Abstract
Malignant pleural mesothelioma (MPM) is an aggressive neoplasm with a poor prognosis. MPM grows from the mesothelial cells lining the surface of the lung and chest wall called Pleura. Exposure to asbestos is mainly linked to the development of MPM. Approximately 80% of the tumors are pleural in origin, and up to 3000 people are diagnosed with MPM in the United States annually. The incidence of MPM is expected to rise in the coming decades particularly in the developing countries. Although there is an increase in the awareness of danger associated with the use of asbestos, its use is still prevalent in Australia and Asia because of its durability and low cost. This further warns and adds to the mortality and morbidity of patients with MPM globally. The traditional treatment strategies have shown only modest improvement towards the disease. MPM is difficult to treat because of the fact that the time between the exposure to asbestos and the appearance of symptoms is extremely delayed, and also due to tumor involvement with the pleural surface and the adjoining tissues such as the chest wall, pericardium and subdiaphragmatic organs. Despite advances in the diagnostic and treatment approaches the median survival rate for MPM is between 9 to 17 months. The standard care with double agent has shown modest improvement however, multimodality approach using novel targets may have potential to achieve the improvement in the survival rate. In this review we give an update on the conventional treatment modalities and discuss about various molecular targets including receptor EphA2, a novel target gene which may be considered as a biomarker for the diagnosis and treatment of MPM.
Keywords: Receptor EphA2, EphrinA1, malignant pleural mesothelioma, receptor tyrosine kinases, asbestos, surgery, chemotherapy, gene therapy
Introduction
Although 50 years have been passed since the discovery of first incidence of MPM, treatment of this neoplasm continues to be a challenge till to date. The diagnosis of patients with MPM remains extremely poor despite several advancements in the strategies and techniques. MPM is an aggressive tumor of the pleura associated with asbestos exposure. Besides asbestos exposure, radiation, simian virus 40 and genetic predisposition to mineral fiber are also considered as causative agents [1-3]. The incidence of MPM is expected to peak globally particularly in Europe and the other developing countries [4-6]. In Western Europe more than 5,000 new cases are diagnosed annually and a death toll of more than quarter million is expected in next 40 years [7]. In Japan peak incidence of death is expected due to MPM in 2025 [8]. In Australia highest incidence of MPM have been reported and the incidence continues to rise with expected peak in 2021 [9]. In India, China, Indonesia and Vietnam since the use of asbestos containing material is still very common, it is predicted that the global burden of MPM is expected to mount in the coming decades [5, 10].
MPM is generally a disease of advance age, the median age at diagnosis ranges from 45 to 85 years in the United States. Because of occupational exposure the incidence is more in men than women (5:1 ratio). MPM is difficult to diagnose and often requires experienced pathologist to differentiate MPM from other benign tumors. MPM is classified into 3 major sub-types based on histology: epithelioid, sarcomatoid, and mixed or biphasic. The epithelioid tumors are most common type and represents of 50% to 70% of all MPM diagnosed. The epithelioid tumor cells are usually more uniform, cuboidal and have easily identifiable nuclei. It is least aggressive and responds to the treatment better compared to sarcomatoid and mixed phenotypes [11]. The sarcomatoid tumors are less common, and it comprises of 7% to 20% of cases diagnosed. It is the most aggressive subtype and most difficult to treat. Normally the cells are spindle shaped with elongated nuclei. Whereas, the mixed subtype as the name implies consists of the combination of both the epithelial and sarcomatoid cells. The mixed subtype makes up about 20% to 35% of mesothelioma cases [8, 11]. The treatment options for MPM depend on the stage of the cancer early or advanced and the patient's age. The options available are: Surgical resection along with, chemotherapy, radiation therapy, immuno-therapy, and gene therapy. However, limitation to the studies such as small sample size, nonrandomized trial, patient selection bias etc. always exits and would have potential effect on the outcome of studies.
Surgery
Surgical treatment for MPM has been controversial. Pleurectomy or palliation surgery is considered as the best option for the treatment of early stage disease. MPM is associated with the development of pleural effusions and palliation is recommended to relieve the symptoms such as dyspnea and chest pain. A common palliation therapy is insufflation of talc as a sclerosing agent into the pleural space to obliterate the pleural cavity in order to prevent reaccumulation of pleural fluid. In some cases talc pleurodesis is combined with video assisted thoracoscopic surgery (VATS) to debulk/decortications of the pleural tumor tissue to control the symptoms with minimal mortality [12, 13]. Radical resection or extrapleural pneumonectomy (EPP) is used at some centers, for the localized disease control but it cannot influence the distant recurrence [14]. EPP have shown improved mortality and morbidity [15, 16]. Surgery alone cannot guarantee the complete resection of tumor suggesting MPM progress in spite of local control. About 54% recurrences have been noticed after EPP at the median interval of 19 months [17, 18]. Albeit surgical techniques currently used to debulk the tumor have been dramatically improved but the treatment of MPM is still difficult. At the main centers, EPP or pleurectomy along with adjunctive therapies such as chemotherapy, immuno-therapy and radiotherapy is most commonly performed for MPM patients for the long-term survival benefit and with acceptable rate of morbidity and mortality [19].
Chemotherapy
Chemotherapy regimens used against MPM has not proven effective till to date because MPM is often resistant to chemotherapy. Chemotherapy in mesothelioma patients is as an option for both patients with unresectable tumors and for patients with resectable tumors. Chemotherapy is provided as single agent or in most of the cases, combination of chemotherapy with neoadjuvent or adjuvant is recommended. Drugs used for the treatment of MPM are classified as: 1) Alkylation agents; 2) Anti-metabolites; 3) Anthracyclines; 4) Platinum Compounds; and 5) Plant alkaloids. Alkylating agents are the earliest chemotherapeutic drugs used to treat cancer. Cyclophosphamide, Ifosfamide, Mitomycin, Mechlorethamine, Thiotepa, and Meiphalan are among the drugs commonly used for treating solid tumors [20-22]. These drugs work directly on DNA and prevent cell division process by cross-linking the DNA strands and causing abnormal base pairing. However, these drugs are toxic and affects cardiac and renal functions and long term use may cause secondary cancers [23, 24]. Combination of Cyclophosphamide with cisplatin was well tolerated for metastatic MPM however; the survival rate was not satisfactory [21].
Anti-metabolites, such as Gemcitabine and Pemetrexed are called folate based drugs. Pemetrexed inhibits the function of enzymes used in purine and pyrimidine synthesis, thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). Pemetrexed prevents the formation of DNA and RNA by inhibiting the formation of precursor for purine and pyrimidine, which are required for the growth and survival of both normal cells and cancer cells [25]. The median survival is 10.3 months after the treatment [26]. Low blood cell counts, mental fatigue, nausea, vomiting, and oral mucositis were noted in patients treated with folate based drugs [27]. In a phase III trial, Pemetrexed plus Cisplatin showed improved survival compared to Cisplatin alone for patients with MPM [28].
