Abstract
Background/Aims
The present study aimed to determine the role of cystatin C as a prognostic factor for acute kidney injury and survival in cirrhotic patients.
Methods
The study investigated 53 liver cirrhosis patients. The renal function was evaluated by serum creatinine, serum and urine cystatin C, and 24-hour creatinine clearance on admission. Acute kidney injury was defined as a serum creatinine level exceeding the normal range (>1.2 mg/dl) and an increase of at least 50% from the baseline value. Multivariate analysis, receiver operating characteristic curve, and survival analysis were used to investigate prognostic factors for acute kidney injury and survival.
Results
Nine of the 53 cirrhotic patients (17.0%) developed acute kidney injury within 3 months. Both serum creatinine and cystatin C were predictive factors for acute kidney injury in univariate analysis, with a diagnostic accuracy of 0.735 (95% confidence interval (CI), 0.525-0.945; p=0.028) for serum cystatin C and 0.698 (95% CI, 0.495-0.901, p=0.063) for creatinine. In multivariate analysis, only serum cystatin C was an independent risk factor for acute kidney injury. The sensitivity and specificity of a serum cystatin C level of >1.23 mg/L to acute kidney injury were 66% and 86%, respectively. Serum cystatin C was positively correlated with the Model for End-Stage Liver Disease (MELD) and MELD-Na scores (r=0.346 and p=0.011, and r=0.427 and p=0.001, respectively). Comparison of the survival rates over the observation period revealed that a serum cystatin C level of >1.23 mg/L was a useful marker for short-term mortality (p<0.001).
Conclusions
The accuracy in predicting acute kidney injury and short-term mortality was higher for a serum cystatin C level of >1.23 mg/L than for the serum creatinine concentration in patients with cirrhosis.
Keywords: Cystatin C, Liver cirrhosis, Acute kidney injury
INTRODUCTION
Acute kidney injury is frequently observed in patients with liver cirrhosis, and is associated with infection, the use of diuretics, reduced effective circulating volume and hepatorenal syndrome.1 In cirrhotic patients, acute kidney injury is particularly dangerous because it poses an obstacle to treatment and has a direct correlation to mortality. In the case of an early-stage decline in renal function, it is possible to achieve recovery, or at least delay the deterioration of renal disease, with active treatment. However, advanced renal failure results in a very high mortality rate.1,2 Therefore, recognizing renal injury during its early stage and beginning aggressive treatment is likely to delay the deterioration of renal function and improve prognosis.
The most frequently used clinical markers of renal failure include serum creatinine, creatinine clearance, and glomerular filtration rate (GFR) measured by dynamic methods.3,4 Unfortunately, in cirrhotic patients, serum creatinine measurements must be interpreted with caution because they can be detected within normal ranges by poor dietary intake, loss of muscle mass, and renal tubular secretion.5 Acute kidney injury is not accompanied by increase in serum creatinine simultaneously in patients with liver cirrhosis and ascites.6,7 Several early markers of renal dysfunction have been proposed, including urine neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, and interleukin-18.8-10 However, these markers require complicated test procedures, as well as additional research regarding their efficacy.11
Cystatin C is known to be a superior marker of renal function than creatinine, especially for cirrhotic patients whose muscle mass tends to be smaller, because it is produced not just in muscle cells but in all nucleated cells.2,12
Gerbes et al. found that serum cystatin C is more effective for detecting kidney injury in advanced cirrhotic patients than serum creatinine.2 There is also a study that reported the efficacy of cystatin C as an early biomarker for predicting the occurrence of acute kidney injury.13 Others have proposed the possibility of using serum cystatin C in patients with diabetes or heart failure as a prognostic factor enabling the early detection of mild renal dysfunction and the prediction of mortality.14,15 However, there are only a limited number of studies that examine the possibility of using cystatin C as an early marker for predicting acute kidney injury and survival in patients with liver cirrhosis.
Therefore, this study observed the changes in renal function in patients with liver cirrhosis to evaluate the role of cystatin C as a prognostic factor for acute kidney injury. It also examined the efficacy of cystatin C measurements in predicting patient survival rates.
