Abstract
Introduction
More than one-fourth of U.S. women are overweight; more than one-third are obese. Maternal obesity has been linked to an increased incidence of stillbirths, fetal macrosomia, fetal intrauterine growth restriction and pre-eclampsia. The placenta plays a key role in the nutrients and oxygen supply to the fetus. The data about structural changes in the placental villous membrane (VM), a major component of the feto-maternal nutrient and oxygen exchange barrier, during obesity are sparse and inconsistent. Our objective was to evaluate the morphometric changes in the placental exchange barrier in a baboon model of obesity.
Materials and methods
The previously described baboon model of maternal obesity was studied. We compared 4 obese to 4 non-obese baboons. Placental stereology with the use of transmission electron microscopy was performed to estimate VM oxygen diffusing capacities and morphometry.
Results
The specific placental oxygen diffusing capacities per unit of fetal weight were similar in baboons and humans. Maternal leptin concentrations correlated negatively with placental basement membrane thickness (r = −0.78, p < 0.05), while fetal leptin levels correlated negatively with endothelial thickness of fetal capillaries (r = −0.78, p < 0.05). The total and specific villous membrane oxygen diffusing capacities were not different between the two groups.
Conclusion
To the best of our knowledge this is the first report of placental oxygen diffusing capacities and placental ultrastructural changes in a baboon model of obesity. Previously reported placental inflammation in maternal obesity is not associated with changes in the VM diffusing capacities and ultrastructure.
Keywords: Placenta, Obesity, Morphometry, Oxygen diffusing capacity, Leptin
1. Introduction
The prevalence of obesity (defined as body mass index, or BMI, ≥ 30 kg/m2) has increased dramatically over the past 20 years [1,2]. Obesity during pregnancy is a serious condition that adversely impacts both maternal and fetal health and is associated with increased risk of gestational hypertension, pre-eclampsia, gestational diabetes and fetal macrosomia [3]. Maternal obesity has been linked to an increased incidence of stillbirths in humans and non-human primates [4–7], intrauterine growth restriction (IUGR) and decreased fetal oxygenation in ruminants [8]. Studies of stillbirths in the Danish British Cohort identified a reduction in median birth weight of stillborn babies in obese women compared with live births suggesting an unrecognized failure to achieve a higher growth potential in these pregnancies [9].
Fetal growth and wellbeing depend heavily on placental nutrient transport and gas exchange which is determined—among other factors—by the structure and function of the placental exchange barrier. The villous membrane (VM) is the major structure of feto-maternal gas exchange in the placenta [10]. Abnormal growth of placental villi has been associated with IUGR and small for gestational age (SGA) fetuses [11,12]. The morphometric structural parameters of the VM have also been described in obesity-related conditions such as pre-eclampsia and diabetes [11,13]. The data regarding placental structure in maternal obesity are sparse and inconsistent [14]. Placental inflammation [6], infiltration by macrophages and neutrophils [15–17], altered placental vascular structure [15,17,18], increased placental weight and other ultra-structural changes [18–20] have been reported. Consumption of higher fat diet in a non-human primate model of obesity decreases utero-placental and feto-placental blood flow [6], however to our best knowledge, no data exist on placental oxygen diffusing capacities in maternal obesity. These data are essential for understanding the link between maternal obesity and stillbirth and for categorizing the origin of fetal hypoxia [21]. The objective of this study was to evaluate the ultrastructural morphometric changes in the placental gas exchange barrier in a baboon model of obesity.
In a previous study of this animal model of obesity, we described that the placenta undergoes inflammatory changes and a decrease in the microvillous surface amplification factor [16]. Presence of inflammatory cells is associated with early overproduction of basement membrane (BM) components as seen in fibrotic conditions [22–24]. In human placenta BM thickening is documented in pre-eclampsia [25] and gestational diabetes [26], both conditions which have been linked to maternal obesity [2]. We hypothesized that the observed inflammatory placental changes seen in obesity would lead to an increased thickness of the exchange barrier and a decrease in oxygen diffusing capacities.
2. Material and methods
2.1. Animal housing and handling
All animals were maintained in a social group environment with partly controlled climate conditions. They were fed and given water ad libitum (LEO5, Purina). The characteristics of the two groups, obese (n = 4) and non-obese (n = 4), of pregnant female baboons (Papio spp.) have been reported previously [16]. Briefly, the animals were selected based on weight (16.7 ± 1.1 kg vs. 15.2 ± 0.7 kg) and obesity index (Rh index) (48.7 ± 1.0 kg/m2 vs 39.1 ± 3.2 kg/m2) at the time of delivery. Cesarean sections were performed at 165 days gestation (0.9 G; term = 185 days). The Animal Care and Use Committee of the Texas Biomedical Research Institute approved all procedures.
2.2. Placental sampling and processing
2.2.1. Stereology
The placenta was removed manually and immediately processed as described previously [27]. Blocks of placenta were randomly selected, using a grid system. Five to eight blocks per placenta were collected, formalin fixed, paraffin embedded, sectioned at 5 mm, and stained with hematoxylin & eosin (H&E). From each section, two randomly selected areas were chosen to assess morphology.
2.2.2. Placental transmission electron microscopy [TEM]
From the above described blocks two slices of placental tissue were randomly selected with the roll of a dice [28] and fixed in 4% glutaraldehyde, 0.5% formaldehyde in 0.1 M Pepers buffer. Postfixation in 1% Zetterqvist's buffered osmium tetroxide occurred for 30 min followed by alcohol dehydration (70–100%). Resin embedding was performed with 1:1 propylene oxide/resin (30 min) and 100% resin (30 min) under 25 psi vacuum. TEM was performed using a JEOL2000EX microscope (JEOL Ltd, Tokyo, Japan).