Anthracycline drugs, such as Doxorubicin, Epirubicin, and Pirubicin are known to interact with DNA by intercalation and inhibition of macromolecular biosynthesis. These drugs act as antitumor agents by inhibiting the progression of the enzyme topoisomerase-II, which relaxes super coils in DNA for transcription. Doxorubicin stabilizes the topoisomerase-II complex after it has broken the DNA chain for replication, preventing the DNA double helix from being resealed and thereby stopping the process of replication in cancer cells [29, 30]. In a phase II trial, combination of Doxorubicin and Mitomycin gave a superior response when compared to Doxorubicin and Cisplatin in phase-II study [31], and about 29% of improved response was observed. In vitro studies with mesothelioma cell line showed synergistic effect with combination of Mitomycin and Cisplatin. Combined Cisplatin and Gemcitabine phase-II trial for advanced MPM resulted in the improvement of the symptoms in the patients who responded to the treatment [32-34]. A multi-center phase-II study used two active regimens in a sequential order as a first line chemotherapy for unresectable MPM. The regimens included Cisplatin, Gemcitabine followed by Mitoxanthrone/Methotrexate/Mitomycin. This trial yielded good results, disease control with improved morbidity [35]. The toxicities were restricted to hematological parameters such as neutropenia, anemia, thrombocytopenia, and vomiting.
Platinum based drugs includes, Cisplatin, Carboplatin, and Oxaliplatin [36]. These drugs bind to DNA of the neoplastic cell and induce programmed cell death. The binding affects both replication and transcription of DNA, as well as mechanisms of DNA repair. Chemotherapy with dual agents produced higher response rate compared to single agent therapy. The combination of cisplatin with pemetrexed yielded significantly longer overall survival (12.1 verses 9.3 months) in a clinical trial of 456 MPM patients with improved quality of life [33, 37]. In addition, patients who received Folate and vitamin B12 supplement had improved survival rate, and lesser toxicity [38]. Moreover greater mean number of cycles of therapy were performed on the patients when compared to group that did not receive supplements. This regimen has become the standard of care and the first line treatment for MPM in advance stage [28]. Nephrotoxicity, neurotoxicity, nausea, vomiting, and electrolyte disturbance were some of the toxic effects noticed with cisplatin based drugs [39]. Plant alkaloids, drugs such as Paclitaxel, Docetaxel are mitotic inhibitors, that affect the formation of microtubules during cell division between metaphase and anaphase, preventing further cancer cell progeny [40]. Paclitaxel also induces programmed cell death or apoptosis [41]. Single agent therapy had limited benefits for the MPM patients. In vitro studies on MPM cell lines with Docetaxel and Paclitaxel indicated that these drugs induced DNA single-strands breaks and cell death [42]. A phase-II trial with Docetaxel produced disappointing results [43, 44]. However, a randomized trial conducted for five years using trimodality chemotherapy (Paclitaxel + Carboplatin) and radiotherapy approach became an acceptable option for the patients for MPM for early stage tumors [45]. The combination of Docetaxel with CPT-11 for stage III- IV MPM patients showed survival of 8.5 months, and the toxicity was noticed in 7 of 15 patients along with neutropenic fever, and diarrhea [46].
Radiation therapy
The treatment of MPM by radiotherapy has been limited, because of its diffuse nature of expansion on the pleural surface. The sensitivity of MPM to radiotherapy has been very modest and the effective doses used would harm the adjacent vital tissues [47]. However, the postsurgical radiotherapy has been proven effective, as it prevented tumor cell seeding at the wound site [48]. The most recent and effective radiotherapy technique used is the intensity modulated radiotherapy (IMRT), in patients who underwent extra pleural pneumonectomy [49]. IMRT might have the potential to cover the dose related concerns and this has been applied with no toxicity in patients with hemithorax [50]. The combined use of radiotherapy and extra pleural pneumonectomy in clinical practice have proven successful to manage local tumor however, patients tend to die with metastatic disease. Hence the outcome of radiation therapy has been disappointment in the management of MPM.
Immunotherapy
MPM patients have been subjected to immunotherapy with limited success. Several animal studies were conducted and large body of evidence indicates that immunotherapy resulted in substantial tumor regression in mouse models of MPM [51, 52]. We earlier reported that antibodies to Interleukin-8 (IL-8) significantly inhibited the tumor growth in a nude mouse model of MPM [53]. The administration of Interleukine-2 showed significant reduction in tumor size and improved survival rate at the initial stage in a mouse model of MPM. However, at the late stage with larger tumors IL-2 failed to inhibit tumor growth [54]. The cytokine therapy involving interleukin-2 or interferon gamma induce a weak response in patients with early stage of disease however, the therapy failed in advanced stage disease of MPM [55, 56]. IFN-α/β induce anti-proliferative effect in tumor cells and modulates CD4+ and CD8+ T cells during initial phase of antigen recognition [57]. A continuous delivery of recombinant granulocyte-macrophage colony stimulating factor (GM-CSF) induced several part responses despite procedure related difficulties [58]. Immuno-modulatory antibodies against B7-H3 a member of B7 family showed promising response in epithelial phenotype [59]. The combination of immunotherapy (IFN-γ) with chemotherapy (Methotrexate) for stage-I and -III disease has shown promising results, with a median survival rate of 17 months and acceptable toxicity [60]. Although, moderate success have been achieved with immunotherapy in management of MPM patients, it is possible that in future certain target cytokine or chemokines may emerge as an effective candidate against MPM and may be included in the standard of care procedures list.
Mesothelioma biomarkers
Till to date, the prognosis of MPM is extremely poor. The available treatment strategies are limited. Identification of a biomarker for the diagnosis may help improve the diagnosis and benefit MPM patient's therapy. Several proteins have been designated as biomarkers for the diagnosis of MPM (Table 1).
Targeted therapy
Conventional therapies have failed to show the improvement in survival of the MPM patients. Treatment remains toxic affecting normal tissues along with tumor tissue. Besides, the selection of patients at appropriate stage of the disease is not easy. Recently several novel biomarkers have been identified which are expressed mostly by tumor tissue and not being expressed by the surrounding normal tissue. The expanding knowledge on molecular mechanisms of the development of mesothelioma and the newer technologies helped identify the novel biomarkers which are considered as potential candidates for MPM therapy are discussed.
The epidermal growth factor receptor
Epidermal growth factor (EGFR) belongs to the ErbB family that has been found to be over expressed in MPM. The activation of EGFR promotes proliferation, cell survival and transformation. Approximately, 60% of the 80% of MM patients express EGFR, yet attempts to use EGFR as a prognostic marker have been futile. A phase-II trial of an EGFR inhibitor, Gefitinib, was conducted on small group of MPM patients revealed no correlation between the expression of receptor and the response to the drug in those patients [61]. Although Gefitinib has showed inhibitory effect on mesothelioma cell lines but the clinical studies on MPM patients showed limited efficacy of the drug suggesting single agent treatment is unsuccessful [62]. Recent report on mutations of EGFR tyrosine kinase of MM patients by Foster, et., al., indicates that 31% of patients had mutations (mut+) and the remaining were wild type (mut-) suggests that these mutations were predictive for optimal resectability [63]. The group of patients with mut+ may be better responsive to TK-inhibitor therapy. However, this needs to be confirmed by future studies.