PATIENTS AND METHODS
1. Patients
This study was conducted by retrospective review on consecutive cirrhotic patients who were hospitalized in Department of Gastroenterology from June to December 2007. The diagnosis of liver cirrhosis was defined as the combination of a physical examination showing ascites, an endoscopy showing the existence of varices, and an abdominal ultrasonography or computed tomography indicating cirrhosis of the liver. At the time of admission, patients with acute infection, gastrointestinal bleeding, or acute renal failure, as well as those undergoing hemodialysis or peritoneal dialysis due to chronic kidney disease, were excluded from the study. Follow-ups were performed every three months for patients with compensated liver cirrhosis, and every 1-2 months for those with decompensated liver cirrhosis. This study was approved by institutional review board (IRB) in Eulji University Hospital.
2. Methods
To evaluate the liver function of cirrhotic patients, a range of tests consisting of serum albumin, bilirubin, prothrombin time, electrolytes, physical examination, and ultrasonography were performed to obtain the Child-Pugh, Model for End-Stage Liver Disease (MELD), and MELD-Na scores. The Child-Pugh score was assigned as a number between 5 and 15 according to serum albumin, bilirubin, prothrombin time extension, ascites, and hepatic encephalopathy, then divided into three classes: A (5-6), B (7-9), and C (10-15).16
The renal function of cirrhotic patients was evaluated by testing for serum creatinine, serum and urine cystatin C (Human Cystatin C ELISA kits; BioVendor LLC, Candler, NC, USA), and 24-hour creatinine clearance. Serum sample was obtained at admission time. Urine sample was collected for 24 hours, from the second hospital day to the next day. Samples were assayed immediately or stored at -20℃. Cystatin C assay employed the quantitative sandwich enzyme immunoassay technique. Cystatin C kits microplates were precoted with monoclonal antibody specific for cystatin C. Dilution buffer was used to dilute samples.
Modification of Diet in Renal Disease (MDRD) equation was used to calculate the estimated GFR (e-GFR) using the following formula:
e-GFRMDRD (mL/min/1.73m2)=186×(serum creatinine)-1.154×(age)-0.203×(0.742 if female).
The following three formulae were used to calculate the estimated GFRs (e-GFRs) using serum cystatin C:
e-GFRHoek (mL/min)=80.35/serum cystatin C-4.32.17
e-GFRLansson (mL/min)=99.43×serum cystatin C-1.5837.18
e-GFRLesley (mL/min)=177.6×serum creatinine-0.65×cystatin C-0.57×age-0.20×(0.82 if female).4
Acute kidney injury was defined as a serum creatinine level was detected over normal range (>1.2 mg/dL) at follow-up sample and showed increase of 50% or more from the baseline value. Instances suggesting obvious prerenal or intrinsic-renal injury caused by dehydration, bleeding, septicemia, drug etc. were excluded from analysis.
3. Statistical analysis
All statistical analysis was performed using spss version 12.0 k (SPSS Inc., Chicago, IL, USA). Data were presented as mean±SD or n (%). The chi-square test and Mann-Whitney U-test were used to compare variables for renal and hepatic functions between different patient groups. The results of comparing the correlation between two continuous variables were indicated by the correlation coefficient (r) using correlation analysis. The reference values for creatinine and serum cystatin C were expressed using a receiver operating characteristic (ROC) curve. Reference values were defined the maximum sum of sensitivity and specificity with over 0.6 of each value. Cross tabulation analysis was used to derive the sensitivity, specificity, positive predictive value, and negative predictive value of the reference values. Survival analysis was used to compare the cumulative incidence of acute kidney injury and mortality rate during the observation period. Logistic regression test was performed to identify independent factors impacting acute kidney injury and mortality. A result was deemed statistically significant when p<0.05.
