Abstract
Objective
To test the hypothesis that harm avoidance, a trait associated with behavioral inhibition, is associated with risk of developing Alzheimer’s disease.
Methods
A total of 791 adults aged 55 years and older without dementia completed a standard self report measure of harm avoidance. They then underwent annual evaluations that included detailed cognitive testing and clinical classification of mild cognitive impairment, dementia and Alzheimer’s disease. In a uniform neuropathologic examination of those who died, counts of neuritic plaques diffuse plaques, and neurofibrillary tangles were standardized and combined to yield a pathologic measure of disease. The relation of harm avoidance to incidence of Alzheimer’s disease and related outcomes was estimated in analyses adjusted for age, sex, and education.
Results
During a mean of 3.5 years of annual observation, 98 people (12.4%) developed incident Alzheimer’s disease. High level of harm avoidance (90th percentile) was associated with a more than twofold increase in risk of Alzheimer’s disease compared to a low score (10th percentile). Higher harm avoidance was also associated with increased incidence of mild cognitive impairment and more rapid decline in episodic memory, working memory, and perceptual speed (but not semantic memory or visuospatial ability). In 116 participants who died and underwent brain autopsy, harm avoidance was not related to a composite measure of plaques and tangles.
Conclusion
High level of the harm avoidance trait, indicating a tendency toward behavioral inhibition, is related to risk of developing Alzheimer’s disease and its precursor, mild cognitive impairment.
Keywords: Harm avoidance, Alzheimer’s disease, mild cognitive impairment, cognitive decline, longitudinal studies, brain autopsy
Harm avoidance is a personality trait indicative of behavioral inhibition (1). Persons with a high level of the trait tend to be pessimistic, apprehensive, shy, and easily fatigued and to avoid new and potentially aversive situations. In prospective studies of children and young adults, higher harm avoidance has been associated with better health-related behavior (2,3) and health outcomes (4,5), possibly because those low in the trait tend to be daring and impulsive. The relation of the trait to health in old age is not well understood, however, with some cross-sectional data linking higher level of the trait to increased likelihood of disability (6) and chronic illness (7–9). In the present study, we test the hypothesis that higher level of the trait is associated with increased risk of Alzheimer’s disease (AD) in old age. The hypothesis is based on the observation that harm avoidance is associated with lifestyle patterns (e.g., reduced physical activity (10) and emotional predispositions (e.g., anxiety proneness [11,12]) which have been associated with more rapid cognitive decline and increased risk of dementia in old age.
To examine the relation of harm avoidance to the development of AD, we used data from the Rush Memory and Aging Project. Participants aged 55 years and older completed a standard self report measure of the trait. At annual intervals thereafter, they had evaluations that included cognitive function testing and clinical classification of AD and its precursor, mild cognitive impairment (MCI). Those who died during the study period had a brain autopsy and uniform neuropathological examination to quantify pathologic burden of AD. In analyses, we tested the hypothesis that higher level of the trait is associated with increased risk of MCI and AD and more rapid decline in cognitive function. We also examined whether higher level of harm avoidance was a symptom of the underlying neuropathologic burden of AD rather than a risk factor for its occurrence.
METHODS
Participants
All subjects are participants from the Rush Memory and Aging Project (13), which involves annual clinical evaluations and brain autopsy at death. They were recruited from retirement communities, subsidized housing facilities, churches, and other social service agencies in the greater Chicago metropolitan area. Eligibility required age of 55 years or older, absence of a previous diagnosis of dementia or AD, and agreement to annual clinical evaluations and brain donation at death.
Data for the present analyses were collected between February of 2004 and December of 2009. During this period, 1,000 participants without dementia completed the harm avoidance scale. Of these, 31 died before the first annual follow-up evaluation and 94 had been in the study less than 12 months. This left 875 eligible for follow-up, and follow-up data were available on 791 (90.4 %). They had a mean age at baseline of 80.6 (SD=7.5), a mean of 14.5 years of education (SD=3.1), 76.2% were women, and 89.1% were white and non-Hispanic. They were followed for a mean of 3.5 years (SD=1.4).
Standard Protocol Approvals, Registrations, and Patient Consents
The Rush Memory and Aging Project was approved by the Institutional Review Board of Rush University Medical Center. After a detailed explanation of the study, written informed consent was obtained from all participants.