Met as a target for MPM
Receptor tyrosine kinases (RTKs) are the high -affinity cell surface receptors with a similar structure which have roles in both normal development and tumorigenesis. The widespread deregulation of RTKs in cancer makes them potential therapeutic targets for a variety of malignancies [64]. The receptor for hepatocyte growth factor (HGF), a transmembrane receptor tyrosine kinase, constituting a 145-κDa β-subunit and a 50-κDa α-subunit is the Met proto -oncogene product. It controls epithelial growth and remodeling through the coordination of cell proliferation, motility, cell migration, and invasion [65] Met signaling pathway has been found to be aberrantly activated as a result of gene mutations, and over expression or structural rearrangements [66, 67] promotes tumorigenesis, especially in the development of invasive and metastasis phenotypes [68].
Table 1.
Malignant mesothelioma markers
| 1 | AUA1 | An antibody with epithelial specificity, against a panel of five cultured human mesothelial cell lines and seven human epithelial ovarian cancer cell lines [101] |
| 2 | Carcinoembryonic antigen (CEA) | A glycoprotein involved in cell Adhesion [102]. Absence of CEA distinguishes MM from lung cancer. |
| 3 | D2-40 | A commercially available monoclonal antibody directed against M2A antigen, a surface sialoglycoprotein originally detected in association with germ cell was positive for 96% of MM which indicates the sensitivity for the cells of mesothelial origin [103]. |
| 4 | Desmin | An intermediate filament present in smooth and striated muscle. Three studies of effusion specimens have demonstrated the value of Desmin in the differentiation of both benign and malignant mesothelial cells from carcinoma cells [104]. |
| 5 | Hyaluronan | An anionic, non sulfated glycosaminoglycan distributed widely throughout connective and epithelial tissues, known to be produced by mesothelial cells. It is a peritoneal fluid marker of mesothelial cells [105]. |
| 6 | MCp130 | A 130-kDa protein, is reactive with mesothelial cells and useful in the diagnosis of epithelial mesotheliomas [106]. |
| 7 | Mesothelin | A 40kDa protein present on normal mesothelial cells and over-expressed in several human tumors as a tumor differentiation antigen [107, 108]. |
| 8 | N-cadherin | A class of type 1 transmembrane proteins which plays important roles in cell adhesions. 78% of malignant mesothelioma cases express N-cadherin [109]. |
| 9 | Protein Phosphatase Inhibitor-1(I-1) | A mesothelial marker which is an endogenous inhibitor of protein phosphatase-1, involved in signal transduction [110]. |
| 10 | Thrombomodulin | A sensitive mesothelial marker and is sufficiently specific to be a useful discriminator, positively identifying, the mesothelial nature of a cell population [111]. |
| 11 | Vimentin | A member of the intermediate filament family of proteins that is especially found in connective tissue as an aid in the identification of cells of mesothelial origin [112]. |
| 12 | WT1 (Wilms' tumor susceptibility gene) | This protein transcribes during the transition of mesenchyme to epithelial tissues. Continuous expression of WT1 through adult life has been described only in the gonads and mesothelial tissues, and it has been suggested as a marker for mesothelial lineage [113]. |
| 13 | Receptor EphA2 | A 120KDa protein, member of RTKs family is over expressed in MPM cells and normal mesothelial does not express the receptor. Thus makes it a potential candidate for the diagnosis of malignant from non-malignant tissue of the pleura [93]. |
Met receptor is uniformly expressed in MPM, however a subset of MPMs was reported to carry a point mutation in Met gene, which suggests that Met would be a good candidate RTK for target therapy of MPM [69]. The expression of Met protein has been detected in 74% to 100% of MPM and HGF/SF expression in 40% to 85% of MPM tumor specimens but not in normal mesothelial cells [70]. HGF/Met signaling is involved in MPM growth and also it can change the rate of migration and invasion in a disease that is clinically characterized by local extension. Although, the effects of Met receptor inhibition in preventing MPM growth are limited to a minority of MPM cell lines, that also produce HGF [71]. Selective small molecular inhibitors of c-Met kinase have been found to induce apoptosis and suppress cell growth both in vitro and in vivo. It is predictable that clinical trials of Met inhibitors, in MPM would be most effective in patients whose tumors express both Met and HGF [70, 72]. C-Met specific siRNA can successfully inhibit c-Met expression resulting in significant inhibition of cell viability, and further inhibition strategies targeting the receptor will be sufficient and more effective for clinical treatment applications for MPM in the future [45, 73].
Angiogenesis inhibitors
Patients with MPM have high levels of VEGF hence monoclonal antibodies against VEGF were considered. VEGF receptor, platelet-derived growth factor receptor and c-KIT are expressed by MPM. Sorafenib, a potent inhibitor of ras/raf/MEK pathway along with VEGFR and c-Kit was evaluated in unresectable mesothelioma in a phase-II trial with limited response in advanced MPM patients [74], and thus warrants further studies. Other anti-angiogenic inhibitor showed modest improvement and are not recommended for clinical use [75]. To date no clinical trial using anti-angiogenic agents have shown efficacy in improvising response or overall survival improvement for MPM.
Figure 1.
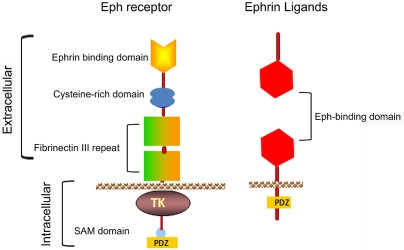
Schematic representation of the domain organization of Eph receptors and Ephrins. SAM = sterile alpha motif; PDZ = PDZ-binding motif; TK = tyrosine kinase domain; GPI = glycosylphosphati-dylinositol linkage.
Histone deacetylase inhibitors
Histone deacetylases (HDACs) are known to play a critical role in cellular differentiation and malignant transformation of MPM [76]. HDACs manipulate chromatin structure and function of various non-histone proteins and transcription factors. The inhibitor of HDAC showed a partial response against advanced MPM in a phase-I trial [77]. Further clinical studies are warranted before these inhibitors could be recommended as treatment option for MPM.
Receptor EphA2 a novel target
Eph (Erythropoietin-producing human hepatocellular carcinoma) receptors consist of the largest known family of RTKs that plays crucial role in several physiological processes, embryonic development, and progression of various human malignancies [78, 79]. Eph receptors form a cell -cell communication system upon interacting with their ligands called Ephrins. To date, 14 Eph receptors are known and have been divided into two subclasses subclass-A and subclass-B. Eph-A subclass includes 9 receptors which bind with five Ephrin-A ligands. Eph-B subclass includes 5 receptors which bind to three Ephrin-B ligands with the exception of EphA4 and EphB2 receptor which can also bind to Ephrin-Bs and Ephrin-A5 respectively, and the Eph-B4 which preferentially binds to Ephrin-B2 [80, 81]. Ephrin ligands (Eph family receptor interacting proteins) consists of eight members, they are also divided into 2 subclasses -A and -B.