2.3. Placental morphometry
2.3.1. Morphometric estimation of placental composition
Placental capillary volume fraction was calculated by dividing placental capillary volume by the villous volume. Placental intervillous space star volume and placental capillary volume fraction were derived from previously published data [16]. 190–200 point intersections were measured per sample, and estimation of villous and capillary surface areas was performed as previously described [27].
2.3.2. Morphometric estimations of maternal–fetal oxygen exchange barrier
2.3.2.1. Villous membrane and syncytiotrophoblast thicknesses
Systematic random uniform sampling was applied to choose the visual fields. The position of the first window was randomly selected with the roll of a dice [28]. The syncytiotrophoblast (ST) and VM thickness were measured by superimposing a series of test lines in a random orientation on the histological images. Intersections were counted over the randomly selected specimens. Twenty villi were randomly measured at 1000fold magnification. The intersection of the grid with the villous surface at the base of the microvillus was taken as a random start point to measure the shortest distance (orthogonal intercept length) to the inner surface of the fetal capillary (for VM) or to the inner surface of ST (for ST) [29]. The measurements (orthogonal intercepts) were performed by the same investigator who was blinded to the specimen origin (obese vs. non-obese).
2.3.2.2. Fetal endothelium and basement membrane thickness
The thickness of the fetal endothelium at the vascular-syncytial membrane (VSM) was measured on randomly selected images of VSM membrane (36 per placenta). The photomicrographs were taken at 7500-fold magnification in order to measure endothelial thickness, as described elsewhere [30]. A grid was superimposed on the images, and the thicknesses of the endothelium and of basement membrane (endothelial and ST laminas and stromal tissue) (BM) were measuredat various points around the extent of the capillary, as demonstrated in Fig. 1.
Fig. 1.
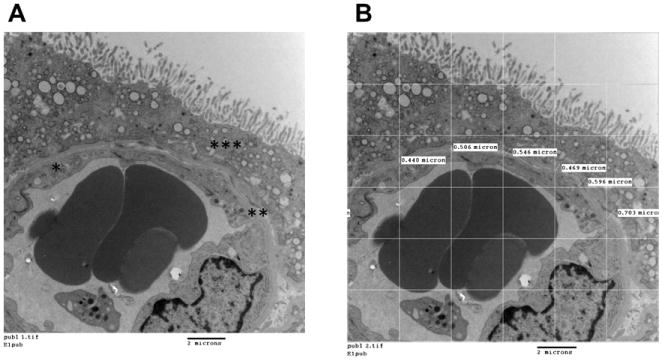
A. Vasculo-syncytial membrane at 7,500× magnification. The following structures have been marked: fetal endothelium (*); basement membrane (**); syncytiotrophoblast (***). B. Vasculo-syncytial membrane with the superimposed grid and randomly selected intersections for measurement of basement membrane thickness. Bar = 2 μm.
2.3.2.3. Arithmetic and harmonic thicknesses calculation
The arithmetic (THa) and harmonic thicknesses (THh) were calculated by multiplying the arithmetic mean intercept length by π/4 and the harmonic mean intercept length by 8/3π respectively. The final measurements were corrected for tissue shrinkage. Additionally, the uniformity index was derived using the formula THa/THh [10].
2.3.2.4. Shrinkage factor
Tissue shrinkage (artifact) from TEM processing was estimated by calculating the diameter of maternal erythrocytes (n = 300). These data were compared to the erythrocyte diameter in a fresh blood smear [31]. Following this calculation, corrections to the TEM measurements were made by multiplying these measurements by 1.63.
2.3.2.5. Villous membrane diffusing capacity (VMDC)
The morphometric VMDC for oxygen is given by the equation:
where K is Krogh's diffusion coefficient with a value of 2.3 · 10−8 cm2 · min−1 · Torr−1 [10]. Specific diffusing capacities were calculated by dividing VMDC by fetal weight.
2.4. Serum leptin measurements
Serum leptin concentration in fetal and maternal serum was measured by radioimmunoassay in a single assay according to the manufacturer's instructions (LINCO Research, Inc., St. Charles, MO), as described previously [32]. The intra-assay coefficients of variation (%CV) were 3.0% at a leptin concentration of 4.9 ng/mL [16].
2.5. Statistical analysis
Comparisons between obese and non-obese groups were made with one-tailed Student's t-tests for fetal and maternal morphometry [16]. Two-tailed test was applied for analyses of placental morphometry. We also calculated the effect size using Cohen's d calculation as a measurement of biological relevance [33]. Correlation of certain variables was performed with linear regression analysis and calculation of Pearson's correlation coefficient. Data throughout are presented as mean ± SEM; n = 4 for all data in each group. Statistical significance was set at p < 5%.
3. Results
3.1. Placental and fetal morphometry
As described previously [16], there were no differences in fetal body weight, fetal organ weight, and placental weight between obese and non-obese animals (Table 1).
Table 1.
Combined fetal and placental morphometry in obese (n = 4) and non-obese (n = 4) baboons.
| Obese (n = 4) |
Non-obese (n = 4) |
Combined | |
|---|---|---|---|
| Fetal weight (g) | 767.5 ± 65.5 | 810.3 ± 17.8 | 788.9 ± 32.4 |
| Fetal Organ weights (g) | |||
| Liver | 25.36 ± 4.85 | 24.93 ±1.57 | 25.14 ± 3.33 |
| Brain | 76.1 ± 6.88 | 80.22 ± 1.54 | 78.16 ± 4.74 |
| Lungs | 22.28 ± 3.63 | 23.01 ± 2.94 | 22.65 ± 3.06 |
| Heart | 4.5 ± 0.5 | 4.4 ±0.6 | 4.4 ± 0.4 |
| Placental weight (g) | 203.0 ± 12.5 | 174.6 ± 20.5 | 188.79 ± 12.35 |
| Thickness of mid-placenta (cm) | 2.2 ± 0.2a | 1.3 ± 0.2 | 1.7 ± 0.2 |
Data represent means ± SEM.
p < 0.05, compared to non-obese group.
