Abstract
Background/Aims
Most patients with acute viral hepatitis A have a favorable course, but a few of them suffer from severe forms of hepatitis such as fulminant hepatitis. This study was carried out to identify the factors influencing the severity of acute viral hepatitis A.
Methods
We retrospectively reviewed the medical records of 713 patients with acute hepatitis A, who were divided into two groups: severe hepatitis A (N=87) and non-severe hepatitis A (N=626). Severe hepatitis was defined as fulminant hepatitis or prolongation of prothrombin time (INR≥1.5). Clinical variables were compared between the two groups.
Results
The incidence of fulminant hepatitis was 1.4% (10/713) in patients with acute hepatitis A. Thirty-three (4.6%) cases exhibited HBsAg positivity. In multivariate analyses, significant alcohol intake and the presence of HBsAg were significant predictive factors of fulminant hepatitis A, and significant alcohol intake and age were significant predictive factors of severe hepatitis A. HBeAg and HBV-DNA status did not affect the clinical course of hepatitis A in chronic hepatitis B carriers.
Conclusions
While most patients with acute hepatitis A have an uncomplicated clinical course, our data suggest that a more-severe clinical course is correlated with being older, significant alcohol intake, and chronic hepatitis-B-virus infection.
Keywords: Hepatitis A, Severity, Liver failure, Fulminant
INTRODUCTION
Hepatitis A is mostly self-limited acute illness and does not progress to chronic hepatitis. In children, hepatitis A virus (HAV) infection is usually asymptomatic whereas symptomatic in adults, and also it rarely develop fulminant hepatitis or accompany serious complication such as acute renal failure.1,2 According to epidemiological research in Korea, anti-HAV positivity increases after birth and becomes 50% around age 10 and most adults had anti-HAV in early 1980s.3 However, after 1990s, with improving standard of living and personal hygiene, anti-HAV positivity in children has decreased significantly resulting in rapid increase of adult hepatitis A infection; 355 in 2004, 2,081 in 2006 and 7,895 in 2008.4,5
Factors influencing on the severity of viral hepatitis A consist of host and viral factors. For the host factors, chronic liver disease, old age, heavy alcohol drinking, human immunodeficiency virus (HIV) infection and pregnancy are being suggested.2,6-13 It has been reported that when hepatitis A is superimposed on chronic hepatitis C, it frequently shows critical clinical course and accompany high rate of fulminant hepatitis and mortality.10 However, the affect of hepatitis B virus (HBV) infection on the severity of viral hepatitis A differs among researches.10,14-17
This study was carried out to identify the factors influencing on the severity of hepatitis A by analyzing several factors including underlying chronic HBV infection.
PATIENTS AND METHODS
1. Subjects and definitions
We retrospectively reviewed the medical records of 713 patients with acute hepatitis A from June 2006 to May 2009. Diagnosis of acute hepatitis A was made by positive serum IgM anti-HAV (radio-Immunoassay, General Biologicals Corp., Hsin Chu, Taiwan), and hepatitis B and hepatitis C by testing HBsAg (RIAKEY, Shin Jin Medics. Inc., Korea), anti-HBs (RIAKEY, Shin Jin Medics. Inc., Korea), and anti-HCV (RIAKEY, Shin Jin Medics. Inc., Korea). Significant alcohol intake was defined as more than 50 g of alcohol for over 10 years.18 Fulminant hepatitis was defined as progression to hepatic encephalopathy within 8 weeks from the onset of jaundice, and severe hepatitis (SH) was defined as fulminant hepatitis or prolongation of prothrombin time (INR) over 1.5.19
2. Methods
A total of 713 patients were divided into two groups; fulminant hepatitis (FH) and non-fulminant hepatitis (Non-FH). Each group was analyzed by age, sex, superimposed hepatitis B or hepatitis C, diabetes mellitus, significant alcohol intake, duration of hospital stay and complete blood count, serum biochemistry and coagulation test at most severe clinical course. In addition, we divided the subjects into severe hepatitis (SH) and non-severe hepatitis (Non-SH) to analyze for the same factors to identify the risk factors of fulminant and severe hepatitis caused by hepatitis A.
3. Statistical analysis
Statistical analysis were performed using SPSS (version 12.0, SPSS Inc., Chicago, IL). To compare baseline variables. Fisher's exact test was used for categorical variables and Mann-Whitney U test and independent t-test were used for continuous variables. Also, to identify the related risk factors for fulminant hepatitis and severe hepatitis, we performed multivariate regression analysis of variables that were significant in univariate analysis. Statistical significance was assumed at p<0.05.