Bidirectional signaling
Eph receptors are transmembrane proteins composed of N-terminal glycosylated ligand-binding domain, a transmembrane region and an intracellular catalytic kinase domain. The cysteine-rich domain facilitates oligomerzation often noticed upon binding with ligands called Ephrins. The intracellular catalytic domain contains highly conserved kinase domain which is involved in phosphorylation of substrates including receptor EphA2 [82]. The sterile a motif (SAM) domain is followed by PDZ binding motif. All Ephrins contain a conserved extracellular receptor binding domain and B-type Ephrins also possess a short but highly conserved kinase domain followed by PDZ binding motif. The Eph signaling is unique in that both receptor and ligand must reorient upon interaction leading to conformational changes which transmits bidirectional signals in both the receptor bearing as well as ligand bearing cells (Figure 1). The cells expressing Eph receptors produce “Forward” signaling. Whereas the cells expressing Ephrins produces “Reverse” signaling. The specificity of ligand binding is dependent on N-terminal glycosylated domain. The Eph receptors and their ligand Ephrins form heterotetramers upon interactions. Ephrin binding leads to clustering of Eph receptor by the activation of kinase domain, and triggers downstream singling pathways [83].
Figure 2.
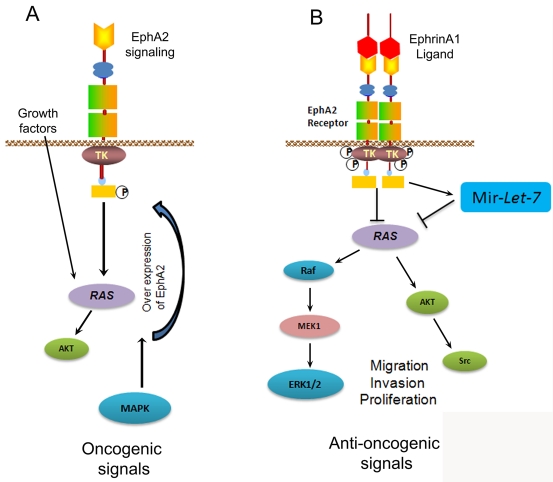
EphA2 signaling pathways in MMC. A. EphA2 signaling promotes oncogenic phenotype. In the absence of ligand, receptor EphA2 kinase function increases by RAS, AKT and MAPK activation which promotes malignancy. B. EphrinA1 binding of EphA2 induces tumor suppressive signals. Activation of EphA2 with EphrinA1 leads to phos-phorylation of EphA2 and inhibits RAS/MAPK pathways. EphrinA1 activation of MMC induced miR-Let-7a which targets RAS signaling and inhibits oncogenic signals in MMC. Phosphorylation = P; inhibition = -L; activation = —.
Expression level of EphA2 receptor activity has a statistically significant involvement in the establishment of carcinogenesis and metastasis through multiple mechanisms [84, 85]. Expression of Eph receptors such as EphA2 is often up regulated in many types of malignancies, yet their exact role in cancer is not well-understood [86]. Studies of Eph receptors in various types of tumors, demonstrate their dual roles in tumor suppression and tumor promotion. In normal cells, binding of Eph2 receptor with EphrinA1 ligand on adjacent cells induces receptor forward signaling, leading to inhibition of Ras/mitogen-activated protein kinase (MAPK) pathway activity, resulting in normal growth. In tumor cells, disruption of cell-cell junctions inhibits EphA2 receptor interaction with endogenous EphrinA1. In addition, Eph receptors are often over expressed and Ephrins are down regulated [87]. Crosstalk between Eph receptors and other receptor tyrosine kinases such as EGFR (Epidermal Growth Factor receptor) and ErbB2 results in more Ras-MAPK pathway activation, which leads to malignant phenotype [88]. In addition, activation of MAPK signaling activates AKT and promotes receptor EphA2 expression and accumulation in tumor cells (Figure 2A). In MM receptor EphA2 is over expressed and it is modulated through the expression of Ras oncogene. RTK pathways including EGFR, IGF (Insulin-Growth Factor) and HGF (Hepatocyte Growth Factor) are activated and these RTKs are known to signal through Ras, and thus Ras signaling pathways play a potential role in MM growth. High levels of EphA2 receptor has been observed in majority of colon cancer samples [89], advanced gastric, ovarian [90, 91] and in non-small cell lung cancers [92]. We reported that MPM cell lines express high levels of receptor EphA2 and normal mesothelial cells did not express these receptors [93]. In addition silencing the receptor with siRNA-EphA2 significantly attenuated the MM growth and induced apoptosis in MPM cells [93, 94]. Furthermore, activation of receptor EphA2 with its ligand EphrinA1 causes phosphorylation of receptor EphA2 and transfer the signal to downstream signaling molecules thereby suppressing tumorogenesis promoting signals and inhibits tumor growth and migration of MPM cells [95]. However, the molecular mechanisms involved in inhibition of tumor growth not clearly understood. Recently we have also reported a novel mechanism that activation of receptor EphA2 with ligand EphrinA1 induces the expression of microRNA let-7 in MPM cells [96]. Micro-RNA let-7a targets Ras oncogene which promotes the malignant phenotype. The expression of let-7a may be responsible for the anti-oncogenic responses induced by EphA2 and EphrinA1 interaction in MPM (Figure 2B). However, further work needs to be performed to understand the molecular mechanisms of EphA2-EphrinA1 signaling which could hold potential for future therapeutic strategies for MPM.
We and others have shown that not only ligand activation causes the down regulation of EphA2 by phosphorylation and proteosomal degradation, but also this change in the receptor status correlates with a decrease in anchorage-independent growth and invasion of tumor cells [97, 98]. It suggests that although the causes of EphA2 over expression in most of the aggressive tumors remain largely unclear, manipulation of EphA2 receptor/EphrinA1 system will aid an opportunity for new therapeutic intervention against MPM. Pasquale et al. and others have highlighted the importance of ephrin-Eph signaling in the hindbrain for the establishment of rhombomeres and malignancy [87, 99, 100]. But we are still far from understanding how best the Eph-Ephrin axis can be a target. Future studies on newer strategies of gene therapy targeting Eph-Ephrin signaling will likely unravel the mechanisms of this unique class of molecules.
Conclusion
Modest success has been achieved so far towards the therapy for MPM. Over the past 2 decades the focus of research to find novel targets led to discovery of the association of EphA2 and its ligand EphrinA1 in the development of malignancy. The mounting evidence from the research involving Eph receptors and their ligands Ephrins puts these molecules as the key players in promoting tumor survival. The mechanisms involved are complex and need further work to fully elucidate their potential in therapeutic strategies against MPM. Now that we know we have identified a target which can be exploited to treat the disease whose cure is still unsuccessful, MPM. The research strategies should be directed to reap the maximum benefit by targeting the EphA2 receptor. However, further work with cell lines and animal models should be carried out to understand the functions of these complex signaling molecules. EphA2 receptor as a novel target may be a potential candidate in future therapies to achieve improved survival benefits for the patients with MPM.