RESULTS
1. Baseline characteristics
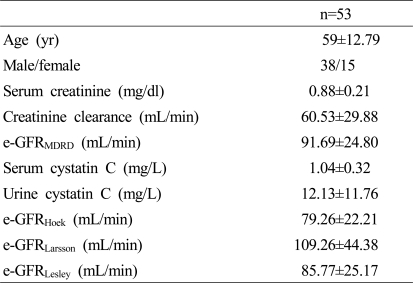
The mean age of 53 cirrhotic patients was 59 years, with 38 males and 15 female patients. Hepatitis B virus-related hepatitis was the most common cause of liver cirrhosis among the patients (39.6%), followed by alcoholic hepatitis (30.2%), hepatitis C virus-related hepatitis (5.7%), non-alcoholic steatohepatitis (5.7%), and unknown causes (18.9%). Ascites was found in 35 patients (66%). The average Child-Pugh score was 9.37±2.78, with Child-Pugh A consisting of 8 patients (15.1%), Child-Pugh B of 19 patients (35.8%), and Child-Pugh C of 26 patients (49.1%). The MELD score was 14.11±6.22, and the MELD-Na score was 16.41±7.48. The mean duration of follow-up was 13±8.9 months (average±standard deviation). The baseline characteristics were shown in Table 1.
Table 1.
Baseline characteristics in all subjects
Data are shown as mean±standard deviation (SD).
yr, years; e-GFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease.
e-GFRMDRD (mL/min/1.73 m2)=186×(serum creatinine)-1.154×(age)-0.203×(0.742 if female).
e-GFRHoek (mL/min)=80.35/serum cystatin C-4.32.
e-GFRLansson (mL/min)=99.43×serum cystatin C-1.5837.
e-GFRLesley (mL/min)=177.6×serum creatinine-0.65×cystatin C-0.57×age-0.20×(0.82 if female).
2. Serum and urine cystatin C in patients with liver cirrhosis
Creatinine clearance and the e-GFRMDRD showed a negative correlation with serum cystatin C [r=-0.532 (p<0.001) and r=-0.691 (p<0.001), respectively], but had no correlation with urine cystatin C and urine cystatin C/urine creatinine ratio [r=-0.129 (p=0.2) and r=-0.124 (p=0.3), respectively]. Serum cystatin C showed a positive correlation with the MELD and MELD-Na scores [r=0.346 (p=0.011) and r=0.427 (p=0.001), respectively] but had no correlation with the Child-Pugh score (r=0.234; p=0.09). Urine cystatin C was not correlated with MELD score (p=0.108), MELD-Na score (p=0.123), or Child-Pugh score (p=0.5).
3. Serum cystatin C and acute kidney injury
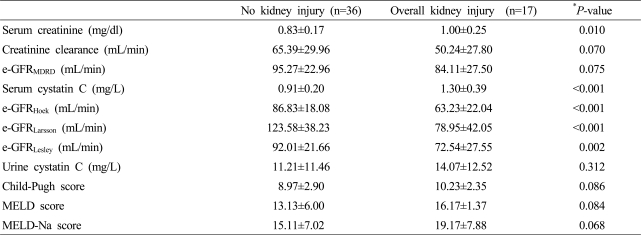
Nine out of the 53 patients with liver cirrhosis developed acute kidney injury during the first three months of follow-up. The e-GFRMDRD did not yield noticeable differences between the groups with and without acute kidney injury (p=0.18), but the formulae utilizing serum cystatin C, i.e., e-GFRHoek and GFRLarsson, differed significantly between two groups (p=0.03 and p=0.03, respectively).
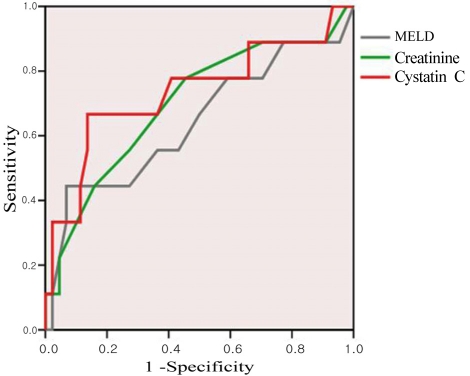
To investigate the efficacy of serum creatinine and serum cystatin C in predicting acute kidney injury, the area under ROC curve was calculated (Fig. 1). The results were 0.735 (95% CI, 0.525-0.945, p=0.028) for serum cystatin C and 0.698 (95% CI, 0.495-0.901, p=0.063) in creatinine. Using the ROC curve, the appropriate cutoff values of serum cystatin C and creatinine for predicting acute kidney injury were 1.23 mg/L (sensitivity 0.667, 1-specificity 0.136) and 0.9 mg/dl (0.85 mg/dl: sensitivity 0.778, 1-specificity 0.455; 0.95 mg/dl: sensitivity 0.556, 1-specificity 0.273), respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of the cystatin C reference value in regard to renal injury occurring within three months were found to be 66.7%, 86.4%, 50%, and 92.7%, respectively. During the whole observation period, 17 patients (32.1%) were developed acute kidney injury. The average initial serum creatinine and cystatin C levels were 1.00 mg/dl and 1.30 mg/L among patients showing acute kidney injury (Table 2). The area under the ROC curve was 0.809 (95% CI, 0.671-0.947, p<0.001) in serum cystatin C and 0.719 (95% CI, 0.568-0.870, p=0.011) in creatinine.