Clinical Evaluation
Participants had a uniform clinical evaluation each year consisting of a medical history, complete neurological examination, and cognitive function testing. On the basis of this evaluation, an experienced clinician diagnosed dementia and MCI, blinded to previously collected data, following previously developed procedures (14, 15). First a neuropsychologist rated impairment in 5 cognitive domains (orientation, attention, memory, language, perception) based on review of all neuropsychological test data. To maintain uniformity in the ratings across time and raters, the neuropsychologist was provided with provisional ratings of each cognitive domain based on educationally adjusted cutoff scores on 11 individual tests (16). Second, a clinician made diagnoses following an in person evaluation and review of all clinical data including the neuropsychologist’s ratings. Dementia was diagnosed using the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (17) which require a history of declining cognition and evidence of impaired function in at least two cognitive domains. For AD, one of the impaired domains had to be memory.
Persons who did not meet dementia criteria but were impaired in one or more cognitive domains were classified as MCI. These criteria are similar to those proposed for cognitive impairment with no dementia (18). They have been shown in this and other cohorts to be associated with subsequent risk of cognitive decline, dementia, and death (16,19) and the neuropathologic hallmarks of dementia (20,21). Further information on the implementation of these criteria in this (22) and other (23) cohorts is published elsewhere.
Assessment of Harm Avoidance
Subjects completed the 35-item Harm Avoidance scale from the Temperament and Character Inventory (12). Items from four subscales were rated as true or false: anticipatory worry (11 items; e.g., “Things often go wrong for me unless I’m careful”; range: 0–11), fear of uncertainty (7 items; e.g., “I usually feel tense and worried when I have to do something new and unfamiliar”; range: 0–7), shyness (8 items; e.g., “I am more shy than most people”; range: 0–8), and fatigability (9 items; e.g., “I have less energy and tire more quickly than most people”; range: 0–9). The score for the full scale (range: 0–35) and each subscale is the number of item responses indicative of the trait in question. These continuous trait measures were used in analyses. The internal consistency of the scale in this cohort has previously been shown to be adequate, with Cronbach’s coefficient alpha equal to .89 for the total score (6).
Assessment of Cognitive Function
Cognition function was assessed annually with a battery of 19 performance tests. There were seven episodic memory measures: Word List Memory, Recall and recognition plus immediate and delayed recall of the East Boston story and story A from Logical Memory. Semantic memory was assessed with a 15-item version of the Boston Naming Test and measures of word reading and verbal fluency. Digit Span Forward, Digit Span Backward, and Digit Ordering assessed working memory. There were four measures of perceptual speed: Symbol Digit Modalities Test, Number Comparison, and word reading and color naming measures from a modified Stroop Neuropsychological Screening Test. Visuospatial ability was assessed with short forms of Judgment of Line Orientation and Standard Progressive Matrices. To minimize floor and ceiling artifacts, psychometrically established composite measures were used in analyses. We used a measure of global cognition based on all 19 tests and measures of episodic memory (7 tests), semantic memory (3 tests), working memory (3 tests), perceptual speed (4 tests), and visuospatial ability (2 tests). We wanted the contribution of each test to the composite measure to be approximately equal. Therefore, scores on individual tests were converted to z scores, using the baseline mean and SD from the entire cohort, and z scores were averaged to yield composite scores. Further information on the individual tests and the derivation of these composite scores is contained in previous publications (24,25).
Other Data Collection
Self report data on affect, personality, and life style was obtained at baseline. Depressive symptoms were assessed with the Center for Epidemiological Studies Depression Scale, using a 10-item (e.g., “I felt sad”) version (26). Loneliness was assessed with a 5-item (e.g., “I miss having people around”) form (27) of the deJong-Gierveld Loneliness Scale (28). The neuroticism trait was measured with the standard 48-item (e.g., “I have a low opinion of myself) scale from the NEO Personality Inventory (29). Physical activity was quantified as hours per week spent in five activities (e.g., walking for exercise), as previously described (30). A composite measure of cognitively stimulating activity was based on frequency of participation in seven mentally demanding activities (e.g., reading a book) (22).
Apolipoprotein E genotype was done by Agencourt Bioscience Corporation (Beverly, MA) using high throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the apolipoprotein gene on chromosome 19. Subjects were divided into those with versus without at least one copy of the ε4 allele.
