCI is a complex medical condition where deterioration in body composition and metabolic profile is defined by the nature or level of injury, completeness of injury, time since injury, and age of the participants.7–10 The homogeneity of any SCI sample is easily questioned because no two injuries are alike. Furthermore, this population is characterized by inherent comorbidities that may vary from one injury to another.7–10 Unless considering an integral approach, all the aforementioned factors could impact the outcomes of longitudinal interventions. For example, the high prevalence of pain and spasticity that may exceed 70% in this population may dampen the outcomes of FES training and interfere with functional independence.11–14 Pain and spasticity medications are well reported to reduce metabolism, energy expenditure, and may further cause overall weakness.15,16 This may further increase stress to a level that is commonly associated with excess release of catabolic hormones (i.e. cortisol), which may further degrade the anabolic processes that evoke muscle hypertrophy and increase lean mass.17–22 Another factor is the hesitancy of individuals with SCI to embrace the importance of starting a long-term lifestyle changes that result in significant adaptations such as reduction in fat mass and improvement in metabolic profile.23,24 Others have shown that dietary control is an effective strategy for prevention of obesity among individuals with SCI.23 However, many individuals with SCI rely on meals that are high in fat and low in protein.24
This is a report of a person with SCI who participated in an FES leg ergometry trial to reduce obesity for 21 weeks. We hypothesized that FES training may result in significant body composition adaptations characterized by increased fat-free mass, reduction in fat mass, as well as improvement in the metabolic profile as determined by intravenous glucose tolerance test and lipid panel profiles.
Case report
A 29-year-old man with T6 ASIA Impairment Scale A injury signed a Veterans Affairs IRB-approved consent form and a Virginia Commonwealth University IRB-approved consent form before undergoing a health history and physical examination with SCI neurological classification assessment and testing. The participant was a student who worked part-time with a past medical history of spasticity (oral Baclofen 20 mg every 6 hours), low back pain (Tramadol 50 mg every 6 hours), and neurogenic bladder (Oxybutynin 10 mg). These conditions were treated as listed above. At the time of enrollment, he reported smoking 5 cigarettes per day. Following physiologic and metabolic testing, he then participated in 4 days per week FES cycling for 21 weeks. The participant completed only 75 sessions of FES-leg ergometry due to holidays, work, and school commitments.
Physiologic and body composition
A VO2 Peak graded exercise test was performed on a Lode upper extremity ergometer (Electro-Med Corporation, Flint, MI, USA) using TrueMax 2400 computerized metabolic measurement system (ParvoMedics, Salt Lake City, UT, USA). Supine and seated circumference measurements were performed in triplicate by a single trained investigator, while total body DXA (GE Lunar, Madison, WI USA) scans were performed and analyzed by a certified DXA operator using Lunar software v13.
Metabolic profile assessment
Following a 12-hour fast, resting metabolic rate was estimated using COSMED K4b2 (Cosmed USA, Chicago, IL, USA) at ∼06:00 hours, followed 1 hour later with a standard 3-hour intravenous glucose tolerance tests (IVGTTs). The IVGTT was used to determine insulin sensitivity and glucose effectiveness. All tests were conducted prior to 21 weeks of training and 72 hours following the final exercise bout following standard procedures. Briefly, an indwelling catheter with an intravenous saline drip (0.9% NaCl) was placed in an antecubital vein, and another intravenous line was placed in a contralateral hand vein between the hours of 8:00 and 8:30 to facilitate infusion of glucose and blood sampling during the IVGTT. Glucose samples were taken at –6, −4, −2, 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 minutes after the rapid glucose injection (0.3 gm/kg IV over 30 seconds at time zero). In addition, 20 minutes after the glucose injection a bolus of insulin (0.02 U/kg) was injected to determine insulin sensitivity. Blood pressure and heart rate will be assessed at minutes 22, 23, and 24 of the protocol. The results of the IVGTT were modeled using MinMod analysis (MinMod Inc., Pasadena, CA, USA). Blood lipid profiles were also assessed during metabolic profile testing using standard analysis procedures.