RESULTS
1. Clinical features of the subjects
Mean age of the patients was 31.1 (±6.8) years, 413 (57.9%) patients were male, 300 (42.1%) patients were female. Duration of hospital stay was 9.6 days. Severe hepatitis occurred in 87 (12.2%) patients and 10 (1.4%) of these patients had fulminant hepatitis. Of the patients with fulminant hepatitis, 2 had liver transplantation, 4 recovered with medical treatment, 3 were expired due to liver failure and 1 expired due to pneumonia and systemic complication after liver transplantation. Chronic HBV carriers were 33 (4.6%), anti-HCV positive patients were 4, diabetes mellitus was in 5 cases and significant alcohol intake was in 25 cases.
2. Difference in clinical features of fulminant and non-fulminant hepatitis
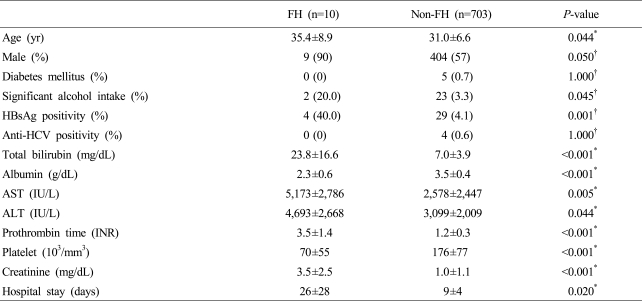
Age of fulminant and non-fulminant hepatitis group were 35.4±8.9 and 31.0±6.6 years which showed significant difference (p=0.044). For each group, 90% and 57% (p=0.05) were male, indicating old age and male have significantly high rate of fulminant hepatitis. HBsAg positivity was 4 (40.0%) and 29 (4.1%), thus it was significantly high in fulminant hepatitis group (p=0.001). Significant alcohol intake history was 2 (20.0%) and 23 (3.3%) showing significantly high in fulminant hepatitis group (p=0.045), whereas anti-HCV positivity (p=1.000) and diabetes mellitus (p=1.0) had no significant difference. Duration of hospital stay was longer in fulminant hepatitis group. Total bililubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), prothrombin time (INR) and creatinine were higher in fulminant hepatitis group, whereas albumin and platelet count were lower in fulminant hepatitis group (Table 1).
Table 1.
Comparison of clinical features between the patients with fulminant and non-fulminant hepatitis
All results were presented as n (%) or mean±SD.
P-value was obtained by *Mann-whitney U test or †Fisher's exact test.
FH, fulminant hepatitis; HBsAg, hepatitis B surface antigen; Anti-HCV, antibody to hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
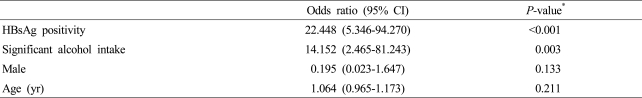
Multivariate analysis of age, sex, HBsAg positivity and significant alcohol intake history which are significant factors in univariate analysis of fulminant hepatitis group and nonfulminant hepatitis group were performed. As a result, HBsAg (OR 22.448, CI 5.346-94.270, p<0.001) and significant alcohol intake history (OR 14.152, CI 2.465-81.243, p=0.003) had significant correlation, whereas sex (OR 0.195, CI 0.023-1.647, p=0.133) and age (OR 1.064, CI 0.965-1.173, p=0.211) had no significant difference (Table 2).
Table 2.
Multivariable analysis of predictive factors associated with fulminant hepatitis
*Multiple logistic regression analysis.
CI, confidence interval; HBsAg, hepatitis B surface antigen.
