Abstract
Cancer cells have developed novel mechanisms for evading chemotherapy-induced apoptosis and autophagy-associated cell death pathways. Upon the discovery that chemotherapeutics could target these cell death pathways in a manner that was not mutually exclusive, new discoveries about the interrelationship between these two pathways are emerging. Key proteins originally thought to be “autophagy-related proteins” are now found to be involved in either inducing or inhibiting apoptosis. Similarly, apoptosis inhibiting proteins can also block autophagy-associated cell death. One example is the complex formed by the autophagy protein, Beclin 1, and anti-apoptotic protein Bcl-2, which leads to inhibition of autophagy-associated cell death. Researchers have been investigating additional mechanisms that form/disrupt this complex in order to better design chemotherapeutics. This review will highlight the role Bcl-2 and Beclin 1 play in cancer development and drug resistance, as well as the role the Bcl-2:Beclin 1 complex in the switch between autophagy and apoptosis.
Keywords: Apoptosis, autophagy, Bcl-2, Beclin 1, Gossypol, BH3 mimetics
Introduction
Autophagy is a catabolic pathway activated by cellular stress, such as starvation or pathogen infection, followed by engulfment of proteins and organelles by double-membrane vesicles, called autophagosomes. Autophagy can result in cellular adaptation, as well as cell survival or cell death. The ability of autophagy to play a role in both survival and cell death is counterintuitive. In addition, autophagy demonstrates tumor suppressive qualities, whereby deficiency in autophagy leads to cellular transformation and tumor development [1-3]. Alternatively, cancer cells can also exploit the benefits of autophagy by inducing cell survival associated with the stressful tumor microenvironment [4], as well as damage caused by chemo- and radiotherapies [5-7]. Therefore, while autophagy demonstrates tumor suppressive actions, it also can confer oncogenicity associated with drug and radioresistance.
Resistance of cancer cells to treatment can be associated with both autophagy and inhibition of the more common apoptosis cell death pathway. Apoptosis resistance in developing novel cancer therapies has been a tremendous challenge with current chemotherapies. However, a greater understanding of the interrelationship between apoptosis and autophagy is providing precedence for developing new drugs, such as BH3 mimetics, that can induce autophagy-associated cell death in apoptotic resistance cells. It is well established that suppression of apoptosis induces autophagy, while autophagy inhibition causes apoptosis [8, 9]. Identifying the “toggle switch” or regulatory mechanisms involved in the interrelationship between apoptosis- and autophagy-associated cell death is imperative for creating optimal chemotherapeutics and inhibiting cancer cell growth. Exploiting these regulatory mechanisms may provide new targets for developing cancer therapeutics.
Throughout this review, we will discuss chemotherapies and mechanisms for inducing cell death via the autophagy cell death pathway. However, it is important to note that while autophagy cell death was previously considered one of the main cell death pathways [10], independent autophagy cell death may only exist in a few exceptional cases, such as Drosophila development [11]. Accumulating evidence is challenging the concept of autophagic cell death in mammalian cells [12-14]. This debate provides precedence for developing additional experiments to truly dissect whether cell death associated with autophagy is actually autophagy cell death or cell death via a combination of death pathways, such as apoptosis, autophagy, and necrosis. Furthermore, this examination has led to re-evaluating modes of cell death and creating additional molecular definitions of cell death subroutines for greater classification and clarity [15].
