Abstract
Objectives
The objective of the study was to evaluate the safety and tolerance of use of the ReWalk™ exoskeleton ambulation system in people with spinal cord injury. Measures of functional ambulation were also assessed and correlated to neurological spinal cord level, age, and duration since injury.
Study design
Case series observational study.
Setting
A national spinal cord injury centre.
Methods
Six volunteer participants were recruited from the follow-up outpatient clinic. Safety was assessed with regard to falls, status of the skin, status of the spine and joints, blood pressure, pulse, and electrocardiography (ECG). Pain and fatigue were graded by the participants using a visual analogue scale pre- and post-training. Participants completed a 10-statement questionnaire regarding safety, comfort, and secondary medical effects. After being able to walk 100 m, timed up and go, distance walked in 6 minutes and 10-m timed walk were measured.
Results
There were no adverse safety events. Use of the system was generally well tolerated, with no increase in pain and a moderate level of fatigue after use. Individuals with lower level of spinal cord injury performed walking more efficiently.
Conclusion
Volunteer participants were able to ambulate with the ReWalk™ for a distance of 100 m, with no adverse effects during the course of an average of 13–14 training sessions. The participants were generally positive regarding the use of the system.
Keywords: Spinal cord injuries, Paraplegia, Rehabilitation, Ambulation, Assistive technology, Exoskeleton, ReWalk™
Introduction
Few conditions are as traumatic or disabling to an individual and society as a spinal cord injury (SCI). Paralysis, loss of sensation, respiratory disturbances, incontinence, sexual dysfunction, spasticity, and pain are among the main potential sequelae.
Being dependent on a wheelchair for mobility is often at the forefront of the concerns of both the individual with SCI and providers of rehabilitation treatment. During the era of modern rehabilitation, multiple compensatory techniques have been developed as potential substitutes for the residual neurological deficits that prevent a return to upright bipedal ambulation. Mechanical, electrical stimulation and exoskeleton devices as well as combinations of these techniques have been used. Examples of orthotics include knee ankle foot orthosis (KAFO) and reciprocating gait orthosis (RGO),1 functional electrical stimulation (FES),2 RGO and FES,3 robots and powered exoskeletal systems.4–6 Factors such as the severity of the SCI (level and completeness), age of the individual, medical/physiological/psychological status, social and economic status all influence the ability to benefit from these types of devices. However, even when all of the factors are positive, the use of such systems is most often for therapeutic purposes rather than for functional walking.
The ReWalk™ (Argo Medical Technologies Ltd., Yokneam Ilit, 20692, Israel) (Figs. 1 and 2) is a unique exoskeletal robotic device that utilizes the user's movements to control externally powered gait. This study was designed to assess the safety, tolerance, and ease of use of this system. Secondary outcome measures were tests of gait performance.
Figure 1.
ReWalk™.
Figure 2.
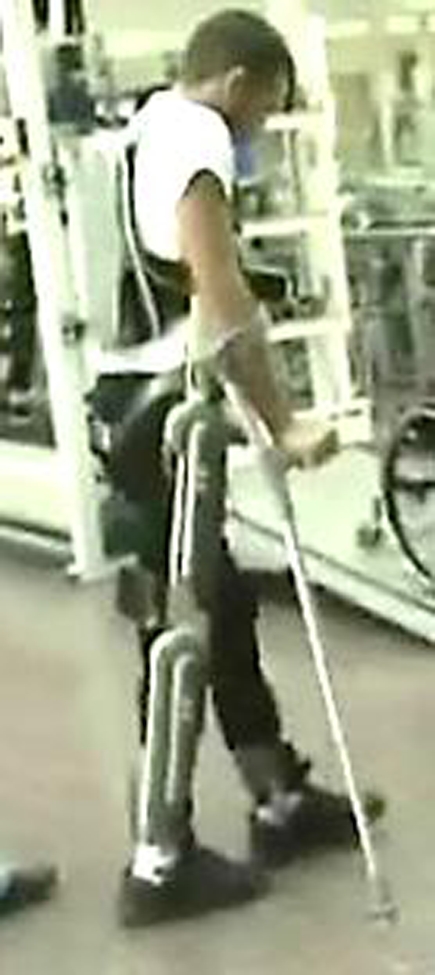
Walking with the ReWalk™
Population
Inclusion criteria were male or non-pregnant, non-lactating female aged 16–70 years, less than 100 kg in weight, and height from 155 to 200 cm, with complete motor, American Spinal Injury Association (ASIA) Impairment Scale A–B; cervical (C7–C8) or thoracic (T1–T12) SCI according to ASIA guidelines;7,8 and at least 6 months since injury. In order to demonstrate orthostatic tolerance to upright posture, all participants had to have been a regular user of a RGO or therapeutic standing frame.