Figure 1.
ROC curves for serum cystatin C, creatinine, and MELD score for predicting the development of acute kidney injury within 3 months. The AUC values of serum cystatin C, creatinine, and MELD score were 0.735, 0.698, and 0.641, respectively.
Table 2.
GFR markers and liver function scores according to overall kidney injury
Data are shown as mean±SD.
*P<0.05 was evaluated by Mann-Whiney U test.
e-GFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; MELD, model for end-stage liver disease.
e-GFRMDRD (mL/min/1.73 m2)=186×(serum creatinine)-1.154×(age)-0.203×(0.742 if female).
e-GFRHoek (mL/min)=80.35/serum cystatin C-4.32.
e-GFRLansson (mL/min)=99.43×serum cystatin C-1.5837.
e-GFRLesley (mL/min)=177.6×serum creatinine-0.65×cystatin C-0.57×age-0.20×(0.82 if female).
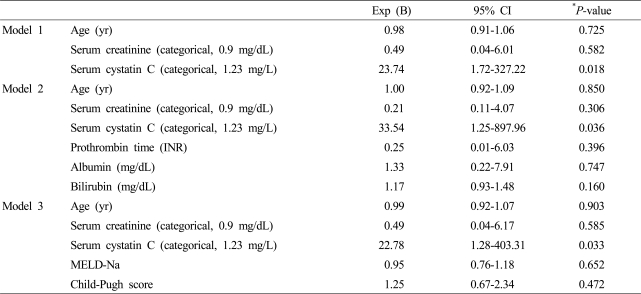
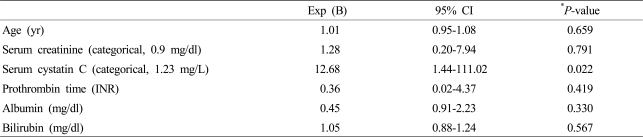
A multivariate logistic regression analysis was performed to discover the factors impacting acute kidney injury during the first three months. Among the variables, which included age, serum creatinine, and serum cystatin C, only serum cystatin C was found to be a significant factor influencing acute kidney injury (Model 1). When the prothrombin time, albumin, and bilirubin in Model 1 were adjusted, serum cystatin C remained an independent prognostic factor (Model 2). Even after correction using the MELD-Na and Child-Pugh scores, serum cystatin C was found to be an independent risk factor for acute kidney injury (Model 3) (Table 3). Serum cystatin C was also the only significant factor for predicting acute kidney injury during whole observation period, among the variables of age, serum creatinine, serum cystatin C, prothrombin time, albumin and bilirubin (Table 4).
Table 3.
Clinical factors impacting acute kidney injury within 3 months
*P<0.05, odds ratios with 95% confidence intervals from logistic regression analysis.
yr, years; Exp, exponential; CI, confidence interval; INR, international normalized ratio; MELD, model for end-stage liver disease.
Table 4.
Clinical factors impacting acute kidney injury over the observation period
*P<0.05, odds ratios with 95% confidence intervals from logistic regression analysis.
yr, years; Exp, exponential; CI, confidence interval; INR, international normalized ratio.