Bcl-2:Beclin 1 interaction regulates autophagy
The Bcl-2 family of proteins, Bcl-2, Bcl-xL and Mcl-1, are well-known anti-apoptotic mediators; however, their roles in inhibiting autophagy are becoming more understood. The cytoprotective function of Bcl-2 proteins stems from their ability to antagonize Bax and Bak, and thus prevent apoptosis. Beclin 1, a Bcl-2 homology 3 (BH3) domain only protein [16], is an essential initiator of autophagy. Beclin 1 recruits key autophagic proteins to a pre-autophagosomal structure, thereby forming the core complex consisting of Beclin 1, Vps34, and Vps15 [17]. In addition, Beclin 1 is a key determining factor as to whether cells undergo autophagy or apoptosis [18]. Beclin 1 has been shown to interact via its BH3 domain with anti-apoptotic Bcl-2 family members [19]. This interaction prevents Beclin 1 from assembling the pre-autophagosomal structure, thereby inhibiting autophagy [19]. The dual role of Bcl-2 and Bcl-xL in inhibiting both apoptosis and autophagic-associated cell death makes these proteins ideal chemotherapeutic targets. BH3 mimetics, such as (-)-gossypol, obatoclax and ABT-737, disrupt the Bcl-2:Beclin1 interaction and currently are undergoing clinical trials [20, 21]. In addition to being promising new therapies, BH3 mimetics are providing additional insight into the autophagy-apoptosis regulatory pathways, as discussed below [22].
Beclin 1 and Bcl-2 expression levels regulate autophagic-apoptotic switch
The expression levels of Beclin 1 and Bcl-2 are key determinants as to whether cells are resistant to apoptosis or autophagy during tumorigenesis and chemotherapy. Early studies investigating the role of autophagy during tumorigenesis, identified Beclin 1 as a tumor suppressor. Initial studies showed Beclin 1 expression was lower in cancer cells versus normal epithelial cells [7]. Heterozygous disruption of BECN1 promotes tumorigenesis [1], while overexpression inhibits tumorigenesis [23]. Clinical studies have demonstrated that BECN1 is lost in 40% to 75% of breast and ovarian cancers [23]. In addition, downregulation of BECN1 has been shown in many types of cancers and associated with poor prognosis [24-29]. Therefore, the reduced expression of Beclin 1 reduces autophagic removal of damaged organelles that generate reactive oxygen species and genotoxic stress, resulting in cellular transformation.
Bcl-2 expression is upregulated in many human cancers and mediates the resistance of cancers to chemotherapeutic agents and radiotherapy [30, 31]. Bcl-2 is overexpressed in 60% of breast cancer patients. Furthermore, breast cancer patients with a positive Bcl-2 expression have shown a poor response to chemotherapy compared with those that had less Bcl-2 expression [32]. Inhibiting Bcl-2 expression has been shown to increase the efficacy of drug treatment by inducing apoptosis and autophagy. In MCF-7 cells, downregulation of Bcl-2 by RNA interference induced cell death 50% above control, however, apoptosis only accounted for 11% of this cell death at 96 hours [33]. It is possible that autophagic cell death was responsible for the additional cell death not measured by TUNEL assay. Furthermore, cell death was enhanced by etoposide and doxorubicin treatment in MCF-7 human breast cancer cells [33]. It was not determined whether this increase was due to apoptotic or autophagic cell death. A more recent study demonstrated that inhibiting Bcl-2 in breast cancer cells via siRNA knockdown did not induce apoptosis as expected, however induced autophagic cell death [34]. This autophagic cell death was increased by combination treatment with doxorubicin, which increased expression of Beclin 1 [34]. Therefore, patient screening for Bcl-2 expression and Beclin 1 is important in determining what type of chemotherapy or combination treatment should be utilized for treating patients.
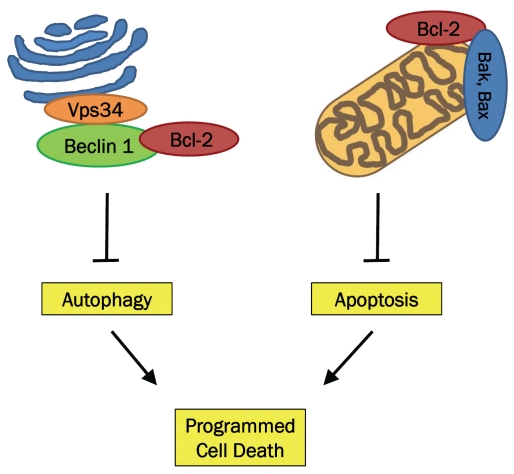
Initial discoveries investigating the interaction between Bcl-2 and Beclin 1 were carried out using overexpression/inhibition studies. HT-29 colon carcinoma cells, which do not express detectable levels of Bcl-2, were stably transfected with Bcl-2 expression vector. It was found that only HT-29 cells stably expressing Bcl-2 could inhibit starvation-induced autophagy via disrupting the Beclin 1/Vps34 complex. Transfecting HeLa cells, an autophagy-competent cell line that expresses Bcl-2, with Bcl-2 siRNA increased the level of starvation-induced autophagy [35]. This demonstrated that Bcl-2 exerts an inhibitory effect on autophagy (Figure 1).