3. Difference in clinical features of severe and non-severe hepatitis
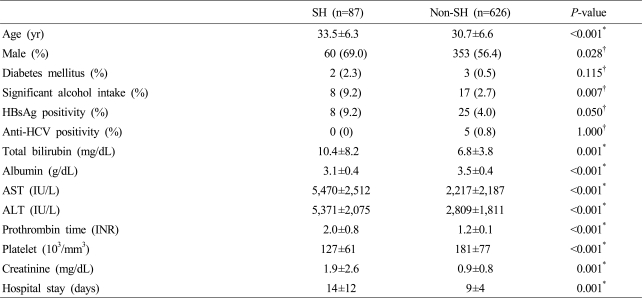
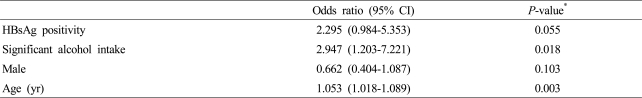
Age of severe and non-severe hepatitis group were 33.5±6.3 and 30.7±6.6 years (p<0.001) which showed significant difference, and 69.0% and 56.4% were male (p=0.028). HBsAg positivity was 8 (9.2%) and 25 (4.0%) indicating significance (p=0.050) and significant alcohol intake history was 8 (9.2%) and 17 (2.7%) showing significantly high in severe hepatitis group (p=0.007), whereas anti-HCV positivity (p=1.000) and diabetes mellitus (p=0.115) had no significant difference. Duration of hospital stay was longer in severe hepatitis group. Total bililubin, AST, ALT, prothrombin time (INR) and creatinine were higher in severe hepatitis group, whereas albumin and platelet count were lower in severe hepatitis group (Table 3). Multivariate analysis showed significant correlation in significant alcohol intake history (OR 2.947, CI 1.203-7.221, p=0.018) and age (OR 1.053, CI 1.018-1.089, p=0.003), and p-value of HBsAg positivity was 0.055 (Table 4).
Table 3.
Comparison of clinical characteristics between the patients with severe hepatitis and non-severe hepatitis
Severe hepatitis was defined as fulminant hepatitis or prothrombin time (INR) over 1.5.
All results were presented as n (%) or mean±SD.
P-value was obtained by *Independent t-test or †Fisher's exact test.
FH, fulminant hepatitis; SH, severe hepatitis A; HBsAg, hepatitis B surface antigen; Anti-HCV, antibody to hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
Table 4.
Multivariate analysis of predictive factors associated with severe hepatitis A
Severe hepatitis was defined as fulminant hepatitis or prothrombin time (INR) over 1.5.
*Multiple logistic regression analysis.
CI, confidence interval; FH, fulminant hepatitis; INR, international normalized ratio; HBsAg, hepatitis B surface antigen.
4. Clinical features of hepatitis A in patients with chronic hepatitis B
Average age was 33.6±7.0 years, 22 (66.7%) were male, 11 (33.3%) were female and duration of hospital stay was 15.1 days. Total bilirubin was 13.3±11.1 mg/dL, AST, 3,023±3,338 IU/L, ALT, 3,108±2,582 IU/L, prothrombin time (INR), 1.46±0.64 and creatinine was 1.5±2.4 mg/dL. HBeAg was positive in 5 patients and their average serum HBV DNA concentration was 1,553,832±5,655,405 copies/mL (310,766±1,131,081 IU/mL). Every patient was newly diagnosed as HBV carriers, thus medical records prior to the onset of acute hepatitis A were unfeasible. Among the 33 HBsAg positive patients, we compared the occurrence of fulminant hepatitis and severe hepatitis according to HBeAg positivity, however, there was no significant difference (p=0.500, p=1.000, respectively). In addition, we compared the incidence of fulminant hepatitis and severe hepatitis according to difference of serum HBV DNA concentration as groups more than 100,000 copies/mL (20,000 IU/mL) and less than 100,000 copies/mL (20,000 IU/mL), and it also showed no statistical difference (p=0.420, p=0.571, respectively).
DISCUSSION
This study demonstrated that host and environmental factors affect the severity of liver disease in patients with acute hepatits A. Acute hepatitis A is mostly self-limited, thus in the past, fulminant hepatitis due to hepatitis A was considered rare in Korea. However, fulminant hepatitis from HAV is increasing constantly because of low anti-HAV positivity resulting in increased number of the patients in adolescents and young adults.20,21 On the other hand, prevalence of fulminant hepatitis from hepatitis B is decreasing; therefore fulminant hepatitis from hepatitis A is increasing relatively.22 Fulminant hepatitis from hepatitis A is known to occur in 0.1~0.3% of entire infection but it was high as 1.4% in this study which might be due to our institute that is tertiary referral hospital with liver transplant center.