Acknowledgments
This work was supported by NIR grant # 09KN-09 from Florida Department of Health (Nasreen N); VA Merit Review (Mohammed, KA). This work was supported by RC1 grant # 09KW-08 from Florida Department of Health to Najmunnisa Nasreen (2009). We are grateful to Professor Francisco Rodrigue- Panadero, MD, Respiratory Endoscopy Unit, University hospital, Sevilla, Espana; for reviewing and providing valuable critique on the review. The authors declare that they have no competing interests.
References
- 1.Testa JR, Carbone M, Hirvonen A, Khalili K, Krynska B, Linnainmaa K, Pooley FD, Rizzo P, Rusch V, Xiao GH. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58:4505–4509. [PubMed] [Google Scholar]
- 2.Roushdy-Hammady I, Siegel J, Emri S, Testa JR, Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357:444–445. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- 3.Carbone M, Albelda SM, Broaddus VC, Flores RM, Hillerdal G, Jaurand MC, Kjaerheim K, Pass HI, Robinson B, Tsao A. Eighth International Mesothelioma Interest Group. Oncogene. 2007;26:6959–6967. doi: 10.1038/sj.onc.1210515. [DOI] [PubMed] [Google Scholar]
- 4.McElvenny DM, Darnton AJ, Price MJ, Hodgson JT. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79–87. doi: 10.1093/occmed/kqi034. [DOI] [PubMed] [Google Scholar]
- 5.Joshi TK, Gupta RK. Asbestos-related morbidity in India. Int J Occup Environ Health. 2003;9:249–253. doi: 10.1179/oeh.2003.9.3.249. [DOI] [PubMed] [Google Scholar]
- 6.Larson T, Melnikova N, Davis SI, Jamison P. Incidence and descriptive epidemiology of mesothelioma in the United States, 1999-2002. Int J Occup Environ Health. 2007;13:398–403. doi: 10.1179/oeh.2007.13.4.398. [DOI] [PubMed] [Google Scholar]
- 7.Leppla SH, Arora N, Varughese M. Anthrax toxin fusion proteins for intracellular delivery of macromolecules. J Appl Microbiol. 1999;87:284. doi: 10.1046/j.1365-2672.1999.00890.x. [DOI] [PubMed] [Google Scholar]
- 8.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 9.Leigh J, Davidson P, Hendrie L, Berry D. Malignant mesothelioma in Australia, 1945-2000. Am J Ind Med. 2002;41:188–201. doi: 10.1002/ajim.10047. [DOI] [PubMed] [Google Scholar]
- 10.Pass HI, Vogelzang N, Hahn S, Carbone M. Malignant pleural mesothelioma. Curr Probl Cancer. 2004;28:93–174. doi: 10.1016/j.currproblcancer.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Hammar SP. Macroscopic, histologic, histochemical, immunohistochemical, and ultra-structural features of mesothelioma. Ultrastruct Pathol. 2006;30:3–17. doi: 10.1080/01913120500313143. [DOI] [PubMed] [Google Scholar]
- 12.Waller DA, Morritt GN, Forty J. Video-assisted thoracoscopic pleurectomy in the management of malignant pleural effusion. Chest. 1995;107:1454–1456. doi: 10.1378/chest.107.5.1454. [DOI] [PubMed] [Google Scholar]
- 13.Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD002916.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Ucar AE, Edwards JG, Rengajaran A, Muller S, Waller DA. Palliative surgical debulking in malignant mesothelioma. Predictors of survival and symptom control. Eur J Cardiothorac Surg. 2001;20:1117–1121. doi: 10.1016/s1010-7940(01)00995-2. [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, Colson Y, Linden P, Chang M, Capalbo L, Oldread E, Neragi-Miandoab S, Swanson SJ, Zellos LS. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg. 2004;128:138–146. doi: 10.1016/j.jtcvs.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Chang MY, Sugarbaker DJ. Extrapleural pneumonectomy for diffuse malignant pleural mesothelioma: techniques and complications. Thorac Surg Clin. 2004;14:523–530. doi: 10.1016/j.thorsurg.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Baldini EH, Recht A, Strauss GM, DeCamp MM Jr, Swanson SJ, Liptay MJ, Mentzer SJ, Sugarbaker DJ. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg. 1997;63:334–338. doi: 10.1016/s0003-4975(96)01228-3. [DOI] [PubMed] [Google Scholar]
- 18.Butchart EG, Ashcroft T, Barnsley WC, Holden MP. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax. 1976;31:15–24. doi: 10.1136/thx.31.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores RM, Pass HI, Seshan VE, Dycoco J, Zakowski M, Carbone M, Bains MS, Rusch VW. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. 626 e621–623. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Krarup-Hansen A, Hansen HH. Chemotherapy in malignant mesothelioma: a review. Cancer Chemother Pharmacol. 1991;28:319–330. doi: 10.1007/BF00685684. [DOI] [PubMed] [Google Scholar]
- 21.Shin DM, Fossella FV, Umsawasdi T, Murphy WK, Chasen MH, Walsh G, Komaki R, McMurtrey MJ, Hong WK. Prospective study of combination chemotherapy with cyclophosphamide, doxorubicin, and cisplatin for unresectable or metastatic malignant pleural mesothelioma. Cancer. 1995;76:2230–2236. doi: 10.1002/1097-0142(19951201)76:11<2230::aid-cncr2820761108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Riehemann K, Schmitt O, Ehlers EM. The effects of thermochemotherapy using cyclo-phosphamide plus hyperthermia on the malignant pleural mesothelioma in vivo. Ann Anat. 2005;187:215–223. doi: 10.1016/j.aanat.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Altinbas M, Er O, Ozkan M, Coskun HS, Gulmez I, Ekici E, Kaplan B, Eser B, Ozesmi M. Ifosfamide, mesna, and interferon-alpha2A combination chemoimmunotherapy in malignant mesothelioma: results of a single center in central anatolia. Med Oncol. 2004;21:359–366. doi: 10.1385/MO:21:4:359. [DOI] [PubMed] [Google Scholar]
- 24.Falkson G, Hunt M, Borden EC, Hayes JA, Falkson CI, Smith TJ. An extended phase II trial of ifosfamide plus mesna in malignant mesothelioma. Invest New Drugs. 1992;10:337–343. doi: 10.1007/BF00944192. [DOI] [PubMed] [Google Scholar]
- 25.McLeod HL, Cassidy J, Powrie RH, Priest DG, Zorbas MA, Synold TW, Shibata S, Spicer D, Bissett D, Pithavala YK, Collier MA, Paradiso U, Roberts JD. Pharmacokinetic and pharmacodynamic evaluation of the glycinamide ribonucleotide formyltransferase inhibitor AG2034. Clin Cancer Res. 2000;6:2677–2684. [PubMed] [Google Scholar]
- 26.Carteni G, Manegold C, Garcia GM, Siena S, Zielinski CC, Amadori D, Liu Y, Blatter J, Visseren-Grul C, Stahel R. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009;64:211–218. doi: 10.1016/j.lungcan.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Joyston-Bechal S. Management of oral complications following radiotherapy. Dent Update. 1992;19:232–234. 236–238. [PubMed] [Google Scholar]