Figure 1.
Bcl-2 can inhibit both autophagy- and apop-tosis-associated cell death. Bcl-2 binds to Bak/Bax, therefore preventing apoptosis. Bcl-2 binds to Beclin 1, preventing assembly of the pre-autophagosomal structure, which results in inhibition of autophagy.
Cellular localization and binding partners
In addition to Beclin 1 and Bcl-2 expression levels, cellular localization is a determining factor regarding the effects of Bcl-2:Beclin 1 interaction on autophagy and apoptosis. Initial studies demonstrated that Bcl-2 and Beclin 1 interact at the endoplasmic reticulum (ER) and mitochondria [35]. However, it appears that only Bcl-2:Beclin 1 localization to the ER results in inhibition of autophagy. Interestingly, a recent study [36] demonstrated that NAF-1 (nutrient-deprivation autophagy factor-1) binds Bcl-2 at the ER, independent of a BH3 domain, and is required for Bcl-2 to inhibit Beclin 1-mediated autophagy. Beclin 1 and Bcl-2 can form complexes with additional autophagy regulatory proteins at different cellular locations. AMBRA1 (activating molecule in Beclin 1-regulated autophagy) is a key regulator of autophagy and has been shown to interact with both Beclin 1 and Bcl-2. In response to autophagic stimuli, unc-51 -like kinase 1 (Ulk1) phosphorylates AMBRA1, which results in AMBRA1 dissociation from the Dynein motor complex [37]. After dissociation, AMBRA1 translocates to the ER, binds to Beclin 1 in the autophagy initiation complex [37], which results in the induction of autophagy. Alternatively, a recent study demonstrated that Bcl-2 localized to the mitochondria can also bind AMBRA1, but ER-localized Bcl-2 does not [38]. The Bcl-2:AMBRA1 interaction at the mitochondria is downregulated during autophagy, as well as during apoptosis. Therefore, Bcl-2 can regulate Beclin 1-induced autophagy by direct binding to Beclin 1, as well as by sequestering the activator of Beclin 1 [38].
Proteins capable of disrupting the Bcl-2:Beclin 1 complex and inducing autophagy are becoming more evident. High mobility group box 1 (HMGB1), a chromatin-associated nuclear protein and extracellular damage-associated molecular pattern molecule (DAMP), is a novel Beclin 1-binding protein important in sustaining autophagy. HMGB1 competes with Bcl-2 for interaction with Beclin 1, and orients Beclin 1 to autophagosomes [39]. BNIP3 is another protein that inhibits the Bcl-2:Beclin 1 complex, conversely via binding to Bcl-2. It was reported that via its BH3 domain, BNIP3 is capable of binding to Bcl-2, thereby disrupting the interaction between Bcl-2 and Beclin 1, and inducing autophagy [40]. Therefore, competitive proteins, such as HMGB1 and BNIP3, demonstrate another mechanism for inducing autophagy by competing with Bcl-2 or Beclin 1.
Phoshorylation regulation
As discussed previously, the cellular localization and complex formation of Beclin 1 and its regulatory proteins is important for determining whether cells undergo apoptosis or autophagy. This cellular location and complex formation is regulated by phosphorylation of key kinases. The dissociation of Bcl-2:Beclin 1 complex can occur by phosphorylation of Bcl-2 by stress-activated c-Jun N-terminal protein kinase 1 (JNK1) [41]. Furthermore, only the ER-enriched pool of Bcl-2 is subjected to regulation of Beclin 1 binding by JNK1-mediated phosphorylation [41], another indication that regulatory mechanisms occur at particular subcellular locations.