FES training protocol
FES cycling performed on an ERGYS 2 ergometer (Therapeutic Alliances, Fairborn, OH, USA) with bilateral stimulation of the quadriceps, hamstring, and gluteal muscles. Muscles were stimulated sequentially at 60 Hz with current amplitude (140 mA) necessary to complete 40 minutes of cycling at a cadence of 50 revolution per minute (RPM) with progressively greater resistance over the course of training. Each session included a 10-minute passive warm-up and cool-down, and heart rate was monitored and recorded throughout the training using a Polar RS400 watch. The passive cycling was performed by one of the research investigators to maintain a cadence of 5 RPM less than the target cadence. Fatigue threshold was set at 10 RPM to allow a longer cycling time despite the fact that muscle fatigue may ensue. Power (W) was calculated using the following formula: W = RPM× KP × D; where RPM is revolutions per minute, KP is the resistance in kilo ponds, and D is the distance traveled in meters per revolution and was measured to be 1.54 m. During the training period, energy expenditure was estimated during rides 1, 40, and 75 using a COSMED K4b2, while average power output was calculated for all sessions.
Analysis section
The case report is a pre–post test design. The values presented in Table 1 are considered the outcomes in body composition and metabolic profile pre and post-FES intervention. % difference was calculated as the difference in post-intervention values minus pre-intervention values divided by pre-intervention outcomes multiplied by 100.
Table 1.
Outcomes of 21-week FES trial on body composition and metabolic profile in a person with complete SCI
| Pre-FES | Post-FES | % difference | |
|---|---|---|---|
| Physical characteristics | |||
| Body weight (kg) | 80 | 88.5 | 11 |
| BMI (kg/m2) | 25.0 | 28 | 12 |
| VO2 (ml/kg/min) | 21.9 | 19.5 | −11 |
| Systolic BP (mmHg) | 107 | 129 | +2 |
| Diastolic BP (mmHg) | 53 | 55 | −36 |
| Anthropometrics | |||
| WC-seated (cm) | 91 | 95 | +4 |
| AB-seated (cm) | 104.5 | 102 | −2 |
| WC-supine (cm) | 89.5 | 91 | +1.5 |
| AB-supine (cm) | 92.5 | 95 | +2.7 |
| DXA | |||
| Whole body %FM | 37 | 42.5 | +5.5 |
| FM (kg) | 29.3 | 37.7 | +29 |
| FFM (kg) | 47 | 48 | +2 |
| BMD (g/cm3) | 1.13 | 1.11 | |
| Metabolic profile | |||
| REE (Kcal/day) IVGTT | 1393 | 1090 | −22 |
| Fasting glucose (mg/dl) | 88 | 100 | +14 |
| Fasting insulin (μu/ml) | 4.5 | 3.4 | −24 |
| Peak glucose (mg/dl) | 262 | 300 | +14.5 |
| Peak insulin (μu/ml) | 209 | 146 | −30 |
| Sg | 0.01 | 0.02 | +100 |
| Si | 6.5 | 20 | +208 |
| Lipid panel | |||
| Total cholesterol (mg/dl) | 222 | 243 | +9.5 |
| HDL-C (mg/dl) | 43 | 38 | −12 |
| LDL-C (mg/dl) | 145 | 132 | −9 |
| TG (mg/dl) | 170 | 364 | +114 |
| VLDL (mg/dl)* | 34 | 73 | +114 |
| Total cholesterol: HDL-C | 5.2 | 6.4 | +23 |
BMI, body mass index; BP, blood pressure; WC, waist circumference; AB, abdominal circumference; FM, fat mass; FFM, fat-free mass; Sg, glucose effectiveness; Si, insulin sensitivity; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; TG, triglycerides.
*VLDL (mg/dl) = TG − HDL − LDL.