Factors which affect the severity of viral hepatitis A consist of host and viral factors. For the viral factors, genetic variation of 5' non-translated region (NTR) was related to the severity of the disease8 whereas genotype of HAV had no relation to the severity of acute hepatitis A.7,9
For the host factor, underlying chronic liver disease, old age, heavy alcohol drinking, HIV infection, pregnancy, etc. are being suggested. It is known to present critical clinical course when hepatitis A is superimposed on preexisting chronic hepatitis. Vento et al. prospectively followed 432 patients with chronic hepatitis C and reported 7 out of patients developed fulminant hepatitis and 6 patients died. However in 163 patients with chronic hepatitis B, 10 acquired HAV superinfection and their clinical course was similar to that of uncomplicated courses of hepatitis A.10 The results showed, unlike chronic hepatitis B who acquired an uncomplicated hepatitis A, patients with chronic hepatitis C had a substantial risk of complicated course. In western countries, chronic hepatitis C with HAV superinfection is an issue since prevalence of hepatitis C is relatively higher than hepatitis B, but we have high prevalence of hepatitis B in Korea. According to a study in Thailand, chronic hepatitis B has significant affect on serious complication such as fulminant hepatitis or mortality. They reported 55% of fulminant hepatitis and 25% of mortality when HAV infection was superimposed on chronic hepatitis B.17 It also has been reported that chronic HBV carriers were 9 times more likely to develop fulminant hepatitis when they acquired acute hepatitis A.16
There has been no domestic report on clinical course and prognosis of HAV superinfection on chronic HBV infection. In this study, we defined severe hepatitis A as fulminant hepatitis or prolongation of prothrombin time (INR) over 1.5.19 When hepatitis A is superimposed on chronic hepatitis B patients, risk of fulminant hepatitis was 22.4 times higher and risk of severe hepatitis was also 2.3 times higher (p=0.055). Therefore, clinical course of the disease seems to be more fatal and severe in HBsAg positive patients. A possible mechanism causing more severe hepatitis in HBV carrier might be that the number of hepatocytes has already been reduced in patients with chronic hepatitis B, thus when hepatitis A occurs, it can cause more severe liver damage. However, medical records on the activities and other prior conditions of chronic hepatitis B of the patients before they acquired hepatitis A were not available in this study and it might have affect on severity of the disease. Thus additional prospective study is needed for this concern. Since we had only 4 patients with hepatitis C included in the research, our study did not show the difference in severity of hepatitis C acquired HAV superinfection unlike the result of Vento et al.10
Age has been known as a risk factor for severe complication and mortality of hepatitis A,23-25 and our study also concluded that the age is an independent factor for severe hepatitis by using multivariate analysis. In addition, we found alcohol intake as an independent factor in development of fulminant hepatitis; alcohol intake causes impairment in host defense system through changing cytokine formation and inducing reactive oxidation. Moreover, alcohol intake and viral hepatitis act synergistically on liver damage by inhibiting the liver regeneration and antiviral reaction with interferon-alpha.26
When HIV patients acquire hepatitis A, HIV infection increases serum concentration of HAV and it also increase hepatitis A related liver damage.6 It has been reported that hepatitis A in second to third trimester of pregnancy is related to maternal complication and preterm labor.13
This study has certain limitations; it showed high prevalence of fulminant hepatitis due to the characteristic of this institute which is tertiary hospital with liver transplant center, and majority of the subjects were referred patients with no prior record on earlier condition of the disease. Also, the number of subjects with fulminant hepatitis and severe hepatitis were small it has limits to determine statistical significance. Furthermore, the study lacks various patient histories since it was done retrospectively. To have accurate analysis on clinical features of acute hepatitis A and incidence of fulminant hepatitis, prospective, multi-institutional and more extensive studies are necessary.
In conclusion, although most patients with acute hepatitis A have uncomplicated clinical course, old age, significant alcohol intake, and underlying chronic HBV infection seems to be related with the development of fulminant or severe hepatitis in acute viral hepatitis A.
Abbreviations
- ALT
alanine aminotransferase
- anti-HAV
antibody to hepatitis A virus
- anti-HBc
antibody to hepatitis B core antigen
- anti-HBs
antibody to hepatitis B surface antigen
- AST
aspartate aminotransferase
- CI
confidence interval
- FH
fulminant hepatitis
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HCV
hepatitis C virus
- anti-HCV
antibody to hepatitis C
- HIV
human immunodeficiency virus
- INR
international normalized ratio
- OR
odds ratio
- PT
prothrombin time
- SH
severe hepatitis
References
- 1.Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980;302:1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- 2.Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128:111–114. doi: 10.7326/0003-4819-128-2-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Hong WS. Seroepilemiology of Type A and Type B Hepatitis in Seoul Area. Korean J Intern Med. 1982;25:19–27. [Google Scholar]
- 4.Yang DW, Lee YA, Shim JY, Park JY, Jung HL, Park MS, et al. A Seroepidemiologic Study on Hepatitis A in Seoul, Korea. J Korean Pediatr Soc. 1999;42:180–185. [Google Scholar]
- 5. Http://cdc.go.kr.