3.2. Morphometry of materno–fetal gas exchange barrier in relationship to placental macrostructure and fetal morphometry
The analyses of placental structure showed a negative correlation between villous capillary volume fraction and villous membrane arithmetic thickness (r = −0.684, p < 0.05) (Fig. 2). Placental VMDC had a weak positive correlation with fetal weight (r = 0.6, p = 0.056) and intervillous space star volume (r = −0.58, p = 0.066).
Fig. 2.
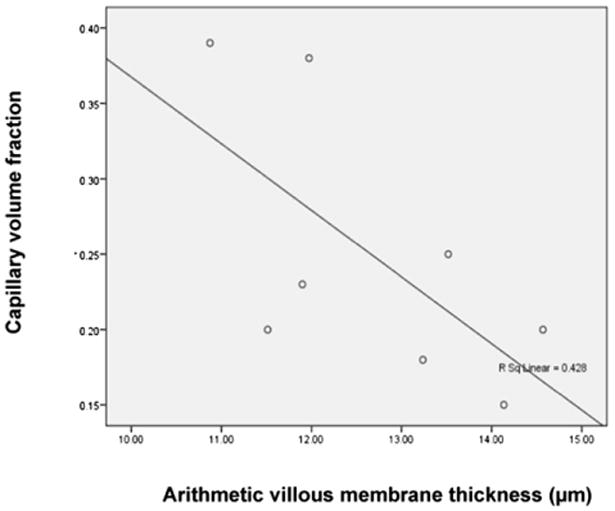
Negative correlation between the villous capillary volume fraction and the arithmetic thickness of villous membrane (r = −0.684, p < 0.05).
3.3. Morphometry of the materno–fetal gas exchange barrier in relation to maternal and fetal leptin concentrations
The fetal leptin concentration correlated positively with fetal weight (r = 0.7, p < 0.05), placental oxygen diffusing capacity (r = 0.77, p < 0.05) and negatively with endothelial thickness of fetal capillaries (r = −0.78, p < 0.05). The thickness of basement membrane negatively correlated with the maternal leptin concentration (r = −0.84, p < 0.01) (Fig. 3).
Fig. 3.
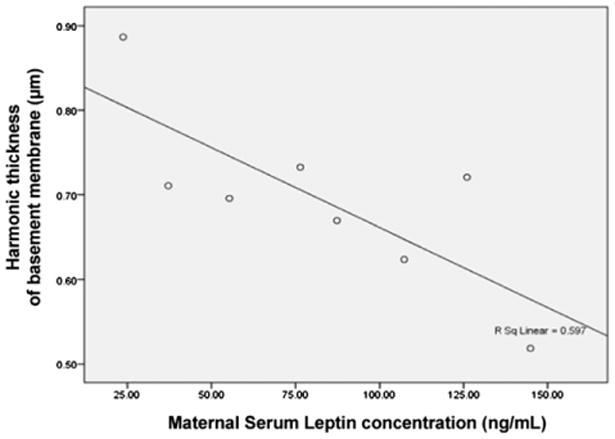
Negative correlation between harmonic thickness of the basement membrane and maternal leptin concentration (r = −0.84, p < 0.01).
3.4. Morphometry of materno–fetal gas exchange barrier in obese and non-obese groups
In the obese group, there was a non-significant decrease in the arithmetic and harmonic thicknesses of the VM (THaVM and THhVM respectively). The effect size was 0.48 for THaVM and 0.75 for THhVM. The decrease in the arithmetic thickness of BM approached significance (p = 0.66). The effect size for this BM thinning was 1.49 and for BM harmonic thickness 1.59. The mean arithmetic and harmonic thicknesses of ST and fetal endothelium did not differ between obese and non-obese groups (Table 2). Calculated VMDC and specific VMDC did not reveal differences between obese and non-obese groups.
Table 2.
Morphometry of the materno—fetal gas exchange barrier in in obese (n = 4) and non-obese (n = 4) baboons. Data represent means ± SEM.
| Obese group (n = 4) |
Non-obese group (n =4) |
p* | |
|---|---|---|---|
| Villous membrane | |||
| Arithmetic thickness (THaVM) (μm) | 12.38 ± 0.65 | 13.05 ± 0.76 | 0.52 |
| Harmonic thickness (THhVM) (μm) | 9.59 ± 0.79 | 10.52 ± 0.87 | 0.46 |
| Uniformity index | 1.3 ± 0.04 | 1.24 ± 0.04 | 0.42 |
| Oxygen diffusing capacity (cm3 · min−1 · Torr−1) | 1.41 ± 0.26 | 1.13 ± 0.19 | 0.42 |
| Specific diffusing capacities (cm3 · min−1 · Torr−1 · kg−1) | 1.80 ± 0.24 | 1.38 ± 0.21 | 0.24 |
| Syncytiotrophoblast | |||
| Arithmetic thickness (THaST) (μm) | 9.09 ± 0.59 | 9.12 ± 0.58 | 0.96 |
| Harmonic thickness (THhST) (μm) | 6.06 ± 0.51 | 6.72 ± 0.76 | 0.50 |
| Uniformity index | 1.51 ± 0.05 | 1.38 ± 0.07 | 0.19 |
| Fetal endothelium | |||
| Arithmetic thickness (μm) | 0.92 ± 0.11 | 0.89 ± 0.03 | 0.76 |
| Harmonic thickness (μm) | 0.81 ± 0.68 | 0.78 ± 0.04 | 0.74 |
| Basement membrane | |||
| Arithmetic thickness (μm) | 0.72 ± 0.058 | 0.89 ± 0.05 | 0.066 |
| Harmonic thickness (μm) | 0.63 ± 0.043 | 0.76 ± 0.04 | 0.092 |
Student's t-test.