Neuropathologic Assessment
The brain was removed in a standard fashion and cut coronally into 1-cm slabs which were fixed in 4% paraformaldehyde. Tissue from 5 brain regions (midfrontal gyrus, superior temporal gyrus, inferior parietal gyrus, entorhinal cortex, CA1/subiculum region of hippocampus) was cut into 0.5-cm blocks, embedded in paraffin, sectioned a 6μm, and then a modified Bielschowsky silver stain was applied. In each region, neuritic plaques, diffuse plaques, and neurofibrillary tangles were separately counted by a neuropathologist or technician blinded to all clinical data. Raw counts of each lesion type in each region were converted to standard scores which were averaged to yield a composite measure of AD neuropathologic burden. Further information on the neuropathological examination and derivation of the composite pathologic measure is published elsewhere (20,21).
Data Analysis
We used Cox proportional hazards models (31) to test the relation of harm avoidance to risk of developing AD with observations censored for persons not diagnosed with AD at the end of the study period. Harm avoidance was treated as a continuous measure in all analyses. Each model included terms to control for the potentially confounding effects of age, sex, and education. Because preliminary analyses suggested that allowing for nonlinear age effects did not influence results, age was treated as a continuous measure. The core model included a term for harm avoidance score. In separate subsequent models, we added terms for the interaction of harm avoidance with sex, age, and education; and for depressive symptoms, loneliness, neuroticism, physical activity, cognitively stimulating activity, and inheritance of an ε4 allele. We repeated the original model with trait subscores in place of the global trait score. In subsequent analyses, we related trait scores to risk of developing MCI. To check the proportional hazard assumption, we examined the correlation between the scaled Schoenfeld residuals and time for each covariate (32).
The relation of trait scores to change in cognitive function was assessed with mixed-effects regression models (33). The initial analysis used a composite measure of global cognition as the outcome. Subsequent analyses adjusted for other affective states or traits, used measures of specific cognitive function, and excluded baseline MCI. Model assumptions about normality and homoscedasticity of errors were assessed by examining the conditional studentized residuals and the effect of removing outliers. In a final set of linear regression models, harm avoidance and its subscores were each regressed on a composite measure of plaques and tangles in separate analyses.
RESULTS
Harm avoidance scores ranged from 0 to 34 (mean=10.5, SD=6.6, skewness=0.7), with higher values indicating more of the trait. Harm avoidance was unrelated to age (r=.06, p=.07) or possession of an apolipoprotein E ε4 allele (χ2 [1]=0.8, p=.36), inversely related to education (r=−.20, p<.001), and higher in women than men (χ2[1]=9.3, p=.002). Higher trait score was associated with higher level of depressive symptoms (r=.42, p<.001), loneliness (r=.34, p<.001), and neuroticism (r=.66, p<.001) and lower level of global cognitive functioning (r=−.17, p<.001), cognitive activity (r=−.17, p<.001), and physical activity (r=−.11, p=.002).
Harm Avoidance and Incidence of Alzheimer’s disease
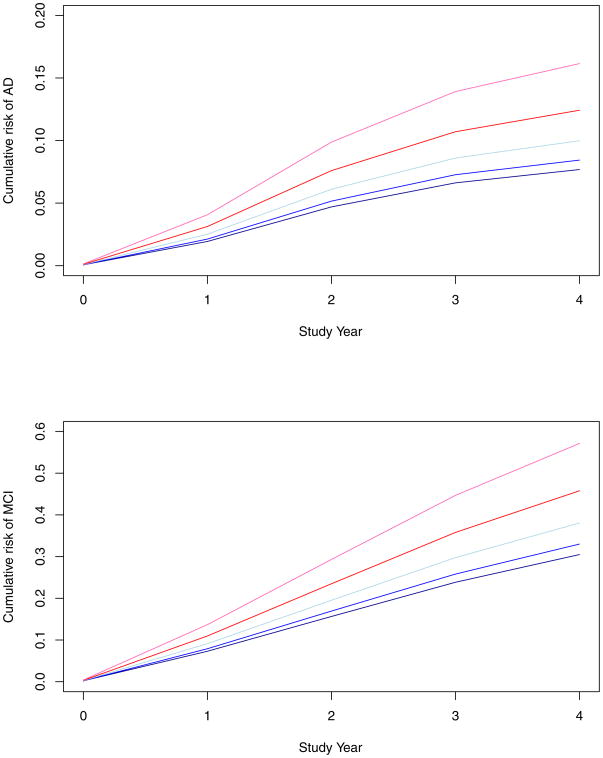
During a mean of 3.5 years of observation, 98 people developed AD. Those who became affected were older, more cognitively impaired, and more likely to have an ε4 allele than those who remained unaffected, and they differed in harm avoidance, loneliness, and cognitive activity (Table 1). We assessed the relation of harm avoidance score to risk of AD in a proportional hazards model that controlled for age, sex, and education. Higher level of the trait was associated with increased risk of AD (hazard ratio=1.045, 95% confidence interval: 1.013, 1.077). To visually examine this effect, we plotted the model-based estimates of risk of developing AD at different levels of harm avoidance (upper panel of Figure 1). A person with a high level of the trait (score=20, 90th percentile, red line) was more than twice as likely to develop AD as a person with a low level of the trait (score=3, 10th percentile, black line).