4. Patients mortality by serum cystatin C and creatinine levels
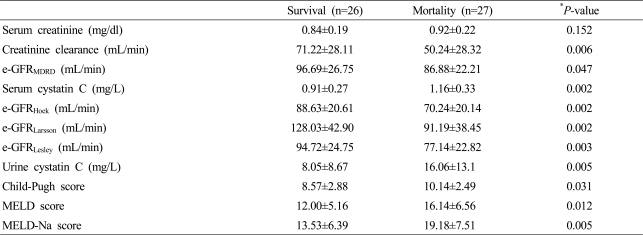
During the study period, a total of 27 cirrhotic patients died as a result of liver related disease. The death group exhibited higher average serum cystatin C (1.16 mg/L vs. 0.91 mg/L, p=0.006) and urine cystatin C (16.06 mg/L vs. 8.05 mg/L, p=0.012) concentrations compared to the survival group. There was no difference between the average serum creatinine concentrations of the death group and the survivor group (Table 5). In multivariate analysis, serum cystatin C and age showed positive correlation with mortality (p=0.029 and p=0.006, respectively).
Table 5.
GFR markers and liver function scores according to overall mortality
Data are shown as mean±SD.
*P<0.05 was evaluated by Mann-Whiney U test.
e-GFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; MELD, model for end-stage liver disease.
e-GFRMDRD (mL/min/1.73 m2)=186×(serum creatinine)-1.154×(age)-0.203×(0.742 if female).
e-GFRHoek (mL/min)=80.35/serum cystatin C-4.32.
e-GFRLansson (mL/min)=99.43×serum cystatin C-1.5837.
e-GFRLesley (mL/min)=177.6×serum creatinine-0.65×cystatin C-0.57×age-0.20×(0.82 if female).
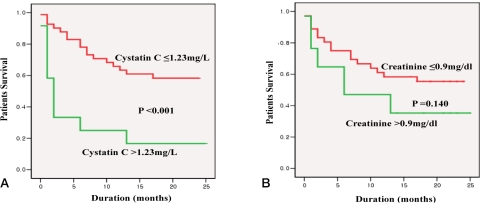
When the cumulative mortality rate for the whole observation period was determined by using serum cystatin C (>1.23 mg/L) serum andcreatinine (>0.9 mg/dl), a difference was found only in the case of serum cystatin C (p<0.001 vs. p=0.140, respectively) (Figure 2A, B).
Figure 2.
Cumulative survival rate according to serum cystatin C (A) and creatinine (B) levels. Reference values of cystatin C and serum creatinine were 1.23 mg/L and 0.9 mg/dL, respectively.
DISCUSSION
Our study showed that serum cystatin C offers a higher level of accuracy in predicting acute kidney injury. Serum cystatin C also displayed a superior discriminating capacity than serum creatinine in predicting short term survival rates.
Renal injury is directly linked to the mortality rate of cirrhotic patients. Moreover, patients with advanced liver disease accompanied by ascites frequently develop hepatorenal syndrome. Thus, there is active ongoing research on the important role played by early markers of renal injury in relation to the mortality rate of cirrhotic patients.5 Because serum creatinine levels in cirrhotic patients with acute kidney injury usually remain within normal range and increase only after the injury has progressed to a certain degree, other prognostic factors have been studied as potential early markers. However, there are as yet no available results involving the clinical use of biomarkers and their efficacy in treatment.11
A recent study reported that serum cystatin C level was useful marker for predicting the prognosis of cirrhotic patients with ascites.19 Serum cystatin C was independent factors predicting kidney injury and mortality. Our study showed similar outcomes to the recent study but with all cirrhotic patients, not only patients with ascites. We presented serum cystatin C was independent predictor of kidney injury and mortality for itself, comparing with urine cystatin C and other formulae using serum cystatin C level.