4. Discussion
4.1. Structure of the placental exchange barrier in the baboon
To our knowledge, this is the first report describing the placental morphometric oxygen diffusing capacity in the baboon (Papio spp). Oxygen transport across the placenta depends on the oxygen diffusing capacities, placental oxygen consumption, maternal-placental blood flow, fetal placental blood flow, oxygen affinity of fetal blood, and the direction of blood flow [34]. While baboon implantation is considered to be shallow when compared to humans [35], the maternal uterine blood flow [36], umbilical blood flow [37], and fetal blood oxygen affinity and capacity [34] are very similar to human values. The calculated THhaVM and THhVM values for baboons are in agreement with [38,39], higher [40–46] or lower [47] than published human data. Interestingly, in the baboon, the uniformity index indicates VM shape closely resembles that estimated in humans (1.24 ± 0.04 vs. 1.26 respectively) [39]. While the VMDC in the baboon is generally lower, compared to humans, the specific oxygen diffusing capacity calculated per unit of fetal weight (1.60 cm3 · min−1 · Torr−1 · Kg−1) is very close to values reported for human pregnancy (1.48 and 1.72 cm3 · min−1 · Torr−1 · Kg−1) [40,48]. The BM harmonic thickness in our study (range 0.52–0.89 μm) is greater than reported BM thickness in human placenta [0.1–0.3 μm] [49,50]. Differences noted between previous studies and our study could be due to species differences or differences in tissue fixation and processing.
Our data regarding the association between villous capillary volume fraction and THaVM agree with human data published by Burton and Feneley [51], indicating that the mechanism of villous membrane [VM] thinning in the baboon involves capillary peripheralization in the same manner as it has been described for the human placenta. The weak correlation of placental VMDC with fetal weight and IVS star volume, compared to the significant correlations found in experimental models and humans in vivo [52] may be explained by the smaller sample size (n = 8) in our study compared to available human data.
4.2. Placental structure and leptin (maternal and fetal) concentrations
Leptin is a hormone released by adipocytes and the placenta [53]. Fetal leptin has been recognized as a unique signaling molecule that can regulate fetal and placental growth [54]. IUGR, for example, is associated with low leptin concentration in the fetal circulation, and treatment of IUGR in piglets with leptin reversed the negative effect of IUGR programming by preventing subsequent obesity [55]. Fetal leptin concentration in sheep inversely correlated with the fetal arterial PO2 [56]. However, the mechanisms of leptin's influence on placental nutrient and oxygen transport to the fetus remain unclear. In human placentae, the fetal leptin concentration did not correlate with villous and intervillous space volumes, villous and placental vascularization [46]. In our study fetal leptin concentration had a direct correlation with placental VMDC and a negative correlation with endothelial thickness of fetal capillaries, while maternal leptin levels had an inverse correlation with basement membrane thickness. In other words, increases in both maternal and fetal leptin concentrations decreases the distance of oxygen travel across the placenta. Interestingly, in vitro studies have shown that leptin decreases the thickness of the BM in ranine lungs [57]. In mammals, the development of the placenta and lung blood-air–tissue exchange barriers comprise fundamental mechanisms of evolution [58,59]. Thinning of this barrier permitted development of hemochorial placentation. There is an increase in leptin concentration among species as you move up the evolutionary tree [60]. Therefore the negative correlation between leptin concentration and basement membrane thickness represents an evolutionary phenomenon. Our data suggest that maternal and fetal leptin might regulate placental oxygen and nutrient transfer to the fetus through the mechanism of placental structural changes.
4.3. Obesity and placental structural changes
Data regarding placental structural changes in maternal obesity remain sparse. In humans, pregnancy weight gain (but not pre-pregnancy weight) correlated positively with placental oxygen diffusing capacities [60]. We expected that inflammatory changes found in placentas from obese women [15,17] and baboons [16] would increase villous membrane thickness and thus decrease placental diffusion capacities as well. For example inflammatory changes have been described for sheep placenta in an overnutritional model of maternal obesity [61]. Maternal over nutrition from early to late gestation leads to IUGR, a 30% reduction in fetal oxygenation, and a 25% reduction in fetal umbilical vein oxygen uptake in sheep [8]. These findings indicate possible changes in placental oxygen diffusion capacities. In our study, we found no difference in the placental oxygen diffusing capacities in the obese group, as compared to non-obese. Our finding parallels the results of the studies in preeclamptic placentas, where despite the inflammatory changes and oxidative stress, placental diffusing capacities were not diminished [11].
Interestingly, Lutsenko [18] reported decreased placental VM thickness in obese women. However, the authors did not estimate surface area and VM volume; therefore it is impossible to make a conclusion regarding VM oxygen diffusing capacities. In humans, diffusing capacity has been reported as either unchanged [46] or increased in maternal diabetes [13], unchanged in pre-eclampsia [11], decreased in small for gestational age fetuses and fetuses with IUGR [11,12], and unchanged or increased with advanced gestation (Table 3) [45,62–72].
Table 3.