Table 1.
Baseline characteristics of participants who developed Alzheimer’s disease and those who did not*
| Characteristics | Incident Alzheimer’s Disease (n=98) | Unaffected persons (n=693) | P Value |
|---|---|---|---|
| Age, y | 84.8 (6.2) | 80.0 (7.4) | <.001 |
| Education | 14.6 (2.6) | 14.5 (3.2) | .79 |
| Women, % | 73.5 | 76.6 | .49 |
| Harm avoidance | 12.3 (7.4) | 10.2 (6.4) | .01 |
| CES-D score | 1.5 (1.8) | 1.2 (1.7) | .06 |
| Loneliness | 2.5 (0.6) | 2.2 (0.6) | <.001 |
| Neuroticism | 72.6 (19.3) | 68.5 (21.7) | .08 |
| Global cognition | −0.36 (0.46) | 0.23 (0.48) | <.001 |
| Physical activity | 3.2 (3.8) | 3.2 (3.7) | .95 |
| Cognitively stim. activity | 3.0 (0.7) | 3.2 (0.7) | .005 |
| APOE ε4 allele, % | 31.6 | 20.7 | .02 |
Data are mean (SD) unless otherwise noted. CES-D is for Center for Epidemiologic Studies Depression scale; APOE is for apolipoprotein E.
Figure 1.
Cumulative risk of developing Alzheimer’s disease (upper panel) and mild cognitive impairment (lower panel) in persons with different levels of harm avoidance (90th percentile, red; 75th percentile, green; 50th percentile, blue; 25th percentile, yellow; 10th percentile, black), adjusted for age, sex, and education.
The scaled Schoenfeld residuals for harm avoidance, age, and education did not vary over time, consistent with the proportional hazard assumption of the Cox regression model. The residuals for sex were related to time, but in a subsequent Cox model stratified for sex, the association of harm avoidance with risk of AD was virtually identical to the original analysis (hazard ratio = 1.043, 95% confidence interval: 1,013, 1.073).
Because women tend to have higher levels of the trait than men (12), we examined the possibility that gender might modify the association of harm avoidance with risk of AD. There was no interaction, however (estimate = −0.038, SE = 0.038, p = .31). In subsequent analyses, there was no evidence that the effect of harm avoidance varied by age (estimate = −0.002, SE = 0.002, p =.31) or education (estimate = 0.000, SE = 0.005, p = .94).
Depressive symptoms (26,34), loneliness (27), and the neuroticism trait (35,36) have been linked to late life dementia and each was associated with harm avoidance in this cohort. To determine whether these associations affected results, we adjusted for each covariate in separate analyses. Harm avoidance continued to be associated with increased risk of AD after controlling for depressive symptoms (hazard ratio=1.038; 95% confidence interval: 1.005, 1.073), loneliness (hazard ratio=1.034; 95% confidence interval: 1.001, 1.068), and neuroticism (hazard ratio=1.046; 95% CI: 1.003, 1.091). With all 3 of these correlated covariates in the same model, the point estimate of the association was similar to previous analyses (hazard ratio = 1.037) but the standard error was increased (95% confidence interval: 0.996, 1.081), likely due to collinearity.
We considered additional factors that might have affected results. First, because physical activity is related to harm avoidance (10) and dementia (37), we repeated the analysis with a term added for self reported level of physical activity at baseline. Second, we repeated the analysis with a term for frequency of participation in cognitively stimulating activities and again with a term for possession of an apolipoprotein E ε4 allele because they are established risk factors for AD. The association of harm avoidance with risk of AD persisted in each analysis (hazard ratios for harm avoidance ranged from 1.028 to 1.045, each p<.05).
Substantial variation was evident in harm avoidance subscores: anticipatory worry (range: 0–11), fear of uncertainty (range: 0–7), shyness (range: 0–8), fatigability (range: 0–9). To determine whether the subscores were differentially associated with AD risk, we analyzed each in a separate model. As shown in Table 2, higher levels of anticipatory worry, fear of uncertainty, and fatigability were each related to increased risk of AD with a nearly significant effect for shyness.