Once Bcl-2 is phosphorylated and dissociates from Beclin 1, autophagy can occur [41]. Upon continued JNK pathway activation and Bcl-2 phosphorylation, the cells will eventually undergo apoptosis via caspase 3 activation [41]. Death-associated protein kinase (DAPK) is also involved in regulating the Bcl-2:Beclin 1 complex. DAPK phosphorylates a threonine within the BH3 domain of Beclin 1, which induces dissociation and allows autophagy to proceed [42].
Apoptosis-induced cleavage of autophagy proteins
Cleavage of key autophagy-related proteins, such as Beclin 1 and ATG5, is another regulatory mechanism involved in disrupting the Bcl-2:Beclin 1 complex. Beclin 1 is a key inducer of autophagy, but can also be cleaved by caspase 8 during chemotherapy-induced apoptosis [43]. Once Beclin 1 is cleaved, it remains cytosolic and cannot induce either autophagy or apoptosis, therefore, rendering the protein non-functioning. Cells expressing a Beclin 1 mutant are incapable of being cleaved and less sensitive to chemotherapy in vivo [43]. Therefore, chemotherapy-associated cleavage of Beclin 1 would ultimately disrupt the Bcl-2:Beclin1 complex, and allow cells to undergo apoptosis upon treatment. Another study demonstrated, that following cleavage of Beclin 1, the C-terminus of cleaved Beclin (Beclin 1-C) can acquire a new apoptosis-promoting function by translocating from the cytosol to the mitochondria, inducing apoptosis in response to interleukin-3 deprivation [44]. Furthermore, using a cell free system, isolated mitochondria were able to release pro-apoptotic factors when combined with recombinant Beclin 1-C protein [44]. Therefore, Beclin 1 can be involved in both autophagy and apoptosis depending upon the presence of the full-length or cleaved form.
ATG5 plays an essential role in inducing autophagy by forming the pre-autophagosome structure. In addition to regulating autophagy, ATG5 also functions as a pro-apoptotic protein. Upon apoptotic stimuli, calpain, a calcium-dependent protease, cleaves ATG5 producing a fragment (tATG5) which contains the amino-terminal portion of ATG5 [45]. tATG5 then translocates to the mitochondria, triggers cytochrome c release and caspase activation [45]. Overexpression of Bcl-2 abrogated the effect of tATG5 on cell death. Furthermore, tATG5 can disrupt the anti-apoptotic Bcl-xL-Bax complex by binding to Bcl-xL which allows the Bax-Bax complex to form, inducing apoptosis [45].
BH3 mimetics: exploiting the interaction between Bcl-2 and Beclin1 and identifying new regulatory pathways
This review has discussed many mechanisms that regulate the Bcl-2:Beclin 1 complex, including Beclin 1 and Bcl-2 expression levels, cellular localization, and phosphorylation. BH3 mimetics are chemotherapies that bind to Bcl-2 and disrupt the Bcl-2:Beclin 1 complex and induce autophagy-associated cell death. In addition to demonstrating promise as therapy in clinical trials, BH3 mimetics have provided beneficial insight into the regulation of the Bcl-2:Beclin 1 interaction as well as identifying additional pathways that are involved in autophagic cell death.