- 28.Burgin M, Gairard-Dory AC, Mennecier B, Molard A, Beretz L, Quoix AE. First-line treatment with pemetrexed in association with cisplatin in patients with non-operable malignant pleural mesothelioma. Rev Pneumol Clin. 2009;65:75–83. doi: 10.1016/j.pneumo.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Magri MD, Veronesi A, Foladore S, De Giovanni D, Serra C, Crismancich F, Tuveri G, Nicotra M, Tommasi M, et al. Epirubicin in the treatment of malignant mesothelioma: a phase II cooperative study. The North-Eastern Italian Oncology Group (GOCCNE)--Mesothelioma Committee. Tumori. 1991;77:49–51. doi: 10.1177/030089169107700112. [DOI] [PubMed] [Google Scholar]
- 30.Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891–2895. [PubMed] [Google Scholar]
- 31.Pennucci MC, Ardizzoni A, Pronzato P, Fioretti M, Lanfranco C, Verna A, Giorgi G, Vigani A, Frola C, Rosso R. Combined cisplatin, doxorubicin, and mitomycin for the treatment of advanced pleural mesothelioma: a phase II FONICAP trial. Italian Lung Cancer Task Force. Cancer. 1997;79:1897–1902. doi: 10.1002/(sici)1097-0142(19970515)79:10<1897::aid-cncr9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Byrne MJ, Davidson JA, Musk AW, Dewar J, van Hazel G, Buck M, de Klerk NH, Robinson BW. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol. 1999;17:25–30. doi: 10.1200/JCO.1999.17.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 34.van Haarst JM, Baas P, Manegold C, Schouwink JH, Burgers JA, de Bruin HG, Mooi WJ, van Klav-eren RJ, de Jonge MJ, van Meerbeeck JP. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer. 2002;86:342–345. doi: 10.1038/sj.bjc.6600118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto C, Marino A, De Pangher Manzini V, Benedetti G, Galetta D, Mazzanti P, Del Conte G, dell'Amore D, Piana E, Giaquinta S, Lopez M, Martoni A. Sequential chemotherapy with cisplatin/gemcitabine (CG) followed by mitoxantrone/methotrexate/mitomycin (MMM) in patients with malignant pleural mesothelioma. A multicenter Italian Phase II Study (SITMP1) Lung Cancer. 2006;52:199–206. doi: 10.1016/j.lungcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Raghavan D, Gianoutsos P, Bishop J, Lee J, Young I, Corte P, Bye P, McCaughan B. Phase II trial of carboplatin in the management of malignant mesothelioma. J Clin Oncol. 1990;8:151–154. doi: 10.1200/JCO.1990.8.1.151. [DOI] [PubMed] [Google Scholar]
- 37.Ellis P, Davies AM, Evans WK, Haynes AE, Lloyd NS. The use of chemotherapy in patients with advanced malignant pleural mesothelioma: a systematic review and practice guideline. J Thorac Oncol. 2006;1:591–601. [PubMed] [Google Scholar]
- 38.Scagliotti GV, Shin DM, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C, Burris H, Gatzemeier U, Blatter J, Symanowski JT, Rusthoven JJ. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol. 2003;21:1556–1561. doi: 10.1200/JCO.2003.06.122. [DOI] [PubMed] [Google Scholar]
- 39.Ceresoli GL, Zucali PA, De Vincenzo F, Gianoncelli L, Simonelli M, Lorenzi E, Ripa C, Giordano L, Santoro A. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer. 2011;72:73–77. doi: 10.1016/j.lungcan.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhauer EA, Vermorken JB. The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs. 1998;55:5–30. doi: 10.2165/00003495-199855010-00002. [DOI] [PubMed] [Google Scholar]
- 41.van Breukelen FJ, Mattson K, Giaccone G, van Zandwijk N, Planteydt HT, Kirkpatrick A, Dalesio O. Mitoxantrone in malignant pleural mesothelioma: a study by the EORTC Lung Cancer Cooperative Group. Eur J Cancer. 1991;27:1627–1629. doi: 10.1016/0277-5379(91)90430-l. [DOI] [PubMed] [Google Scholar]
- 42.Ollikainen T, Knuuttila A, Suhonen S, Taavitsainen M, Jekunen A, Mattson K, Linnainmaa K. In vitro sensitivity of normal human mesothelial and malignant mesothelioma cell lines to four new chemotherapeutic agents. Anticancer Drugs. 2000;11:93–99. doi: 10.1097/00001813-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Vorobiof DA, Rapoport BL, Chasen MR, Abratt RP, Cronje N, Fourie L, McMichael G, Hacking D. Malignant pleural mesothelioma: a phase II trial with docetaxel. Ann Oncol. 2002;13:412–415. doi: 10.1093/annonc/mdf046. [DOI] [PubMed] [Google Scholar]
- 44.Misset JL, Bleiberg H, Sutherland W, Bekradda M, Cvitkovic E. Oxaliplatin clinical activity: a review. Crit Rev Oncol Hematol. 2000;35:75–93. doi: 10.1016/s1040-8428(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 45.Jagadeeswaran R, Ma PC, Seiwert TY, Jagadeeswaran S, Zumba O, Nallasura V, Ahmed S, Filiberti R, Paganuzzi M, Puntoni R, Kratzke RA, Gordon GJ, Sugarbaker DJ, Bueno R, Janamanchi V, Bindokas VP, Kindler HL, Salgia R. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res. 2006;66:352–361. doi: 10.1158/0008-5472.CAN-04-4567. [DOI] [PubMed] [Google Scholar]
- 46.Knuuttila A, Ollikainen T, Halme M, Mali P, Kivisaari L, Linnainmaa K, Jekunen A, Mattson K. Docetaxel and irinotecan (CPT-11) in the treatment of malignant pleural mesothelioma–a feasibility study. Anticancer Drugs. 2000;11:257–261. doi: 10.1097/00001813-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Carmichael J, Degraff WG, Gamson J, Russo D, Gazdar AF, Levitt ML, Minna JD, Mitchell JB. Radiation sensitivity of human lung cancer cell lines. Eur J Cancer Clin Oncol. 1989;25:527–534. doi: 10.1016/0277-5379(89)90266-6. [DOI] [PubMed] [Google Scholar]
- 48.Baldini EH. External beam radiation therapy for the treatment of pleural mesothelioma. Thorac Surg Clin. 2004;14:543–548. doi: 10.1016/S1547-4127(04)00108-2. [DOI] [PubMed] [Google Scholar]
- 49.Ahamad A, Stevens CW, Smythe WR, Vaporciyan AA, Komaki R, Kelly JF, Liao Z, Starkschall G, Forster KM. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2003;55:768–775. doi: 10.1016/s0360-3016(02)04151-2. [DOI] [PubMed] [Google Scholar]
- 50.Forster KM, Smythe WR, Starkschall G, Liao Z, Takanaka T, Kelly JF, Vaporciyan A, Ahamad A, Dong L, Salehpour M, Komaki R, Stevens CW. Intensity-modulated radiotherapy following extrapleural pneumonectomy for the treatment of malignant mesothelioma: clinical implementation. Int J Radiat Oncol Biol Phys. 2003;55:606–616. doi: 10.1016/s0360-3016(02)04150-0. [DOI] [PubMed] [Google Scholar]