Table 2.
Relation of harm avoidance subscales to risk of developing Alzheimer’s disease*
| Model Term | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Anticipatory worry | 1.117 | 1.019, 1.225 |
| Age | 1.109 | 1.070, 1.149 |
| Sex | 1.265 | 0.788, 1.077 |
| Education | 1.017 | 0.947, 1.092 |
| Fear of uncertainty | 1.153 | 1.019, 1.305 |
| Age | 1.115 | 1.075, 1.155 |
| Sex | 1.309 | 0.812, 2.111 |
| Education | 1.017 | 0.946, 1.093 |
| Shyness | 1.073 | 0.987, 1.166 |
| Age | 1.110 | 1.072, 1.150 |
| Sex | 1.217 | 0.761, 1.945 |
| Education | 1.008 | 0.939, 1.081 |
| Fatigability | 1.111 | 1.020, 1.210 |
| Age | 1.108 | 1.070, 1.148 |
| Sex | 1.211 | 0.757, 1.939 |
| Education | 1.012 | 0.943, 1.087 |
From four separate proportional hazards models.
Harm Avoidance and Incidence of Mild Cognitive Impairment
We conducted additional analyses to assess whether harm avoidance is associated with incidence of MCI, one of the earliest clinical manifestations of AD (38). Of 588 people without evidence of any cognitive impairment at baseline, 208 (35.4%) developed MCI during the study period. Higher level of harm avoidance was associated with increased incidence of MCI (hazard ratio =1.038; 95% confidence interval: 1.015, 1.061). The lower panel of Figure 1 shows the model-based estimates of risk of developing MCI at different levels of harm avoidance. Risk of MCI was increased by more than 80% with a high level of the trait (score =20, 90th percentile, red line) compared to a low level (score=3, 10th percentile, black line). In line with the proportional hazards assumption, there was no evidence that model coefficients varied over time.
Similar associations were observed for all four trait subscores (hazard ratio for anticipatory worry =1.109; 95% confidence interval: 1.036, 1.188; hazard ratio for fear of uncertainty =1.100; 95% confidence interval: 1.007, 1.202; hazard ratio for shyness = 1.066; 95% confidence interval: 1.001, 1.134; hazard ratio for fatigability =1.086; 95% confidence ratio: 1.022, 1.055).
Harm Avoidance and Cognitive Decline
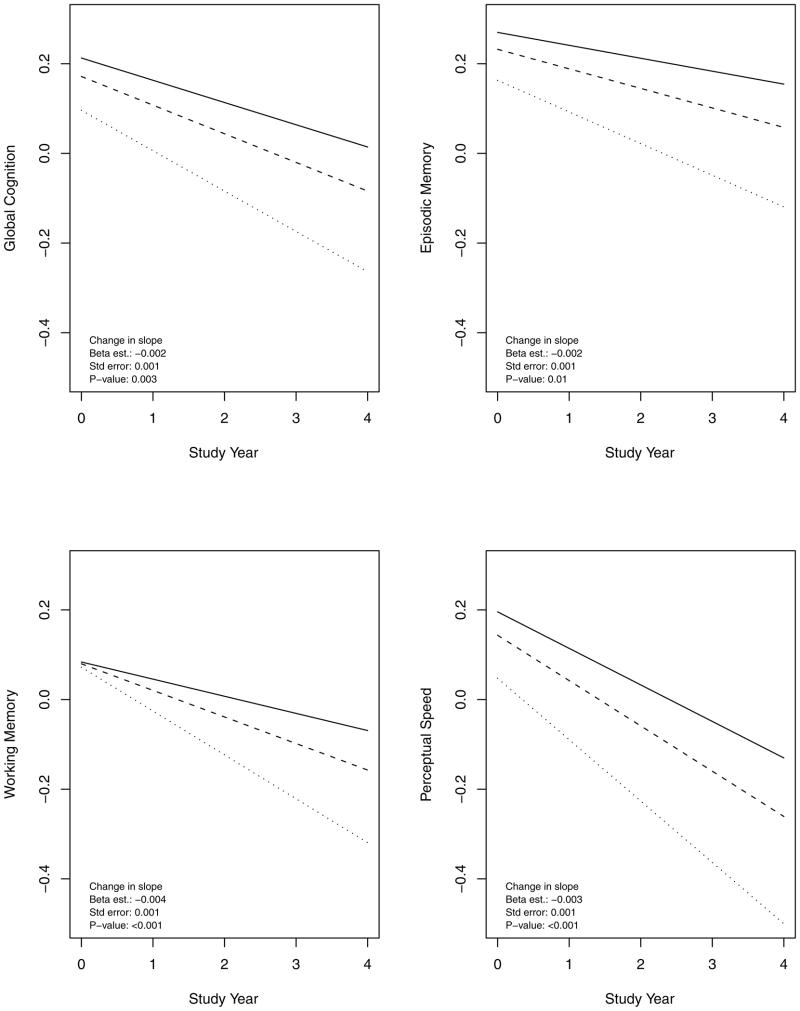
Because of the insidious onset and gradual progression of AD, separating MCI from normality and dementia from MCI can be difficult. To ensure that results obtained with MCI and AD were not the result of diagnostic bias or baseline differences in level of cognitive function, we examined the relation of harm avoidance to cognitive decline, the primary manifestation of the disease. In a mixed-effects model adjusted for age, sex, and education, harm avoidance was associated with lower baseline score on a composite measure of global cognition (beta estimate = −0.007, SE=0.003, p=.007) and, with this baseline effect accounted for, more rapid global cognitive decline (beta estimate =−0.002, SE=0.001, p=.003). Rate of global cognitive decline was approximately 50% faster in a person with a high level of the trait (90th percentile, dotted line) compared to a low level (10th percentile, solid line), as shown in the upper left portion of Figure 2. Plots of the conditional studentized residuals suggested adequate model fit though there were some outliers. The association of harm avoidance with MCI risk was unchanged, however, when the analysis was repeated with the outliers removed (beta estimate = −0.002, SE = 0.001, p < .001).