Recent studies from our lab compared treatment with the natural BH3-mimetic, (-)-gossypol in treating apoptosis-resistant prostate cancer cells with high levels of Bcl-2 versus prostate cancer cells with low Bcl-2 expression [46]. (-)-Gossypol induces similar levels of total cell death in both prostate cancer cell lines, however, the mode of cell death depends upon the expression of the Bcl-2 family of proteins [20]. In prostate cancer cells with low Bcl-2 expression, more than 80 percent of cells died via apoptotic cell death. Conversely, in prostate cancer cells with high Bcl-2 expression, more than 60 percent died via induction of the autophagy pathway. This death can be blocked by the apoptosis inhibitor Z-VAD in low Bcl-2 expressing cells and the autophagy inhibitor 3-MA or Atg5/Beclin 1 siRNAs in high Bcl-2 expressing cells. Thus, the level of Bcl-2 determines which type of cell death will be dominant in prostate cancer cells after treatment with the Bcl-2 inhibitor (-)-gossypol. Furthermore, overexpressing Bcl-2 decreased the level of (-)-gossypol-induced autophagy, possibly due to the stoichiometric abundance of Bcl-2 sequestering Beclin 1 and inhibiting autophagy induction [46]. Similar results were also observed with other BH3-mimetics, ABT-236 and ABT-737 ([47] and Lian and Marquez, manuscript in preparation), indicating that this phenomenon is not compound specific. These data suggests that Bcl-2 expression levels may be a key marker in determining whether to utilize BH3 mimetics for chemotherapy when treating patients.
Our research also demonstrated that the BH3-mimetic (-)-gossypol induces autophagy via blocking Bcl-2:Beclin 1 interaction at the ER [46]. Upon overexpression of Bcl-2, the complex of Bcl-2:Beclin 1 on ER membranes is interrupted by (-)-gossypol prior to the complex of Bcl -2:Bak/Bax on mitochondria [46]. After treatment with (-)-gossypol, Beclin 1 is liberated from Bcl-2 in conjunction with transcriptional upregulation [46]. Together, these events trigger the autophagic cascade. Therefore, autophagic cell death via (-)-gossypol is Beclin 1-dependent [20]. Our data are consistent with that of the BH3 mimetic, ABT-737, which also induces autophagy by disrupting the Bcl-2:Beclin 1 complex formed at the ER and not mitochondria [47]. In addition, our lab has also found that (-)-gossypol can induce JNK signaling and subsequent phosphorylation of Bcl-2, disrupting the Bcl-2:Beclin 1 complex (Lian and Marquez, manuscript in preparation).
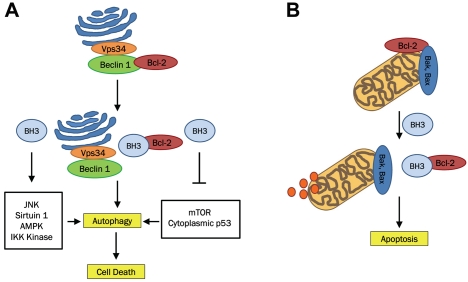
As previously discussed, BH3 mimetics induce autophagy by disrupting the Bcl-2:Beclin 1 inhibitory complex. A recent compelling study has demonstrated that BH3 mimetics, ABT-737 and HA14-1, also stimulate other pro-autophagic pathways and hence activate the nutrient sensors Sirtuin 1 and AMPK, inhibit mTOR, deplete cytoplasmic p53, and trigger the IKK Kinase [22]. Activation of autophagy was independent of reduced oxidative phosphorylation or reduced cellular ATP concentrations. Furthermore, induction of autophagy by ATBT-737 and HA14-1 was completely inhibited by knockdown of Beclin 1 or Vps34. This suggests that BH3 mimetics can act across multiple pathways, eliciting a coordinated effort to maximally induce autophagy. In addition, disrupting the Bcl-2:Beclin 1 complex, elicits a signaling response that induces multiple signals activating autophagy (Figure 2).
Figure 2.
BH3 mimetics (i.e. (-)-Gossypol, ABT-236, ABT-737, HA14-1) bind Bcl-2 and prevent Bcl-2 inhibition of autophagy- and apoptosis-associated cell death. BH3 mimetics can also induce autophagy by activating JNK, Sirtuin 1, AMPK, IKK Kinase as well as inhibiting mTOR and depleting cytoplasmic p53.