- 51.Murai KK, Pasquale EB. ‘Eph'ective signaling: forward, reverse and crosstalk’. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 52.Kruklitis RJ, Singhal S, Delong P, Kapoor V, Sterman DH, Kaiser LR, Albelda SM. Immuno-gene therapy with interferon-beta before surgical debulking delays recurrence and improves survival in a murine model of malignant mesothelioma. J Thorac Cardiovasc Surg. 2004;127:123–130. doi: 10.1016/j.jtcvs.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 53.Galffy G, Mohammed KA, Nasreen N, Ward MJ, Antony VB. Inhibition of interleukin-8 reduces human malignant pleural mesothelioma propagation in nude mouse model. Oncol Res. 1999;11:187–194. [PubMed] [Google Scholar]
- 54.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 55.Castagneto B, Zai S, Mutti L, Lazzaro A, Ridolfi R, Piccolini E, Ardizzoni A, Fumagalli L, Valsuani G, Botta M. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: Results of a phase II study on 31 consecutive patients. Lung Cancer. 2001;31:303–310. doi: 10.1016/s0169-5002(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 56.Boutin C, Nussbaum E, Monnet I, Bignon J, Vanderschueren R, Guerin JC, Menard O, Mignot P, Dabouis G, Douillard JY. Intrapleural treatment with recombinant gamma-interferon in early stage malignant pleural mesothelioma. Cancer. 1994;74:2460–2467. doi: 10.1002/1097-0142(19941101)74:9<2460::aid-cncr2820740912>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 57.Odaka M, Sterman DH, Wiewrodt R, Zhang Y, Kiefer M, Amin KM, Gao GP, Wilson JM, Barsoum J, Kaiser LR, Albelda SM. Eradication of intraperitoneal and distant tumor by adenovirus-mediated interferon-beta gene therapy is attributable to induction of systemic immunity. Cancer Res. 2001;61:6201–6212. [PubMed] [Google Scholar]
- 58.Davidson JA, Musk AW, Wood BR, Morey S, Ilton M, Yu LL, Drury P, Shilkin K, Robinson BW. Intralesional cytokine therapy in cancer: a pilot study of GM-CSF infusion in mesothelioma. J Immunother. 1998;21:389–398. doi: 10.1097/00002371-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Calabro L, Sigalotti L, Fonsatti E, Bertocci E, Di Giacomo AM, Danielli R, Cutaia O, Colizzi F, Covre A, Mutti L, Natali PG, Maio M. Expression and regulation of B7-H3 immunoregulatory receptor, in human mesothelial and mesothelioma cells: immunotherapeutic implications. J Cell Physiol. 2011;226:2595–2600. doi: 10.1002/jcp.22600. [DOI] [PubMed] [Google Scholar]
- 60.Halme M, Knuuttila A, Vehmas T, Tammilehto L, Mantyla M, Salo J, Mattson K. High-dose methotrexate in combination with interferons in the treatment of malignant pleural mesothelioma. Br J Cancer. 1999;80:1781–1785. doi: 10.1038/sj.bjc.6690597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Govindan R, Kratzke RA, Herndon JE 2nd, Niehans GA, Vollmer R, Watson D, Green MR, Kindler HL. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res. 2005;11:2300–2304. doi: 10.1158/1078-0432.CCR-04-1940. [DOI] [PubMed] [Google Scholar]
- 62.Janne PA, Taffaro ML, Salgia R, Johnson BE. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res. 2002;62:5242–5247. [PubMed] [Google Scholar]
- 63.Foster JM, Gatalica Z, Lilleberg S, Haynatzki G, Loggie BW. Novel and existing mutations in the tyrosine kinase domain of the epidermal growth factor receptor are predictors of optimal resectability in malignant peritoneal mesothelioma. Ann Surg Oncol. 2009;16:152–158. doi: 10.1245/s10434-008-0206-6. [DOI] [PubMed] [Google Scholar]
- 64.Dediol I, Buljan M, Vurnek AIM, Bulat V, M AI, A AU. Psychological burden of anogenital warts. J Eur Acad Dermatol Venereol. 2009;23:1035–1038. doi: 10.1111/j.1468-3083.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 65.Sangwan V, Paliouras GN, Abella JV, Dube N, Monast A, Tremblay ML, Park M. Regulation of the Met receptor-tyrosine kinase by the protein-tyrosine phosphatase 1B and T-cell phosphatase. J Biol Chem. 2008;283:34374–34383. doi: 10.1074/jbc.M805916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canadas I, Rojo F, Arumi-Uria M, Rovira A, Albanell J, Arriola E. C-MET as a new therapeutic target for the development of novel anti-cancer drugs. Clin Transl Oncol. 2010;12:253–260. doi: 10.1007/s12094-010-0501-0. [DOI] [PubMed] [Google Scholar]
- 68.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 69.Kawaguchi K, Murakami H, Taniguchi T, Fujii M, Kawata S, Fukui T, Kondo Y, Osada H, Usami N, Yokoi K, Ueda Y, Yatabe Y, Ito M, Horio Y, Hida T, Sekido Y. Combined inhibition of MET and EGFR suppresses proliferation of malignant mesothelioma cells. Carcinogenesis. 2009;30:1097–1105. doi: 10.1093/carcin/bgp097. [DOI] [PubMed] [Google Scholar]
- 70.Kuhnen C, Tolnay E, Steinau HU, Voss B, Muller KM. Expression of c-Met receptor and hepatocyte growth factor/scatter factor in synovial sarcoma and epithelioid sarcoma. Virchows Arch. 1998;432:337–342. doi: 10.1007/s004280050175. [DOI] [PubMed] [Google Scholar]
- 71.Mukohara T, Civiello G, Davis IJ, Taffaro ML, Christensen J, Fisher DE, Johnson BE, Janne PA. Inhibition of the met receptor in mesothelioma. Clin Cancer Res. 2005;11:8122–8130. doi: 10.1158/1078-0432.CCR-05-1191. [DOI] [PubMed] [Google Scholar]
- 72.Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leelawat K, Leelawat S, Tepaksorn P, Rattanasinganchan P, Leungchaweng A, Tohtong R, Sobhon P. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res. 2006;136:78–84. doi: 10.1016/j.jss.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 74.Dubey S, Janne PA, Krug L, Pang H, Wang X, Heinze R, Watt C, Crawford J, Kratzke R, Vokes E, Kindler HL. A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. J Thorac Oncol. 2010;5:1655–1661. doi: 10.1097/JTO.0b013e3181ec18db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dowell JE, Kindler HL. Antiangiogenic therapies for mesothelioma. Hematol Oncol Clin North Am. 2005;19:1137–1145. viii. doi: 10.1016/j.hoc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–168. doi: 10.1016/S0065-230X(04)91004-4. [DOI] [PubMed] [Google Scholar]