% difference = [(Post-intervention outcomes – Pre-intervention outcomes)/Pre-intervention outcomes] × 100.
Results
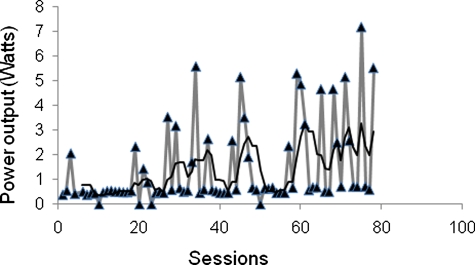
This participant completed 75 sessions over 21 weeks. Energy expenditure was 3.54 kcal/minute (Ride 1), 3.77 kcal/minute (Ride 40), and 3.98 kcal/minute (Ride 75); during this time, session power outputs increased as highlighted in Fig. 1. Physiological, body composition, and metabolic characteristics deteriorated during this same period and are outlined in Table 1. Anecdotally, the participant reported that shortly after engaging in the study his lifestyle and eating habits degenerated; he routinely ate meals that were calorically dense, high in saturated fat, and low nutrition. Table 2 lists the self-reported factors that may have caused the unexpected outcomes after 21 weeks of FES-cycling. He also reported increasing cigarette use and caffeine consumption 6–10 cups of coffee daily.
Figure 1.
Progression in power output over 75 sessions during 21 weeks of FES-leg ergometry. The black line is the trend line that represents the average of power output every five sessions. The figure clearly highlights that power output has significantly increased over the course of the training.
Table 2.
Self-report of potential factors that may have attenuated the outcomes of FES-cycling on body composition and metabolic profile
| Pain – low back pain |
|
| Spasms* |
|
| Dietary habits |
|
| Stress | Stress level has increased due to |
| •Studying for school and extra working hours | |
| •Family problems and disturbance in personal relationships | |
| •Need to cope up with the FES-training schedule for 20 weeks |
*Frequency of spasms was evaluated using the Spasms Frequency Score; 0 is no spasms; 1 is one ore fewer spasms per day; 2 is between 1 and 5 spasms per day; 3 is five to less than 10 spasms per day, and 4 is ten or more spasms per day or continuous contraction.
Discussion
The current case report has anticipated positive outcomes to FES-cycling intervention on body composition and metabolic profile. However and contrary to the proposed hypothesis, the findings showed deterioration in body composition, metabolic profiles, and highlight potential deleterious impacts despite frequent exercise. Clinicians and researchers planning to pursue similar interventional trials need to consider factors similar to increasing pain, adhere to appropriate dietary habits, and physiological stress throughout the trial. These factors were based on self-report from interaction with the participant during the course of the study. Therefore, it is extremely important to exercise caution that the current case report cannot point to a specific factor that has contributed to the observed changes, but the purpose is to provide awareness of potential emerging factors that may limit the outcomes in future investigations.