Exclusion criteria included: history of severe neurological disorder other than SCI (multiple sclerosis, cerebral palsy, amyotrophic lateral sclerosis, traumatic brain injury, stroke, etc.); concurrent severe medical disease; pressure sores; unstable spine, unhealed limb or pelvic fractures; and psychiatric or cognitive status that may interfere with the trial.
Methods
The protocol for the study was approved by the hospital's Helsinki Committee. The volunteer participants were recruited from a SCI outpatient follow-up clinic. The one-tailed t test was used to compare the gait performances of the participants according to the level of lesion, age, and time from injury. Population was grouped by medians. In addition, two-tailed Pearson coefficients were calculated in order to explore the correlations between the variables.
The device (ReWalk™)
The ReWalk™ comprised a motorized exoskeleton, a battery unit, and a computer-based controller contained in a backpack, a wireless mode selector, and an array of sensors that measure upper-body tilt angle, joint angles, and ground contact. There is a built-in backup system for both the battery and the main computer. The exoskeleton has bilateral lateral uprights for the thigh and leg, hinged knee joints, and is articulated to foot plates distally and to a sacral band proximally.
It uses a closed-loop algorithm software control. The motors control the movements at the hip and knee joints, but not the ankles that are articulated using a mechanical joint with spring-assisted dorsiflexion. When in the ‘walk’ mode, forward flexion of the upper body is detected by the tilt sensor and triggers a step. The resulting gait is a three-point pattern, advancing one step at a time.
There are four additional modes: sit-stand, stand-sit, up steps, and down steps. The maximal walking velocity is 0.6 m/second (2.2 km/hour).
As the activation of movements is under the voluntary control and initiation of the user, the device is inherently safer than a pure robotically driven control. Software design prevents rapid hip and knee flexion as may occur in a fall, and provides a controlled speed stand to sit. A manual mode allows for adjustment of the position of the lower limbs. User stability and safety during standing and ambulation are achieved by concurrent use of walking aids such as crutches, walker, and/or railings for stair climbing.
Training
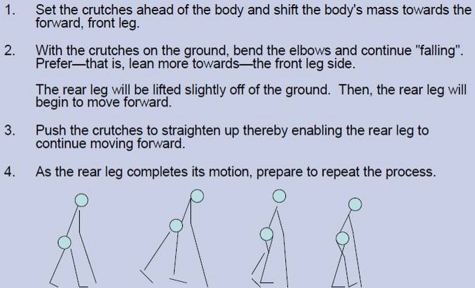
The participants were initially provided with both the technical information of the device as well as written instructions on how it is to be used and activated. The device was then fitted and adjusted to each participant. The education and fitting took approximately 30 minutes per participant. Training then proceeded under the direction of a physical therapist. The gait training progressed from parallel bars to walker to crutches. The unique technique of crutch walking with the ReWalk™ was taught to the participants as illustrated in Fig. 3.
Figure 3.
Technique of walking with the ReWalk™.
The participants also worked on sit-stand and stand-sit and learned to use the various modes of the system. Testing was done once they were able to walk unassisted with crutches for 100 m.
The training process during this pilot study was dynamic and evolved during the course of the study. As an example, kinematic data during walking with ReWalk™ were collected from two participants in the study using a three-dimensional motion tracking CODA CX1 active marker sensor system. The data were compared with velocity-matched normative data from the MossRehab Gait & Motion Analysis Laboratory9 and used to refine the computer algorithm to synchronize motion between the hip and the knee to achieve a more natural gait pattern.
A number of the participants were initially skeptical that they would be able to use the device, and others were concerned about the external controls. These concerns were addressed with a demonstration of walking with the system by a person with paraplegia who was already trained in the use of the device.
Data collection
The following parameters were monitored: incidence of falls, status of the skin, status of the spine and joints, blood pressure, pulse, and electrocardiography (ECG). The spine X-rays that included the original injury site and/or fixation, as well as ECGs were done before and after completing the study. For each session, baseline and post-training measurements were done for pulse, blood pressure, and skin and joint integrity. Visual analogue scales (VASs) for pain10 and fatigue were also measured pre- and post-training session.
The participants were asked to respond to 10 statements concerning the use of the device. They provided their subjective opinion by indicating in a Likert scale the number that best represents how they feel: (1) strongly disagree, (2) disagree, (3) somewhat agree, (4) agree, and (5) strongly agree. All of the statements were phrased in a positive manner regarding the training process, comfort, safety of use, and medical issues (pain, spasticity, bowel movements, breathing).