Conclusions and future directions
As this review outlines, multiple mechanisms regulate the autophagy- inhibiting Bcl-2:Beclin 1 complex. The expression levels of Bcl-2 and Beclin 1 have demonstrated the importance of the affinity for each component in maintaining or disrupting this inhibitory complex. The discovery of binding partners that alter the affinity of Bcl-2:Beclin 1 for one another, are also becoming more evident. Cellular location, whereby the complex is formed at the ER or mitochondria, are also important contributing factors as to whether this complex affects autophagy. More drug treatment research studies are demonstrating that overriding and disrupting the Bcl-2:Beclin 1 complex occurs by phosphorylation of key kinases, especially JNK phosphorylation of Bcl-2. Developing combination therapies between BH3 mimetics and JNK activators will provide a greater “double-hit” effect in disrupting the Bcl-2:Beclin 1 complex, eventually leading cancer cells to autophagic/apoptotic cell death. Identifying the major mechanisms that maintain/disrupt this complex will allow us to develop additional drug treatments that can target this complex from many approaches ensuring a greater therapeutic outcome.
References
- 1.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. The Journal of clinical investigation. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer cell. 2003;4:422–424. doi: 10.1016/s1535-6108(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 3.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouschop KM, Wouters BG. Regulation of autophagy through multiple independent hypoxic signaling pathways. Current molecular medicine. 2009;9:417–424. doi: 10.2174/156652409788167131. [DOI] [PubMed] [Google Scholar]
- 5.Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T, Brodie C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. International journal of cancer. Journal international du cancer. 2009;125:717–722. doi: 10.1002/ijc.24402. [DOI] [PubMed] [Google Scholar]
- 6.Kuwahara Y, Oikawa T, Ochiai Y, Roudkenar MH, Fukumoto M, Shimura T, Ohtake Y, Ohkubo Y, Mori S, Uchiyama Y. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell death & disease. 2011;2:e177. doi: 10.1038/cddis.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nature reviews. Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 8.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature reviews. Molecular cell biology. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 9.Platini F, Perez-Tomas R, Ambrosio S, Tessitore L. Understanding autophagy in cell death control. Current pharmaceutical design. 2010;16:101–113. doi: 10.2174/138161210789941810. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosk-lonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell death and differentiation. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denton D, Shravage B, Simin R, Baehrecke EH, KumarS Larval midgut destruction in Drosophila: not dependent on caspases but suppressed by the loss of autophagy. Autophagy. 2010;6:163–165. doi: 10.4161/auto.6.1.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen HM, Codogno P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy. 2011;7:457–465. doi: 10.4161/auto.7.5.14226. [DOI] [PubMed] [Google Scholar]
- 13.Shen S, Kepp O, Kroemer G. The end of autophagic cell death? Autophagy. 2012:8. doi: 10.4161/auto.8.1.16618. [DOI] [PubMed] [Google Scholar]
- 14.Shen S, Kepp O, Michaud M, Martins I, Minoux H, Metivier D, Maiuri MC, Kroemer RT, Kroemer G. Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene. 2011;30:4544–4556. doi: 10.1038/onc.2011.168. [DOI] [PubMed] [Google Scholar]
- 15.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell death and differentiation. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. The Journal of biological chemistry. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 17.He C, Levine B. The Beclin 1 interactome. Current opinion in cell biology. 2010;22:140–149. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell death and differentiation. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. Journal of virology. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian J, Karnak D, Xu L. The Bcl-2-Beclin 1 interaction in (-)-gossypol-induced autophagy versus apoptosis in prostate cancer cells. Autophagy. 2010;6:1201–1203. doi: 10.4161/auto.6.8.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 22.Malik SA, Orhon I, Morselli E, Criollo A, Shen S, Marino G, BenYounes A, Benit P, Rustin P, Maiuri MC, Kroemer G. BH3 mimetics activate multiple pro-autophagic pathways. Oncogene. 2011;30:3918–3929. doi: 10.1038/onc.2011.104. [DOI] [PubMed] [Google Scholar]