Participant's self-report
Pain is a common complaint in individuals with SCI with a prevalence that can range from 77 to 86% and interfere with activities of daily living and returning to work.11 Types of pain could be neuropathic pain, musculoskeletal pain, and visceral pain. Regardless of the type of pain, individuals with SCI have to rely on variable dosages and mixtures of analgesics to allow them to be functional. A recent study that investigated the relationship between medication prescription and ambulatory distance following incomplete SCI showed that individuals on high doses of pain medication are more likely to be limited in ambulation to less than 150 feet.14 Additionally, it is well documented that pain is associated with increasing levels of stress, anxiety, and altered sleep pattern. Stress level is higher in men with SCI compared to the general population.17 Moreover, stress level is associated with impaired immune system function and poor healing of pressure ulcers, development of obesity, metabolic diseases, and elevated levels of cortisol.18–22 Excess plasma cortisol is known to cause deterioration in body composition and metabolic profiles.20–21 Daily exercise routine has been shown to provide neurobiological stimuli to reduce stress and anxiety.25 The mechanisms have been attributed to metabolic, neuro-chemical pathways of exercising skeletal muscles, afferent feedback to the spinal cord, and the brain which offer plausible mechanisms that might help explain effects of exercise on the central nervous system.26 The absence of these mechanisms in individuals with SCI may result in FES exercise having a lesser effect on mental and psychosocial stress. Finally, reliance on high-energy high-fat diets during the course of the training is known to disintegrate the metabolic and body composition profiles.27 It is reported that a high-fat diet has the capability to evoke dyslipidemia, glucose intolerance, and negatively impact body composition.27
Positive adaptations
Despite breakdown in the physiological and metabolic profiles, there were observed increases in work output, caloric expenditure, and improved insulin sensitivity. The case report underscores that FES training of large muscles can induce increased insulin sensitivity in the absence of anticipated body composition adaptations or dietary control intervention. The observation has been recently confirmed following 12 weeks of lower extremity resistance training, which resulted in improvement in insulin profile without changes in glucose concentration in men with SCI.3 Following SCI, skeletal muscles undergo fiber-type conversion from slow to fast twitch.28 However, training can reverse this process and cause improvement in insulin sensitivity, because slower fibers are more insulin sensitive compared to fast twitch ones.28,29
Future studies
Future studies should consider the potential impact of the self-reported variables on body composition and metabolic profile in persons with SCI. It is hypothesized that controlling for pain and stress in conjunction with healthy dietary habits may improve the outcomes of training on body composition and metabolic profile. Moreover, measuring these antagonistic factors will allow accurate calculation of the true effect size of exercise on body composition and metabolic profile. For instance, twice weekly for 12 weeks of resistance training in conjunction with dietary control has superior effects compared with dietary control only on body composition and metabolic profile after SCI.3 Another important aspect is the dosing of the prescribed exercise and how pacing can attenuate these emerging negative factors. A 9-month randomized control trial twice weekly of aerobic and resistance training for 90–120 minutes has been shown to reduce the level of pain, stress, and improved quality of life in persons with SCI.30 Therefore, future trials may consider reduction of the frequency of training per week to reduce the associated stress and pain observed in the current report and to corroborate or deny the aforementioned hypothesis in persons with SCI.
Limitations
The case report relies on observation rather than formally testing the hypothesis that the aforementioned factors may have attenuated anticipated effects on body composition and metabolic profile. It is important to note that the findings are descriptive and caution should be noted not to draw general conclusion. However, we sought to share our experience to gain insight into possible factors that can alter the outcomes of FES training. Furthermore, the rehabilitation community can benefit from the outcome of this case report through anticipation of confounding factors, particularly through the careful monitoring of pain, stress, and dietary habits during future training trials involving populations with physical disabilities.
Conclusion
This case underscores the necessity of combined dietary modifications and lifestyle changes with an exercise intervention in order to reduce obesity. Moreover, regular diary records are needed to monitor participants in order to maximize intervention outcomes. This participant was engaged in an extended weight loss training trial, but due to work and school commitments was unable to adhere to study parameters. However, he was permitted to complete the study due to his commitment to finish. This observation is meant to raise awareness among researchers and clinical specialists of the importance of considering environmental, social, and physical factors when designing longitudinal exercise interventions among individuals with SCI. Future studies are warranted to investigate the factors that may impede exercise improvement to cardiovascular risk factors following SCI.
Acknowledgements
The project described was supported by VHA RR&D #B3918R, VHA RR&D #B6757R, and award number UL1RR031990 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Veterans Health Administration, National Center for Research Resources, or the National Institutes of Health.