The number of training sessions required to reach the ability to walk 100 m unassisted on a level surface with crutches was recorded.
The following tests were done at the completion of the training: timed up and go (TUG); distance in a 6-minute walk, and a 10-m timed walk.18
Results
Eight male participants were recruited for the study. Six completed the study. Both of the drop outs were due to transportation logistics, i.e. distance from training centre or lack of transportation. There were several technical problems related to the device (sensor and connector failure, etc.) that caused no adverse effects and were subsequently corrected.
The average age of the participants was 33.2 years (SD = 10.5); ranging from 21 to 48 years old; the average time from injury was 5 years (SD = 1.3). The levels of neurological injury were relatively evenly spread from T5 to T12.
Safety
There were no falls, skin or joint injuries, cardiovascular episodes, or changes on spine radiographs. Changes in blood pressure and pulse rate were typical for physical activity, without reaching abnormal levels. The fatigue level after the activity was considered to be moderate, as defined by the use of a VAS fatigue scale of 1 to 10. The volunteers did not report any increased pain after use.
Results are summarized in Table 1.
Table 1.
Average and standard deviation of cardio-vascular, pain and fatigue pre- and post-training sessions
| Pre-training blood pressure (mmHg) | Post-training blood pressure (mmHg) | Pre-training heart rate (beats/minute) | post-training heart rate (beats/minute) | Pre-training pain (VAS) | post-training pain (VAS) | Pre-training fatigue (VAS) | Post-training fatigue (VAS) | |
|---|---|---|---|---|---|---|---|---|
| Average | 121/77 | 129/83 | 68 | 92 | 1.77 | 1.71 | 1 | 4.6 |
| SD | 1.43/7.4 | 4.09/7.4 | 7.36 | 17.97 | 0.92 | 1.02 | 3.32 | 2.02 |
Performances
Participants’ demographics and performances are shown in Table 2. They required an average of 13.7 training sessions (SD = 5.8) to achieve readiness for the walking tests. Each session lasted for an average of 50 minutes.
Table 2.
Demographics and performances
| Age | Time from injury (years) | NLOI* | TUG† (seconds) | Six minutes (m) | 10 m (seconds) | Number of training sessions to testing | |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 3 | T10 | 107 | 72 | 44 | 18 |
| 2 | 47 | 6 | T7 | 90 | 35 | 67 | 24 |
| 3 | 48 | 7 | T12 | 72 | 72 | 42 | 13 |
| 4 | 21 | 5 | T5 | 98 | 30 | 87 | 12 |
| 5 | 30 | 4 | T9 | 80 | 55 | 55 | 8 |
| 6 | 25 | 5 | T7 | 156 | 18 | 103 | 7 |
*Neurological level of injury.
†Timed up and go.
The test phase included three tasks: TUG, number of metres walked in 6 minutes, and the time needed to walk 10 m. On average, it took the participants 101 seconds (SD = 27.3) to complete the TUG task and 66 seconds (SD = 22.3) to complete the 10-m walk. The average distance walked during 6 minutes was 47 m (SD = 20.8). The three participants with lower lesions (T9–T12), walked longer distances (mean 66.3 m (SD = 8)) than the three with higher lesions (T5–T7) (mean, 22.7 m (SD = 7)) (P < 0.01). In the 10-m walk test, participants with lower levels of injury walked faster (mean 47 seconds (SD = 5.8)) than those with higher levels of injury (mean 85.7 seconds (SD = 14.7)) (P < 0.05). The level of injury did not influence the results in the TUG test or the number of training sessions needed to be ready for the tests (t(4) = 1.2 (P = 0.15) and t(4) = 0.23 (P = 0.42)), respectively. In this small sample, age and time from injury did not influence any of the test measures.
In addition, we calculated two-tailed Pearson correlations between the variables. Level of lesions was correlated with test measures of distance completed in 6 minutes, r(6) = −0.88, (P < 0.05) and time to complete 10 m (r(6) = −0.83 (P < 0.05)). The later two were highly correlated one to each other (r(6) = −0.96 (P < 0.01)).
Satisfaction questionnaire
The statements and responses are listed in Table 3. We considered responses averaging 1–2 as clearly disagreeing with the statement, 2–3 tending to disagree, 3–4 tending to agree, and 4–5 clearly agreeing. The participants showed a tendency towards positive feelings regarding the training process (statements 1 and 3). Regarding the medical issues, the participants clearly agreed that the use did not cause considerable pain or breathing problems, tended to agree that spasticity was diminished and that there was no excessive fatigue. However, they tended towards no improvement in their bowel programmes. The participants did clearly feel safe and comfortable with the device at the end of the training (numbers 6 and 10). In contrast, they tended to feel that wearing or/ adjusting the device was not relatively simple.