Figure 2.
Rate of decline in different cognitive domains in persons with different levels of harm avoidance (90th percentile, dotted line; 50th percentile, dashed line; 10th percentile, solid line), adjusted for age, sex, and education.
In subsequent models, we controlled for other affective states and traits. The association of harm avoidance with global cognitive decline persisted in separate analyses that adjusted for depressive symptoms (beta estimate = −0.003, SE = 0.001, p = .004), loneliness (beta estimate = −0.002, SE = 0.001, p =.03), and neuroticism (beta estimate = −0.002, SE = 0.001, p = .049) and in an analysis that simultaneously adjusted for all 3 of these covariates (beta estimate=−0.002, SE=0.001, p=0.047).
To determine whether harm avoidance was related to decline in some cognitive systems but not others, we repeated the analysis with composite measures of specific cognitive functions in place of the global measure. Harm avoidance was associated with decline in episodic memory, working memory and perceptual speed (figure 2) but not semantic memory or visuospatial ability (Table 3). Results for working memory were especially notable. As shown in Figure 2 (lower left), trait score was unrelated to baseline level of working memory, but those with a high level of the trait (90th percentile, dotted line) experienced twice the rate of working memory decline as persons low in the trait (10th percentile, solid line).
Table 3.
Relation of harm avoidance to change in different domains of cognitive function*
| Cognitive Domain | Model Term | Beta Estimate | SE | P Value |
|---|---|---|---|---|
| Episodic memory | Time | −0.045 | 0.013 | <.001 |
| Harm avoidance | −0.006 | 0.003 | .07 | |
| Harm avoidance x time | −0.002 | 0.001 | .01 | |
| Semantic memory | Time | −0.039 | 0.011 | <.001 |
| Harm avoidance | −0.007 | 0.003 | .02 | |
| Harm avoidance x time | −0.001 | 0.001 | .26 | |
| Working memory | Time | −0.101 | 0.015 | <.001 |
| Harm avoidance | −0.000 | 0.004 | .91 | |
| Harm avoidance x time | −0.004 | 0.001 | <.001 | |
| Perceptual speed | Time | −0.112 | 0.013 | <.001 |
| Harm avoidance | −0.009 | 0.004 | .02 | |
| Harm avoidance x time | −0.003 | 0.001 | <.001 | |
| Visuospatial ability | Time | −0.075 | 0.018 | <.001 |
| Harm avoidance | −0.010 | 0.004 | .005 | |
| Harm avoidance x time | −0.001 | 0.001 | .19 |
From five separate mixed-effects models adjusted for age, sex, and education. SE is for standard error.
To see if the association of harm avoidance with cognitive decline was due to an association with MCI, we repeated analyses excluding those with MCI at baseline. Higher harm avoidance was associated with more rapid decline in global cognition (beta estimate = −0.003, SE = 0.001, p = .001), working memory (beta estimate = −0.004, SE = 0.001, p<.001) and perceptual speed (beta estimate = −0.003, SE = 0.001, p = .001) but not with decline in other cognitive measures.