Changes in placental morphometry of blood-tissue barrier under pathological conditions (selected publications).
| Maternal pathology/ experimental conditions |
Basement membrane thickness (μm) |
VM Harmonic thickness (μm) |
VM Arithmetics thickness (μm) |
VM oxygen diff capacity (cm−3 · min−1 · Torr−1) |
VM specific oxygen diffusing capacity (cm−3 · min−1 · Torr−1 · kg−1) |
References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute value (in controls) |
Changes | Absolute value (in controls) |
Changes | Absolute value (in controls) |
Change | Absolute value (in controls) |
Changes | Absolute value (in controls) |
Changes | ||
| Obesity | ↑ | [60] | |||||||||
| 0.76 ± 0.04* | ↔ | 10.52 ±0.87 | ↔ | 13.05 ±0.76 | ↔ | 1.24 ±0.04 | ↔ | 1.38 ±0.21 | ↔ | Present study | |
| ↓* | [18] | ||||||||||
| Diabetes | 0.18 ± 0.05 | ↑ | [62] | ||||||||
| ↓ | [63] | ||||||||||
| 8.76** | ↑ | 1.06* | ↑ | [13] | |||||||
| Type 1 diabetes | 9.3 ±1.2 | ↔ | 2.7 ± 1.2 | ↔ | 0.8 ± 0.04 | ↔ | [46] | ||||
| 5.7 ± 1.9 | ↑ with fetal macrosomia |
5.6 ±1.5 | ↔ | 1.72 | ↓ | [64] | |||||
| ↓ | [65] | ||||||||||
| Tendency to ↑ | [66] | ||||||||||
| Pre-eclampsia | ↓ | [67] | |||||||||
| 3.81 ± 0.19 | ↔ | 5.3 2 ± 0.33 | ↔ | 46.6 ± 4.3 | ↔ | 13.5 ± 1.04 | ↔ | [11] | |||
| Higher altitude | 3.56 ± 0.79 | ↓ theoretically | 4.4 ± 0.817 | ↓ theoretically | [39] | ||||||
| 6.9 | ↓ | 4.22 | ↑ | [68] | |||||||
| Maternal Anemia | 7.15 ± 0.46 | ↓ | 5.31 ± 0.66 | ↔ | 1.48 ± 0.16 | ↔ | [48] | ||||
| Maternal smoking | trophoblast BL =·0.18 ± 0.04 |
↑ | ↔ | [38,69,70] | |||||||
| IUGR | ↓ | 0.8–0.9 | ↔ | [11] | |||||||
| SGA | 2.7 ±·0.1 | ↓ | 2.97 | ↓ | ↔ | [12] | |||||
| Placental ultrasound maturity | 4.26 ±·0.16 | ↔ | 4.76 ± 0.19 | ↔ | 5.6 ± 0.8* | ↔ | 1.80 ± 0.21* | ↔ | [71] | ||
| ↑ by 40% | [72] | ||||||||||
| In vitro hypoxia | 7.4 | ↓ | 5.5 | [44] | |||||||
| In vitro hyperoxia | 7.4 | ↑ | 5.5 | [44] | |||||||
| Timing of biopsy | 3.87 ±·0.2 | ↑ after 20 Min |
4.85 ± 0.2 | ↑ after 20 Min |
[45] | ||||||
VM: Villous membrane; IUGR: intrauterine growth retardation; SGA: small for gestational age;
Harmonic thickness
calculated units.
The decrease of BM thickness in obese animals in our study had “large” biological relevance (Cohen's d > 0.8) [33]; however the difference was not statistically significant. We expected to find the BM thickening, associated with inflammatory changes in the obese placentas. Such thickening has been described in the chronic inflammation associated with asthma (lungs), and higher fat diet (kidney) in humans and rodents [24,73]. In the human placenta, BM thickening has been associated with diabetes [74], advanced gestational age [75] maternal smoking [69] and pre-eclampsia [25]. Emmrich etal. [63] found thinning of the endothelial basal laminae associated with delay of villi maturation in obesity in diabetes mellitus. In the placenta, the BM is an important component of the transport structure and might act to filter or possibly provide transient storage capacity [76]. The thinning of the placental BM in the obese mother might facilitate the passive diffusion of nutrients (e.g., fatty acids) and oxygen to the fetus, contributing to the fetal macrosomia and lipid overload observed in maternal over nutrition and obesity [77].
5. Conclusion
To the best of our knowledge this is the first report of placental oxygen diffusing capacities and placental ultrastructural parameters in a baboon model of obesity. Despite differences in the THaVM, THhVM and the VM oxygen diffusing capacities, the specific VM oxygen diffusing capacities (per unit of fetal weight) are essentially the same in baboons and humans. Maternal leptin concentration in our study correlated negatively with placental basement membrane thickness, while fetal leptin concentration correlated negatively with endothelial thickness of fetal capillaries. The total and specific villous membrane diffusing capacities were not influenced by maternal obesity in this non-human primate model.
Acknowledgments
The authors are deeply thankful to Dr. Graham Burton for his expertise and advice in placental stereology. We are grateful to personnel of Center for Pregnancy and Newborn Research (UTHSC—San Antonio) for their support. The authors also thank Mrs. Kathy Troughton (UTHSC) for her EM expertise, Mrs. Lauren Chestnut (UTHSCSA) for her work with the electron microscopy specimens, and Mrs. LaShea Bridges (UTHSC Pediatrics) for her administrative support. Authors would like to thank Dr. Juan Carlos Lopez–Alvarenga (Texas BioMed) for his help with the statistical analyses. This study was partially supported by the UTHSC Leonard Share Young Investigator's Award to J.S; Texas Biomedical Research Institute Grant C06 RR013556 and NIH grant HD21350 to Dr. Peter Nathanielsz (UTHSC—San Antonio), UTHSCSA ERC New Investigator Award to N.S.-L., NIH grant R21 HD059292 to R.F., NCRR grant P51 RR013986 to the Southwest National Primate Research Center, and the Pediatric Endocrine Fund at Le Bonheur Foundation to R.F. R.F. discloses unrelated research support within the past three years from Tolerx, MacroGenics, NIH T35 DK007405, Gabrielle's Angel Foundation, Eli Lilly & Co., Diamyd, Pfizer, and Novo Nordisk A/S.