Table 3.
Satisfaction questionnaire
| Statement | Likert scale AV (SD) | |
|---|---|---|
| 1 | Training/learning to use the device is not complicated | 3.00 (1.63) |
| 2 | Wearing/adjusting the device is relatively simple | 2.57 (1.51) |
| 3 | It was comfortable to exercise with the device | 3.71 (1.38) |
| 4 | The usage of the device did not cause considerable pain | 4.71 (0.49) |
| 5 | I did not feel excessive fatigue while exercising with the device | 3.57 (1.51) |
| 6 | After completing the training period I felt comfortable using the device | 4.29 (0.95) |
| 7 | Training with the device diminished spasticity in my legs | 3.71 (1.50) |
| 8 | I did not have breathing difficulties while training with the device | 4.86 (0.38) |
| 9 | I felt improvement in my bowel movement during the training program | 2.86 (1.35) |
| 10 | After completing the training I felt safe using the device | 4.29 (0.76) |
AV, average.
Discussion
Safety is the primary concern when a new device for mobility is introduced for individuals with SCI. In this pilot study, the ReWalk™ was shown to be safe and well tolerated. There were no significant complications related to cardiovascular stresses, fatigue, excessive skin pressure, pain, or musculoskeletal problems.
Previous studies involving training with active (RGO, FES) or passive (Lokomat) devices did not specifically address the issue of excessive skin pressure. This may be due to the common occurrence of signs of excessive pressure from externally applied devices requiring fitting and adjustment, and therefore, was not felt to be worthy of study or report. Nevertheless, attention to potential skin problems in a prospective manner is mandatory when evaluating a new type of external device designed for functional walking.
There were no falls during the course of this study. In our review of the literature, we were able to find only a single study, of an FES system that did not have an orthotic or exoskeletal component, which included information on falls. In that study of three participants, four falls were reported.11 We did not find any specific mention of falls or the prevention of falls in studies of the RGO or in other walking assistance devices.
The Satisfaction Survey that we used was graded with a Likert scale that had five points, including the mid-point of ‘somewhat agree’. In the analysis, this midpoint was taken as the dividing line between a favourable response versus a non-favourable response; with the two sides being symmetrical and balanced. Overall, the participants were satisfied with the device other than the previously mentioned relative difficulty with wearing and adjusting the device.
There were no problems related to increased pain. One participant with chronic high-level neuropathic pain at baseline (VAS 8–9 at rest), showed a repeated improvement of 4–6 points on the VAS after each training session. This is similar to the findings reported by others that partial weight-bearing treadmill training has a beneficial effect on neuropathic pain.12 In that study of rodents with induced incomplete SCI with neuropathic pain, exercise training while weight bearing significantly reduced pain. The authors’ hypothesis was that this type of training decreased the neuropathic pain by normalizing the brain-derived neurotrophic factor mRNA levels in the cord and periphery. If that hypothesis is correct, we assume that the same mechanism would likely be the cause for the improvement in this individual's neuropathic pain.
The volunteer participants reached the level of being able to walk 100 m with crutches and proceeding with the test after an average of 13–14 sessions. However, they still had not attained the proficiency for functional daily use of the device. As the ReWalk™ requires subject input, achieving proficiency necessitates assimilation of user capabilities into the control system. The human user interface results in a unique pattern of control for walking in individuals with SCI. As expected, those with lower levels of SCI had better walking performances and also progressed more rapidly to being ready for testing.
The motor learning process was dependent on the amount of practice, with all of the subjects progressing in developing this cognitive–motor skill. The limited number of sessions needed to achieve walking 100 m was not sufficient to allow the individuals to develop the skill for more rapid walking. Furthermore, although motor learning of new skills is a normal process, it has been suggested that there may be factors that inhibit the process in individuals with SCI. In a study of individuals with SCI, several abnormal features of brain activation were detected, such as not modulating brain activity with change in task load or type. These findings may indicate that people with paraplegia have altered motor cortex function, possibly making motor learning more difficult,13 and perhaps implicating the need for specially tailored training programmes including for individuals learning to use the ReWalk™.
The use of the ReWalk™ as a training tool to induce neuronal activity of the central pattern generator (CPG) circuits may be feasible. Studies on spinal cord plasticity after SCI have shown that gait CPG can be induced by specific gait training programmes.14 The use of existing devices (Lokomat,15 Gait Trainer,16 etc.) for passive walking with body weight support is thought to induce the CPG; however, there is no functional component to the training. The individual with incomplete, but severe SCI, using the ReWalk™ would achieve the same goal with active, functional walking.