Harm Avoidance and AD Pathology
We conducted a final series of analyses to determine whether harm avoidance was a subtle manifestation of the neuropathologic changes underlying AD. At the time of these analyses, 220 study participants had died; 182 (82.7%) underwent brain autopsy, the results of which were available in 116 of whom 74.1% were women. They had a mean age at death of 88.2 (SD = 5.9), a mean postmortem interval of 7.9 hours (SD = 7.6), and mean of 7.5 months (SD = 4.8) from last clinical evaluation to death. In separate linear regression models adjusted for age at death, sex, and education, a composite measure of plaques and tangles (mean = 0.52, SD = 0.49) was not related to harm avoidance (beta estimate = 1.37, SE = 1.37, p= .32) or its component scores (beta estimate for anticipatory worry = 0.12, SE = 0.47, p = .81; beta estimate for fear of uncertainty = 0.51, SE = 0.31, p = .10; beta estimate for shyness = 0.68, SE = 0.51, p = .19; beta estimate for fatigability = 0.08, SE = 0.50, p= .87).
DISCUSSION
Harm avoidance is a broad anxiety-related trait. We found that older people with a high level of the trait, indicating a tendency to be excessively risk averse, were about twice as likely to develop MCI and dementia as people with a low level of the trait. The results suggest that harm avoidance is a risk factor for late life dementia.
We are not aware of prior research on the association of harm avoidance with dementia. Factors may be related to the development of AD through an association with level of cognitive function, rate of cognitive decline, or, as in the case of harm avoidance, both. Importantly, the association of harm avoidance with cognitive decline was adjusted for baseline level of cognitive function.
The basis of the association between harm avoidance and AD is uncertain. One possibility is that a high score is a subtle early sign of the disease. However, harm avoidance was not related to the underlying neuropathologic burden of AD unlike other early signs of the disease such as olfactory impairment (39,40), gait dysfunction (41), and low body mass (42). Further, among those without evidence of cognitive impairment at baseline, harm avoidance predicted subsequent cognitive decline and development of MCI, linking the trait to the emergence of the earliest signs of late life dementia.
Statistical adjustment for negative affect and lifestyle activities did not substantially affect the association of harm avoidance with risk of AD. This suggests that other factors are involved. Lower harm avoidance is associated with resilience, optimism, composure, and energy (12,43). These attributes may facilitate adaptation to accumulating neurodegeneration and other vicissitudes of late life. In addition, willingness to take risks and tolerate uncertainty is associated with better cognitive function (44). Over the life span, exposure to such trial and error learning opportunities might enhance executive cognitive functions in those able to tolerate risk and uncertainty. In this regard, it is noteworthy that harm avoidance was mainly related to change in working memory and perceptual speed, executive functions that involve manipulating and rapidly processing information.
Several factors increase confidence in these findings. The diagnoses of AD and MCI were based on a uniform evaluation and accepted criteria applied by experienced clinicians, making it unlikely that results were affected by clinical misclassification. Participation in follow-up and autopsy was high, minimizing the likelihood that selective attrition influenced findings. Results were consistent with multiple outcomes (i.e., AD, MCI, cognitive decline) and with multiple measures of the harm avoidance trait (i.e., total score, subscores).
This study also has important limitations. In particular, the cohort is selected. It will be important, therefore, to replicate these findings in a defined population of older people. It is possible that results might differ with a longer observation period. Because most covariates were assessed with brief measures, some residual confounding by these variables may have occurred.
Acknowledgments
This research was supported by grants from the National Institutes of Health.