- 6.Laurence JC. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am J Med. 2005;118:75S–83S. doi: 10.1016/j.amjmed.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara K, Yokosuka O, Imazeki F, Saisho H, Saotome N, Suzuki K, et al. Analysis of the genotype-determining region of hepatitis A viral RNA in relation to disease severities. Hepatol Res. 2003;25:124–134. doi: 10.1016/s1386-6346(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara K, Kojima H, Yonemitsu Y, Yasui S, Imazeki F, Miki M, et al. Phylogenetic analysis of hepatitis A virus in sera from patients with hepatitis A of various severities. Liver Int. 2009;29:838–845. doi: 10.1111/j.1478-3231.2008.01919.x. [DOI] [PubMed] [Google Scholar]
- 9.Rezende G, Roque-Afonso AM, Samuel D, Gigou M, Nicand E, Ferre V, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38:613–618. doi: 10.1053/jhep.2003.50366. [DOI] [PubMed] [Google Scholar]
- 10.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 11.Coppola N, Genovese D, Pisaturo M, Taffon S, Argentini C, Pasquale G, et al. Acute hepatitis with severe cholestasis and prolonged clinical course due to hepatitis A virus Ia and Ib coinfection. Clin Infect Dis. 2007;44:e73–e77. doi: 10.1086/513430. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001;14:38–58. doi: 10.1128/CMR.14.1.38-58.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elinav E, Ben-Dov IZ, Shapira Y, Daudi N, Adler R, Shouval D, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology. 2006;130:1129–1134. doi: 10.1053/j.gastro.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90:201–205. [PubMed] [Google Scholar]
- 15.Tassopoulos N, Papaevangelou G, Roumeliotou-Karayannis A, Kalafatas P, Engle R, Gerin J, et al. Double infections with hepatitis A and B viruses. Liver. 1985;5:348–353. doi: 10.1111/j.1600-0676.1985.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 16.Chu CM, Liaw YF. Increased incidence of fulminant hepatic failure in previously unrecognized HBsAg carriers with acute hepatitis independent of etiology. Infection. 2005;33:136–139. doi: 10.1007/s15010-005-4094-4. [DOI] [PubMed] [Google Scholar]
- 17.Pramoolsinsap C. Acute hepatitis A and acquired immunity to hepatitis A virus in hepatitis B virus (HBV) carriers and in HBV- or hepatitis C virus-related chronic liver diseases in Thailand. J Viral Hepat. 2000;7:11–12. doi: 10.1046/j.1365-2893.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- 18.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27:914–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- 19.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 20.Weitz M, Siegl G. Variation among hepatitis A virus strains. I. Genomic variation detected by T1 oligonucleotide mapping. Virus Res. 1985;4:53–67. doi: 10.1016/0168-1702(85)90020-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Lee YS, Lee JH, Kim W, Lim KS. Clinical outcomes and predictive factors of spontaneous survival in patients with fulminant hepatitis A. Korean J Hepatol. 2008;14:474–482. doi: 10.3350/kjhep.2008.14.4.474. [DOI] [PubMed] [Google Scholar]
- 22.Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 23.Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985;122:226–233. doi: 10.1093/oxfordjournals.aje.a114093. [DOI] [PubMed] [Google Scholar]
- 24.Brown GR, Persley K. Hepatitis A epidemic in the elderly. South Med J. 2002;95:826–833. [PubMed] [Google Scholar]
- 25.Sjogren M. Immunization and the decline of viral hepatitis as a cause of acute liver failure. Hepatology. 2003;38:554–556. doi: 10.1053/jhep.2003.50401. [DOI] [PubMed] [Google Scholar]
- 26.Gao B. Interaction of alcohol and hepatitis viral proteins: implication in synergistic effect of alcohol drinking and viral hepatitis on liver injury. Alcohol. 2002;27:69–72. doi: 10.1016/s0741-8329(02)00201-x. [DOI] [PubMed] [Google Scholar]