References
- 1.ACOG. Obesity in pregnancy. Obsterics Gynecol. 2005;106:671–5. doi: 10.1097/00006250-200509000-00054. Committee Opinion Number 315. [DOI] [PubMed] [Google Scholar]
- 2.Poston L, Harthoorn LF, Van Der Beek EM. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Contributors to the ILSI Europe Workshop. Pediatr Res. 2011;69:175–80. doi: 10.1203/PDR.0b013e3182055ede. [DOI] [PubMed] [Google Scholar]
- 3.Weiss J, Malone F, Emig D, Ball R, Nyberg D, Comstock C, et al. Obesity, obstetric complications and cesarean delivery rate- a population-based screening study. FASTER Research Consortium. Am J Obstet Gynecol. 2004;190:1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, McClure EM, Bhutta ZA, Belizán JM, Reddy UM, Rubens CE, et al. Stillbirths: the vision for 2020. Lancet's Stillbirths Series steering committee. Lancet. 2011;377:1798–805. doi: 10.1016/S0140-6736(10)62235-0. [DOI] [PubMed] [Google Scholar]
- 5.Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–40. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 6.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–64. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlabritz-Loutsevitch NE, Moore CM, Lopez-Alvarenga JC, Dunn BG, Dudley D, Hubbard GB. The baboon model (Papio hamadryas) of fetal loss: maternal weight, age, reproductive history and pregnancy outcome. J Med Primatol. 2008;37:337–45. doi: 10.1111/j.1600-0684.2008.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony R, Scheaffer A, Wrigh CD, Regnault T. Ruminent models of prenatal growth restriction. Reprod Suppl. 2003;61:183–94. [PubMed] [Google Scholar]
- 9.Nohr E, Bech B, Davies M, Frydenberg M, Henriksen T, Olsen J. Pregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol. 2005;106:250–9. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- 10.Mayhew T, Joy C, Haas J. Structure-function correlation in the human placenta: the morphometric diffusing capacity for oxygen at full term. J Anat. 1984;139:691–708. [PMC free article] [PubMed] [Google Scholar]
- 11.Mayhew T, Manwani R, Ohadike J, Wijesekara J, Baker P. The placenta in preeclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta. 2007;28:233–8. doi: 10.1016/j.placenta.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Ansari T, Fenlon S, Pasha S, O'Neil B, Gillian J, Green C, et al. Morphometric assessment of the oxygen diffusion conductance in placentae from pregnancies complicated by intra-uterine growth restriction. Placenta. 2003;24:618–26. doi: 10.1016/s0143-4004(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 13.Mayhew T, Sorensen E, Kiebe J, Jackson M. Oxygen diffusive conductance in placentae from control and diabetic women. Diabetologia. 1993;36:955–60. doi: 10.1007/BF02374479. [DOI] [PubMed] [Google Scholar]
- 14.Higgins L, Greenwood S, Wareing M, Sibley C, Mills T. Obesity and the placenta: a consideration of nutrient exchange mechanism in relation to aberrant fetal growth. Placenta. 2011;32:1–7. doi: 10.1016/j.placenta.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Challier J, Basu S, Binten T, Minium J, Hotmire K, Catalano P, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–81. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farley D, Tejero M, Comuzzie A, Higgins P, Cox L, Werner S, et al. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752–60. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts K, Riley S, Reynolds R, Barr S, Evans M, Statham A, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32:247–54. doi: 10.1016/j.placenta.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Lutsenko N. Obstetric pathology and morphologic features of the placenta in obese parturients. Akush Ginekol [Mosk] 1984;1:30–2. [PubMed] [Google Scholar]
- 19.Chernuka G, Volobuev A, Malysheva V. Status of the feto-placental system in obese pregnant women. Akush Ginekol. 1988;10:40–3. Mosk. [PubMed] [Google Scholar]
- 20.Garn S, Hoff K, McCabe K. Maternal fatness and placental size. Am J Clin Nutr. 1979;32:277–9. doi: 10.1093/ajcn/32.2.277. [DOI] [PubMed] [Google Scholar]
- 21.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–21. 623–6. doi: 10.1016/s0143-4004(97)90000-x. discussion. [DOI] [PubMed] [Google Scholar]
- 22.Ramos BF, Zhang Y, Jakschik BA. Mast cells contribute to fibrin deposition in reverse passive Arthus reaction in mouse skin. Eur J Immunol. 1992;22:2381–5. doi: 10.1002/eji.1830220930. [DOI] [PubMed] [Google Scholar]
- 23.Thompson HL, Burbelo PD, Gabriel G, Yamada Y, Metcalfe DD. Murine mast cells synthesize basement membrane components. A potential role in early fibrosis. J Clin Invest. 1991;87:619–23. doi: 10.1172/JCI115038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, et al. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002;57:309–16. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soma H, Yoshida K, Mukaida T, Tabuchi Y. Morphologic changes in the hypertensive placenta. Contrib Gynecol Obstet. 1982;9:58–75. [PubMed] [Google Scholar]
- 26.Okudaira Y, Kirota K, Chohen S, Strauss L. Ultrstructure of the human placenta in matemal diabetes mellitus. Lab Invest. 1966;15:910–26. [PubMed] [Google Scholar]