The authors thank the many Illinois residents for participating in the Rush Memory and Aging Project; Traci Colvin, MPH, and Karen Skish for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; and Donna Esbjornson, MS, for statistical programming. This research was supported by the National Institute on Aging grants R01AG17917, R01AG24480, R01AG24871, and R01AG33678, and by the Illinois Department of Public Health. The sponsors have no role in the design and conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Dr. Wilson had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Glossary
- AD
Alzheimer’s disease
- MCI
mild cognitive impairment
- SD
standard deviation
- SE
standard error
References
- 1.Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- 2.Masse LC, Tremblay Re. Behavior ob boys in kindergarten and the onset of substance use during adolescence. Arch Gen Psychiatry. 1997;54:62–68. doi: 10.1001/archpsyc.1997.01830130068014. [DOI] [PubMed] [Google Scholar]
- 3.Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res. 1998;12:494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Sovio U, King V, Miettunen J, Ek K, Laitinen J, Joukamaa M, Vieijola J, Jarvelin Cloninger’s temperament dimensions, socio-economic and lifestyle factors and metabolic syndrome markers at age 31 years in the Northern Finland Birth Cohort 1966. J Health Psychol. 2007;12:371–382. doi: 10.1177/1359105307074301. [DOI] [PubMed] [Google Scholar]
- 5.Hintsanen M, Pulkki-Raback L, Juonala M, Viikari JSA, Raitakari OT, Keltikangas-Jarvinen L. Cloninger’s temperament traits and preclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study. J Psychosom Res. 2009;67:77–84. doi: 10.1016/j.jpsychores.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Buchman AS, Arnold SE, Shah RC, Tang Y, Bennett DA. Harm avoidance and disability in old age. Exp Aging Res. 2006;32:243–261. doi: 10.1080/03610730600699142. [DOI] [PubMed] [Google Scholar]
- 7.Fujii C, Harada S, Ohkoshi N, Hayashi A, Yoshizawa K. Cross-cultural traits for personality of patients with Parkinson’s disease in Japan. Am J Med Genetics. 2000;96:1–3. [PubMed] [Google Scholar]
- 8.Jacobs H, Heberlein I, Vieregge A, Vieregge P. Personality traits in young patients with Parkinson’s disease. Acta Neurol Scand. 2001;103:82–87. doi: 10.1034/j.1600-0404.2001.103002082.x. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee A, Jurewicz EC, Applegate LM, Louis ED. Personality in essential tremor: further evidence of non-motor manifestations of the disease. J Neurol Neurosurg Psychiatry. 2004;75:958–961. doi: 10.1136/jnnp.2004.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkers AC, Tulen JH, Duivenvoorden HJ, Gieteling MJ, Wegewijs-de Jong M, van den Broek WW, Passchier J, Pepplinkhuizen L. Effect of personality dimensions on the diurnal pattern of motor activity. J Pers. 2002;70:233–247. doi: 10.1111/1467-6494.05004. [DOI] [PubMed] [Google Scholar]
- 11.Caseras X, Avila C, Torrubia R. The measurement of individual difference in behavioral inhibition and behavioral activation systems: a comparison of personality scales. Personality and Individual Differences. 2003;34:99–1013. [Google Scholar]
- 12.Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A guide to its development and use. St. Louis, MO: Center for Psychobiology of Personality; 1994. [Google Scholar]
- 13.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiol. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer’s disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 15.Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, Hebert LE, Aggarwal NT, Beckett LA, Joglekar R, Berry-Kravis E, Schneider JA. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Tuokko HA, Frerichs RJ. Cognitive impairment with no dementia: longitudinal studies, the findings, and the issues. Clin Neuropsychol. 2000;14:504–525. doi: 10.1076/clin.14.4.504.7200. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer’s disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Mild cognitive impairment is related to Alzheimer’s disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. The relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Hebert LE, Evans DA. Cognitive decline in incident Alzheimer disease in a community population. Neurology. 2010;74:951–955. doi: 10.1212/WNL.0b013e3181d64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- 25.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA. Loneliness and risk of Alzheimer’s disease. Arch Gen Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 28.de Jong-Gierveld J, Kamphuis F. The development of a Rasch-type loneliness scale. Appl Psychol Measurement. 1985;9:289–299. [Google Scholar]
- 29.Costa PT, McCrae RR. Professional manual. Lutz, FL: Psychological Assessment Resources; 1992. NEO Personality Inventory-revised. [Google Scholar]
- 30.Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle Nerve. 2007;35:354–362. doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- 31.Cox DR. Regression models and life tables (with discussion) J R Soc Stat Soc B. 1972;74:187–220. [Google Scholar]
- 32.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer-Verlag; 2000. [Google Scholar]
- 33.Laird N, Ware J. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 34.Devanand DP, Sano M, Tang M-X, Taylor S, Gurland BJ, Wilder G, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proness to psychological distress and risk of Alzheimer’s disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 37.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann NY Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 42.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer’s disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 43.Simeon D, Yehuda R, Cunill R, Knutelska M, Putnam F, Smith LM. Factors associated with resilience in health adults. Psychoneuroendocrinol. 2007;32:1149–1152. doi: 10.1016/j.psyneuen.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Dohmen T, Falk A, Huffman D, Sunde U. IZA Discussion Paper No 2735. Apr, 2007. Are risk aversion and impatience related to cognitive ability? [Google Scholar]