References
- 1.Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med 2010;33(1):90–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gater DR, Jr., Dolbow D, Tsui B, Gorgey AS. Functional electrical stimulation therapies after spinal cord injury. Neuro Rehabilitation 2011;28(3):231–48 [DOI] [PubMed] [Google Scholar]
- 3.Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165–74 [DOI] [PubMed] [Google Scholar]
- 4.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22 [DOI] [PubMed] [Google Scholar]
- 5.Gater DR., Jr. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18(2):333–51 [DOI] [PubMed] [Google Scholar]
- 6.Carlson KF, Wilt TJ, Taylor BC, Goldish GD, Niewoehner CB, Shamliyan TA, et al. Effect of exercise on disorders of carbohydrate and lipid metabolism in adults with traumatic spinal cord injury: systematic review of the evidence. J Spinal Cord Med 2009;32(4):361–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56 [DOI] [PubMed] [Google Scholar]
- 8.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr., Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 9.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46(7):466–76 [DOI] [PubMed] [Google Scholar]
- 10.Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab 2011;36(1):107–14 [DOI] [PubMed] [Google Scholar]
- 11.Donnelly C, Eng JJ. Pain following spinal cord injury: the impact on community reintegration. Spinal Cord 2005;43(5):278–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord 2006;44(12):708–22 [DOI] [PubMed] [Google Scholar]
- 13.Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord 2005;43(10):577–86 [DOI] [PubMed] [Google Scholar]
- 14.Kohout RK, Saunders LL, Krause JS. The relationship between prescription medication use and ability to ambulate distances after spinal cord injury. Arch Phys Med Rehabil 2011;92(8):1246–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terao Y, Miura K, Saito M, Sekino M, Fukusaki M, Sumikawa K. Quantitative analysis of the relationship between sedation and resting energy expenditure in postoperative patients. Crit Care Med 2003;31(3):830–3 [DOI] [PubMed] [Google Scholar]
- 16.McCall M, Jeejeebhoy K, Pencharz P, Moulton R. Effect of neuromuscular blockade on energy expenditure in patients with severe head injury. JPEN J Parenter Enteral Nutr 2003;27(1):27–35 [DOI] [PubMed] [Google Scholar]
- 17.Tate DG, Maynard F, Forchheimer M. Predictors of psychologic distress one year after spinal cord injury. Am J Phys Med Rehabil 1993;72(5):272–5 [DOI] [PubMed] [Google Scholar]
- 18.DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol 1988;54(3):486–95 [DOI] [PubMed] [Google Scholar]
- 19.Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS. Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med 2000;23(2):129–35 [DOI] [PubMed] [Google Scholar]
- 20.Raikkonen K, Keltikangas-Jarvinen L, Adlercreutz H, Hautanen A. Psychosocial stress and the insulin resistance syndrome. Metabolism 1996;45(12):1533–8 [DOI] [PubMed] [Google Scholar]
- 21.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev 2001;2(2):73–86 [DOI] [PubMed] [Google Scholar]
- 22.Chrousos GP, Gold PW. A healthy body in a healthy mind – and vice versa – the damaging power of uncontrollable stress. J Clin Endocrinol Metab 1998;83(6):1842–5 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal Cord 2006;44(2):82–91 [DOI] [PubMed] [Google Scholar]
- 24.Groah SL, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med 2009;32(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc 1997;29(1):63–74 [DOI] [PubMed] [Google Scholar]
- 26.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–56 [DOI] [PubMed] [Google Scholar]
- 27.Buckley AJ, Keserü B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism 2005;54(4):500–7 [DOI] [PubMed] [Google Scholar]
- 28.Kriketos AD, Pan DA, Lillioja S, Cooney GJ, Baur LA, Milner MR, et al. Interrelationships between muscle morphology, insulin action, and adiposity. Am J Physiol 1996;270(6):R1332–9 [DOI] [PubMed] [Google Scholar]
- 29.Zierath JR, He L, Guma A, Odegoard WE, Klip A, Wallberg HH. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 1996;39(10):1180–9 [DOI] [PubMed] [Google Scholar]
- 30.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 2003;41(1):34–43 [DOI] [PubMed] [Google Scholar]