- 77.Krug LM, Curley T, Schwartz L, Richardson S, Marks P, Chiao J, Kelly WK. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006;7:257–261. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 78.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 79.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 80.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 81.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 82.Boyd AW, Lackmann M. Signals from Eph and ephrin proteins: a developmental tool kit. Sci STKE. 2001;2001 doi: 10.1126/stke.2001.112.re20. [DOI] [PubMed] [Google Scholar]
- 83.Himanen JP, Nikolov DB. Eph signaling: a structural view. Trends Neurosci. 2003;26:46–51. doi: 10.1016/s0166-2236(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 84.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 85.Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc Res Tech. 2002;59:58–67. doi: 10.1002/jemt.10177. [DOI] [PubMed] [Google Scholar]
- 86.Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–368. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- 87.Beauchamp A, Debinski W. Ephs and ephrins in cancer: Ephrin-A1 signalling. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaught D, Brantley-Sieders DM, Chen J. Eph receptors in breast cancer: roles in tumor promotion and tumor suppression. Breast Cancer Res. 2008;10:217. doi: 10.1186/bcr2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saito T, Masuda N, Miyazaki T, Kanoh K, Suzuki H, Shimura T, Asao T, Kuwano H. Expression of EphA2 and E-cadherin in colorectal cancer: correlation with cancer metastasis. Oncol Rep. 2004;11:605–611. [PubMed] [Google Scholar]
- 90.Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Konno H, Shinmura K, Tanaka M, Sugimura H. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–47. doi: 10.1111/j.1349-7006.2005.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol. 2008;214:27–37. doi: 10.1002/jcp.21158. [DOI] [PubMed] [Google Scholar]
- 92.Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA, Kinch MS. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63:7907–7912. [PubMed] [Google Scholar]
- 93.Nasreen N, Mohammed KA, Antony VB. Silencing the receptor EphA2 suppresses the growth and haptotaxis of malignant mesothelioma cells. Cancer. 2006;107:2425–2435. doi: 10.1002/cncr.22254. [DOI] [PubMed] [Google Scholar]
- 94.Mohammed KA, Wang X, Goldberg EP, Antony VB, Nasreen N. Silencing receptor EphA2 induces apoptosis and attenuates tumor growth in malignant mesothelioma. Am J Cancer Res. 2011;1:419–431. [PMC free article] [PubMed] [Google Scholar]
- 95.Nasreen N, Mohammed KA, Lai Y, Antony VB. Receptor EphA2 activation with ephrinA1 supresses growth of malignant mesothelioma (MM) Cancer Lett. 2007;258:215–222. doi: 10.1016/j.canlet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Khodayari N, Mohammed KA, Goldberg EP, Nasreen N. EphrinA1 inhibits malignant mesothelioma tumor growth via let-7 microRNA-mediated repression of the RAS oncogene. Cancer Gene Ther. 2011;18:806–816. doi: 10.1038/cgt.2011.50. [DOI] [PubMed] [Google Scholar]
- 97.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 98.Lai YM, Mohammed KA, Nasreen N, Baumuratov A, Bellew BF, Antony VB. Induction of cell cycle arrest and apoptosis by BCG infection in cultured human bronchial airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L393–401. doi: 10.1152/ajplung.00392.2006. [DOI] [PubMed] [Google Scholar]
- 99.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 100.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 101.Radford H, Wilson AP. A comparison of immunohistochemical staining of human cultured mesothelial cells and ovarian tumour cells using epithelial and mesothelial cell markers. Anal Cell Pathol. 1996;11:173–182. [PubMed] [Google Scholar]
- 102.Wang NS, Huang SN, Gold P. Absence of carcinoembryonic antigen-like material in mesothelioma: an immunohistochemical differentiation from other lung cancers. Cancer. 1979;44:937–943. doi: 10.1002/1097-0142(197909)44:3<937::aid-cncr2820440322>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 103.Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105–110. doi: 10.1038/modpathol.3800259. [DOI] [PubMed] [Google Scholar]
- 104.Davidson B, Nielsen S, Christensen J, Asschenfeldt P, Berner A, Risberg B, Johansen P. The role of desmin and N-cadherin in effusion cytology: a comparative study using established markers of mesothelial and epithelial cells. Am J Surg Pathol. 2001;25:1405–1412. doi: 10.1097/00000478-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 105.Ho-dac-Pannekeet MM. Peritoneal fluid markers of mesothelial cells and function. Adv Ren Replace Ther. 1998;5:205–211. doi: 10.1016/s1073-4449(98)70033-0. [DOI] [PubMed] [Google Scholar]
- 106.Singh G, Singh J, Ordonez NG, Kasper M, Katyal SL, Wong-Chong ML, McCloskey CA. Expression of a 130-kDa mesothelial and ciliated cell Ag (MCp130) in normal and developing human and rat lung and its role as a diagnostic marker for mesotheliomas and tumors of the female reproductive system. Lab Invest. 1995;73:48–58. [PubMed] [Google Scholar]
- 107.Kachali C, Eltoum I, Horton D, Chhieng DC. Use of mesothelin as a marker for mesothelial cells in cytologic specimens. Semin Diagn Pathol. 2006;23:20–24. doi: 10.1053/j.semdp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abutaily AS, Addis BJ, Roche WR. Immunohistochemistry in the distinction between malignant mesothelioma and pulmonary adenocarcinoma: a critical evaluation of new antibodies. J Clin Pathol. 2002;55:662–668. doi: 10.1136/jcp.55.9.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McLaren L, Boyle S, Mason JO, Bard JB. Expression and genomic characterization of protein phosphatase inhibitor-1: a novel marker for mesothelium in the mouse. Mech Dev. 2000;96:237–241. doi: 10.1016/s0925-4773(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 111.Kennedy AD, King G, Kerr KM. HBME-1 and antithrombomodulin in the differential diagnosis of malignant mesothelioma of pleura. J Clin Pathol. 1997;50:859–862. doi: 10.1136/jcp.50.10.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaRocca PJ, Rheinwald JG. Coexpression of simple epithelial keratins and vimentin by human mesothelium and mesothelioma in vivo and in culture. Cancer Res. 1984;44:2991–2999. [PubMed] [Google Scholar]
- 113.Gulyas M, Hjerpe A. Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol. 2003;199:479–487. doi: 10.1002/path.1312. [DOI] [PubMed] [Google Scholar]