One of the major obstacles for functional use of the commercially available ambulation devices for paraplegics (e.g. RGO, KAFO) is the high-energy demands imposed. In an Italian survey, the RGO was abandoned for functional walking by 43 of 74 users primarily due to the high-energy costs.17 In our clinical experience of more than 20 years of following individuals using the RGO, the percentage that continue functional walking is even less than in the above study.
The issues of energy demands and therapeutic versus functional walking must be addressed in further studies with the ReWalk™.
Limitations
The potential benefits of the ReWalk™ are many, but efficacy still needs to be demonstrated in a larger study. Among the potential benefits are improved functional mobility, cardio-vascular and respiratory status, bone metabolism, and bowel and bladder function, as well as reduction of spasticity and neuropathic pain. Our study did not include any female participants, individuals with tetraplegia, children, or older adults. Future large-scale inclusive studies are needed.
Conclusion
Although the ReWalk™ was designed specifically for people with SCI, this technology may potentially be adapted in the future for others with walking dysfunction from stroke, cerebral palsy, myelomeningocele, traumatic brain injury, and Guillain Barré syndrome.
Acknowledgement
This study was financially supported by Argo Medical Technologies Ltd.
References
- 1.Winchester PK, Carollo JJ, Parekh RN, Lutz LM, Aston JW., Jr. A comparison of paraplegic gait performance using two types of reciprocating gait orthoses. Prosthet Orthot Int 1993;17(2):101–6 [DOI] [PubMed] [Google Scholar]
- 2.Stallard J, Major RE. A review of reciprocal walking systems for paraplegic patients: factors affecting choice and economic justification. Prosthet Orthot Int 1998;22(3):240–7 [DOI] [PubMed] [Google Scholar]
- 3.Kobetic CS, To JR, Schnellenberger ML, Audu TC, Bulea R, Gaudio G, et al. Development of hybrid orthosis for standing, walking, and stair climbing after spinal cord injury. J Rehabil Res Dev 2009;46(3):447–62 [PubMed] [Google Scholar]
- 4.Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev 2000;37(6):693–700 [PubMed] [Google Scholar]
- 5.Banala S, Kim S, Agrawal SK, Scholz JP. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst Rehabil Eng 2009;17(1):2–8 [DOI] [PubMed] [Google Scholar]
- 6.Banala S, Agrawal SK, Fattah A, Krishnamoorthy V, Hsu WL, Scholz JP, et al. Gravity-balancing leg orthosis and its performance evaluation. IEEE Trans Robot 2006;22(6):1228–37 [Google Scholar]
- 7.American Spinal Injury Association/International Medical Society of Paraplegia International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients. Chicago, IL: American Spinal Injury Association/International Medical Society of Paraplegia; 2000 [Google Scholar]
- 8.Waring WP, Biering-Sorenson F, Burns S, Donovan W, Graves D, Jha A, et al. 2009 review and revisions of the International Standards for the Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2010;33(4):346–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esquenazi A, Talaty M. Gait analysis: technology and clinical application. In: Braddom RL. (ed.) Physical medicine and rehabilitation. 3rd edn. Philadelphia, PA: Saunders, Elsevier Inc.; 2007. Chapter 5, p. 93–110 [Google Scholar]
- 10.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 2001;8(12):1153–7 [DOI] [PubMed] [Google Scholar]
- 11.Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisné D, Beillot J, et al. Clinical experience with functional electrical stimulation-assisted gait with parastep in spinal cord-injured patients. Spine 2000;25:501–8 [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004;127:1403–14 [DOI] [PubMed] [Google Scholar]
- 13.Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain function after spinal cord injury. Brain 2005;128:2941–50 [DOI] [PubMed] [Google Scholar]
- 14.Molinari M. Plasticity properties of CPG circuits in humans: impact on gait recovery. Brain Res Bull 2009;78:22–5 [DOI] [PubMed] [Google Scholar]
- 15.Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey K, et al. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair 2005;19:313–24 [DOI] [PubMed] [Google Scholar]
- 16.Hesse S, Uhlenbrock DA. Mechanized gait trainer for restoration of gait. J Rehabil Res Dev 2000;37(6):701–8 [PubMed] [Google Scholar]
- 17.Franceschini M, Baratta S, Zampolini M, Loti D, Lotta S. Reciprocating gait orthoses: a multicenter study of their use by spinal cord injured patients. Arch Phys Med Rehabil 1997;178:582–6 [DOI] [PubMed] [Google Scholar]
- 18.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil 2005;86:190–6 [DOI] [PubMed] [Google Scholar]