In the present study, both serum creatinine and serum cystatin C concentrations, as measured during hospitalization, were significantly high for the group exhibiting acute kidney injury during the course of the study period. Nonetheless, in the case of creatinine, the averages for the group showing a renal event within the first three months (1.03 mg/dl) and the group showing a renal event within the entire observation period (1.00 mg/dl) both fell within the reference range. Therefore, when using serum creatinine as an early marker for assessing renal injury in cirrhotic patients, a different standard than the clinically used reference range is required. Serum cystatin C, along with the MELD score and the MELD-Na score, proved to be an independent factor relating to the survival rate of cirrhotic patients. Additional research is needed to ascertain precisely how cystatin C affects patient survival rates. Recent studies have suggested its use as a biomarker of chronic inflammation. It has already been reported that serum cystatin C is linked to the mortality rate of patients with heart failure, regardless of objective renal function,15 and that it yields a higher rate of accuracy than serum creatinine in predicting the mortality rate of diabetic patients.14
The correlation between urinary cystatin C and the GFR has been reported by Tian et al.20 Urinary cystatin C, which is strongly basic and has a low molecular weight, is mostly absorbed in the proximal tubule after passing through the glomerular basement membrane, thus resulting in the correlation noted above.12 There has also been a study proposing a direct relationship with the GFR using urinary cystatin C and the urine creatinine ratio,21 and another proposing the increase of initial urinary cystatin C as well as urine cystatin C/creatinine ratio as prognostic factors for acute kidney injury in post-cardiothoracic surgery patients.22 However, in the present study, urinary cystatin C did not demonstrated a significant difference in patients displaying acute kidney injury. The group that developed renal injuries within the first three months had an average urinary cystatin C level of 11.44 mg/L, while the group that did not show acute kidney injury had an average level of 15.50 ng/mL, thus yielding no significant difference (p=0.3). Likewise, no difference was observed in regard to the assessment of acute kidney injury during the whole observation period. Urinary cystatin C may show a correlation with acute kidney injury when used in cross-sectional GFR calculation or repetitive measurement, but it has no efficacy as a singular prognostic factor and requires repeated follow-ups or large-scale research.
In cirrhotic patients with serum cystatin C concentrations of 1.23 mg/L or higher, there is an increased frequency of acute kidney injury as well as a higher rate of mortality. Therefore, active effort should be devoted to identifying and correcting the causative factors for renal dysfunction in such patients. In conclusion, serum cystatin C has superior diagnostic value than serum creatinine as an early marker for predicting acute kidney injury, and is related to mortality in patients with liver cirrhosis.
Abbreviations
- GFR
glomerular filtration rate
- MELD
Model for End-Stage Liver Disease
- MDRD
Modification of Diet in Renal Disease
References
- 1.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 2.Gerbes AL, Gulberg V, Bilzer M, Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50:106–110. doi: 10.1136/gut.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 6.Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201–205. [PubMed] [Google Scholar]
- 7.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 8.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 9.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Hoyos M, San Segundo D, Benito MJ, Fernandez-Fresnedo G, Ruiz JC, Rodrigo E, et al. Association between serum soluble CD30 and serum creatinine before and after renal transplantation. Transplant Proc. 2008;40:2903–2905. doi: 10.1016/j.transproceed.2008.08.087. [DOI] [PubMed] [Google Scholar]
- 11.Bonventre JV. Diagnosis of acute kidney injury: from classic parameters to new biomarkers. Contrib Nephrol. 2007;156:213–219. doi: 10.1159/000102086. [DOI] [PubMed] [Google Scholar]
- 12.Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta. 2002;323:121–128. doi: 10.1016/s0009-8981(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 13.Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 14.de Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care. 2009;32:1833–1838. doi: 10.2337/dc09-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 18.Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 19.Seo YS, Jung ES, An H, Kim JH, Jung YK, Yim HJ, et al. Serum cystatin C level is a good prognostic marker in patients with cirrhotic ascites and normal serum creatinine levels. Liver Int. 2009;29:1521–1527. doi: 10.1111/j.1478-3231.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 20.Tian S, Kusano E, Ohara T, Tabei K, Itoh Y, Kawai T, et al. Cystatin C measurement and its practical use in patients with various renal diseases. Clin Nephrol. 1997;48:104–108. [PubMed] [Google Scholar]
- 21.Hellerstein S, Berenbom M, Erwin P, Wilson N, DiMaggio S. The ratio of urinary cystatin C to urinary creatinine for detecting decreased GFR. Pediatr Nephrol. 2004;19:521–525. doi: 10.1007/s00467-003-1373-0. [DOI] [PubMed] [Google Scholar]
- 22.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]