- 27.Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, Jenkins S, Hubbard G, Burton G, et al. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon [Papio sp] Placenta. 2007;28:783–93. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 2008;29:1–14. doi: 10.1016/j.placenta.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Jensen E, Gundersen H, Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc. 1979;115:19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 30.Burton G, Tham W. Formation of vaculo-syncytial membranes in the human placenta. J Dev Physiol. 1992;18:43–7. [PubMed] [Google Scholar]
- 31.Burton GJ, Palmer ME. Eradicating fetomaternal fluid shift during perfusion fixation of the human placenta. Placenta. 1988;9:327–32. doi: 10.1016/0143-4004(88)90040-9. [DOI] [PubMed] [Google Scholar]
- 32.Schlabritz-Loutsevitch NE, Lopez-Alvarenga JC, Comuzzie AG, Miller MM, Ford SP, Li C, et al. The prolonged effect of repeated maternal glucocorticoid exposure on the maternal and fetal leptin/insulin-like growth factor axis in Papio species. Reprod Sci. 2009;16(3):308–19. doi: 10.1177/1933719108325755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2/e) Hillsdale NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 34.Carter A. Evolution of factors affecting placental oxygen transfer. Placenta. 2009 Suppl. A:19–25. doi: 10.1016/j.placenta.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey E, Houston M, Harris J. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol. 1976;124:647–52. doi: 10.1016/0002-9378(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 36.Balasuria H, Bell P, Waugh R, Thompson J, Gillin A, Hennessy A, et al. Primate maternal placental angiography. Placenta. 2010;31:32–6. doi: 10.1016/j.placenta.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Paton JB, Fisher DE, Peterson EN, DeLannoy CW, Behrman RE. Cardiac output and organ blood flows in the baboon fetus. Biol Neonate. 1973;22:50–7. doi: 10.1159/000240539. [DOI] [PubMed] [Google Scholar]
- 38.Laga E, Driscoll S, Munro H. Quantitative studies of human placenta. I. Morphometry. Biol Neonate. 1973;23:231–59. doi: 10.1159/000240605. [DOI] [PubMed] [Google Scholar]
- 39.Jackson M, Joy C, Mayhew T, Hass J. Stereological studies on the true thickness of the villous membrane in human term placentae: a study of placentae from high-altitude pregnancies. Placenta. 1985;6:249–58. doi: 10.1016/s0143-4004(85)80054-0. [DOI] [PubMed] [Google Scholar]
- 40.Jauniaux E, Burton G. The effect of smoking in pregnancy on early placental morphology. Obstet Gynecol. 1992;79:645–8. [PubMed] [Google Scholar]
- 41.Mayhew T, Jackson M, Haas J. Microscopic morphology of the human placenta and its effects on oxygen diffusion: a morphometric model. Placenta. 1986;7:121–31. doi: 10.1016/s0143-4004(86)80003-0. [DOI] [PubMed] [Google Scholar]
- 42.Critchley G, Burton G. Intralobular variations in barrier thickness in the mature human placenta. Placenta. 1987;8:185–94. doi: 10.1016/0143-4004(87)90021-x. [DOI] [PubMed] [Google Scholar]
- 43.Burton G, Palmer M, Dalton K. Morphometric differences between the placental vasculature of non-smokers, smokers and ex-smokers. Brit J Obstet Gynaecol. 1989;96:907–15. doi: 10.1111/j.1471-0528.1989.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 44.Burton G, Mayhew T, Robertson L. Stereological re-examination of the effects of varying oxygen tensions on human lacental villi maintained in organ culture for up to 12 h. Placenta. 1989;10:263–73. doi: 10.1016/0143-4004(89)90027-1. [DOI] [PubMed] [Google Scholar]
- 45.Feneley M, Burton G. Villous composition and membrane thickness in the human placenta at term: a stereological study using unbiased estimators and optimal fixation techniques. Placenta. 1991;12:131–42. doi: 10.1016/0143-4004(91)90017-a. [DOI] [PubMed] [Google Scholar]
- 46.Nelson S, Coan P, Burton G, Lindsay R. Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes. 2009;58:2634–41. doi: 10.2337/db09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jauniaux E, Burton G, Moscoso G, Hustin J. Development of the early human placenta: a morphometric study. Placenta. 1991;12:269–76. doi: 10.1016/0143-4004(91)90008-4. [DOI] [PubMed] [Google Scholar]
- 48.Reshetnikova O, Burton G, Teleshova O. Placenta histomorphometry and morphometric diffusing capacity of the villous membrane in pregnancies complicated by maternal iron deficiency anemia. Am J Obstet Gynecol. 1995;173:724–7. doi: 10.1016/0002-9378(95)90330-5. [DOI] [PubMed] [Google Scholar]
- 49.Rhodin J, Terzakis J. The ultrastructure of the human full-term placenta. J Ultrastruct Res. 1962;6:88–106. doi: 10.1016/s0022-5320(62)90063-1. [DOI] [PubMed] [Google Scholar]
- 50.Strauss F. Functional morphology of the human placenta. Arkh Anat Gistol Embriol. 1971;61:11–34. [PubMed] [Google Scholar]
- 51.Burton G, Feneley M. Capillary volume fraction is the principal determnant of villous membrane thickness in the normal human placenta at term. J Dev Physiol. 1992;17:39–45. [PubMed] [Google Scholar]
- 52.Karimu A, Burton G. Star volume estimates of the intervillous clefts in the human placenta: how changes in umbilical arterial pressure might influence the maternal placental circulation. J Dev Physiol. 1993;19:137–42. [PubMed] [Google Scholar]
- 53.Misra VK, Trudeau S. The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity. 2011;19:416–21. doi: 10.1038/oby.2010.172. [DOI] [PubMed] [Google Scholar]