- 23.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 24.Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, Mannucci S, De Nisi MC, Toscano M, Malagnino V, Falzarano SM, Pirtoli L, Tosi P. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. International journal of oncology. 2007;30:429–436. [PubMed] [Google Scholar]
- 25.Shi YH, Ding ZB, Zhou J, Qiu SJ, Fan J. Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy. 2009;5:380–382. doi: 10.4161/auto.5.3.7658. [DOI] [PubMed] [Google Scholar]
- 26.Ahn CH, Jeong EG, Lee JW, Kim MS, Kim SH, Kim SS, Yoo NJ, Lee SH. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2007;115:1344–1349. doi: 10.1111/j.1600-0463.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 27.Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, Guo L, Wu XY, Xu J, Long ZJ, Zhao Y, Zhou WH, Mai HQ, Liu Q, Hong MH. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy. 2010;6:395–404. doi: 10.4161/auto.6.3.11303. [DOI] [PubMed] [Google Scholar]
- 28.Huang JJ, Li HR, Huang Y, Jiang WQ, Xu RH, Huang HQ, Lv Y, Xia ZJ, Zhu XF, Lin TY, Li ZM. Beclin 1 expression: a predictor of prognosis in patients with extranodal natural killer T-cell lymphoma, nasal type. Autophagy. 2010;6:777–783. doi: 10.4161/auto.6.6.12784. [DOI] [PubMed] [Google Scholar]
- 29.Shen Y, Li DD, Wang LL, Deng R, Zhu XF. Decreased expression of autophagy-related proteins in malignant epithelial ovarian cancer. Autophagy. 2008;4:1067–1068. doi: 10.4161/auto.6827. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19:6627–6631. doi: 10.1038/sj.onc.1204087. [DOI] [PubMed] [Google Scholar]
- 31.Karnak D, Xu L. Chemosensitization of prostate cancer by modulating Bcl-2 family proteins. Curr Drug Targets. 2010;11:699–707. doi: 10.2174/138945010791170888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchholz TA, Garg AK, Chakravarti N, Aggarwal BB, Esteva FJ, Kuerer HM, Singletary SE, Hortobagyi GN, Pusztai L, Cristofanilli M, Sahin AA. The nuclear transcription factor kappaB/bcl -2 pathway correlates with pathologic complete response to doxorubicin-based neoadjuvant chemotherapy in human breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:8398–8402. doi: 10.1158/1078-0432.CCR-05-0885. [DOI] [PubMed] [Google Scholar]
- 33.Lima RT, Martins LM, Guimaraes JE, Sambade C, Vasconcelos MH. Specific downregulation of bcl-2 and xIAP by RNAi enhances the effects of chemotherapeutic agents in MCF-7 human breast cancer cells. Cancer gene therapy. 2004;11:309–316. doi: 10.1038/sj.cgt.7700706. [DOI] [PubMed] [Google Scholar]
- 34.Akar U, Chaves-Reyez A, Barria M, Tari A, Sanguino A, Kondo Y, Kondo S, Arun B, Lopez-Berestein G, Ozpolat B. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy. 2008;4:669–679. doi: 10.4161/auto.6083. [DOI] [PubMed] [Google Scholar]
- 35.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Chang NC, Nguyen M, Germain M, Shore GC. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. The EMBO journal. 2010;29:606–618. doi: 10.1038/emboj.2009.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, D'Amelio M, Nardacci R, Romagnoli A, Piacentini M, Cecconi F, Fimia GM. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. The Journal of cell biology. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strappazzon F, Vietri-Rudan M, Campello S, Nazio F, Florenzano F, Fimia GM, Piacentini M, Levine B, Cecconi F. Mitochondrial BCL-2 inhibits AMBRA1-induced autophagy. The EMBO journal. 2011;30:1195–1208. doi: 10.1038/emboj.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. The Journal of cell biology. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Molecular and cellular biology. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Molecular cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zalckvar E, Berissi H, Eisenstein M, Kimchi A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009;5:720–722. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, Stolz DB, Yu J, Zhang L. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer research. 2011;71:3625–3634. doi: 10.1158/0008-5472.CAN-10-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, Agostinis P, Vanden Berghe T, Lippens S, Vandenabeele P. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell death & disease. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nature cell biology. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 46.Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, Xiang D, Ji M, Lawrence TS, Xu L. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell death and differentiation. 2011;18:60–71. doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. The EMBO journal. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]