- 54.Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. Diabetes. 2009;58:2634–41. doi: 10.1113/jphysiol.2008.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djiane J, Attig L. Role of leptin during perinatal metabolic programming and obesity. J Physiol Pharmacol. 2008;59(Suppl. 1):55–63. [PubMed] [Google Scholar]
- 56.Forhead AJ, Thomas L, Crabtree J, Hoggard N, Gardner DS, Giussani DA, et al. Plasma leptin concentration in fetal sheep during late gestation: ontogeny and effect of glucocorticoids. Endocrinology. 2002;143:1166–73. doi: 10.1210/endo.143.4.8762. [DOI] [PubMed] [Google Scholar]
- 57.Torday J, Ihida-Stanbury K, Rehan V. Leptin stimulates Xenopus lung development: evolution in a dish. Evol Dev. 2009;11:219–24. doi: 10.1111/j.1525-142X.2009.00321.x. [DOI] [PubMed] [Google Scholar]
- 58.Mess A, Ferner K. Evolution and development of gas exchange structures in Mammalia: the placenta and the lung. Respir Physiol Neurobiol. 2010;173 Suppl:S74–82. doi: 10.1016/j.resp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Torday J, Powell F, Farmer C, Orgeig S, Nielsen H, Hall A. Leptin integrates vertebrate evolution: from oxygen to the blood-gas barrier. Respir Physiol Neurobiol. 2010;173:S37–42. doi: 10.1016/j.resp.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens-Simon C, Metlay L, McAnarney E. Maternal prepregnant weight and weight gain: relationship to placental microstructure and morphometric oxygen diffusion capacity. Am J Perinatol. 1995;12:407–12. doi: 10.1055/s-2007-994509. [DOI] [PubMed] [Google Scholar]
- 61.Zhu M, Du M, Nathanielsz W, Ford S. Maternal obesity up regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta. 2010;5:387–91. doi: 10.1016/j.placenta.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Honda K, Toyoda C, Nakabayashi M, Omori Y. Quantitative investigations of placental terminal villi in maternal diabetes mellitus by scanning and transmission electron microscopy. Tohoku J Exp Med. 1992;167:247–57. doi: 10.1620/tjem.167.247. [DOI] [PubMed] [Google Scholar]
- 63.Emmrich P, Fuchs U, Heinke P, Jutzi E, Godel E. The epithelial and capillary basal laminae of the placenta in maternal diabetes mellitus. Lab Invest. 1976;35:87–92. [PubMed] [Google Scholar]
- 64.Jauniaux E, Burton G. Villous histomorphometry and placental bed biopsy investigation in Type I diabetic pregnancies. Placenta. 2006;27:468–74. doi: 10.1016/j.placenta.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Jirkovska M. Comparison of the thickness of the capillary basement membrane of the human placenta under normal conditions and in type 1 diabetes. Funct Dev Morphol. 1991;1:9–16. [PubMed] [Google Scholar]
- 66.Mayhew TM, Sorensen FB, Klebe JG, Jackson MR. Growth andmaturation of villi in placentae from well-controlled diabetic women. Placenta. 1994;15:57–65. doi: 10.1016/s0143-4004(05)80236-x. [DOI] [PubMed] [Google Scholar]
- 67.Reshetnikova OS, Burton GJ, Teleshova OV. Morphometric diffusing capacity of the human placenta in cases of pre-eclampsia. Placenta. 1995;16 doi: 10.1016/0002-9378(95)90330-5. A. [DOI] [PubMed] [Google Scholar]
- 68.Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am J Obstet Gynecol. 1994;171:1560–5. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 69.Van der Velde WJ, Peereboom-Stegeman JH, Treffers PE, James J. Basal lamina thickening in the placentae of smoking mothers. Placenta. 1985;6:329–40. doi: 10.1016/s0143-4004(85)80042-4. [DOI] [PubMed] [Google Scholar]
- 70.Bush PG, Mayhew TM, Abramovich DR, Aggett PJ, Burke MD, Page KR. Maternal cigarette smoking and oxygen diffusion across the placenta. Placenta. 2000;21:824–33. doi: 10.1053/plac.2000.0571. [DOI] [PubMed] [Google Scholar]
- 71.Yin T, Loughna P, Ong S, Padfield J, Mayhew T. No correlation between ultrasound placental grading at 31–34 weeks of gestation and a surrogate estimate of organ function at term obtained by stereological analysis. Placenta. 2009;30:726–30. doi: 10.1016/j.placenta.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Burton G, Jauniaux E. Sonographic, stereological and Doppler flow veloci-metric assessments of placental maturity. Brit J Obstet Gynaecol. 1995;102:818–25. doi: 10.1111/j.1471-0528.1995.tb10849.x. [DOI] [PubMed] [Google Scholar]
- 73.Altunkaynak ME, Ozbek E, Altunkaynak BZ, Can I, Unal D, Unal B. The effects of high-fat diet on the renal structure and morphometric parametric of kidneys in rats. Anatomy. 2008;212:845–52. doi: 10.1111/j.1469-7580.2008.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones C, Fox H. Placental changes in gestational diabetes. An ultrastructural study. Obstet Gynecol. 1976;48:274–80. [PubMed] [Google Scholar]
- 75.Jones CJ, Fox H. Ultrastructure of the placenta in prolonged pregnancy. J Pathol. 1978;126:173–9. doi: 10.1002/path.1711260306. [DOI] [PubMed] [Google Scholar]
- 76.Sölder E, Rohr I, Kremser C, Hutzler P, Debbage PL. Imaging of placental transport mechanisms: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144:S114–20. doi: 10.1016/j.ejogrb.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 77.Merzouk H, Meghelli-Bouchenak M, Loukidi B, Prost J, Belleville J. Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biol Neonate. 2000;77:17–24. doi: 10.1159/000014190. [DOI] [PubMed] [Google Scholar]


