Abstract
Background/Aims
Clevudine is a pyrimidine analogue with potent activity against hepatitis B virus (HBV) replication in vitro. In a previous pivotal phase III clinical study, 24 weeks treatment with clevudine 30 mg has been shown to profoundly suppress HBV replication and normalize serum alanine aminotransferase level.
Methods
In this study, we compare the efficacy and safety of clevudine (30 mg daily) versus lamivudine (100 mg daily) for 48 weeks in treatment-naive chronic hepatitis B e antigen (HBeAg) positive patients.
Results
Ninety-two chronic HBeAg positive patients were randomized to receive clevudine 30 mg daily or lamivudine 100 mg daily in a 1:1 ratio. The clevudine group demonstrated greater viral suppression at week 48 when compared with the lamivudine group (median reduction: 4.27 vs. 3.17 log10 copies/ml at week 48, p<0.0001). At week 48, serum HBV DNA level was below 300 copies/mL in 73% and 40% in the clevudine and lamivudine groups, respectively (p=0.001). HBeAg seroconversion occurred in 18% of patients in the clevudine group versus 12% in the lamivudine group at week 48. Lamivudine-resistant mutations were detected in 11 (24%) patients in the lamivudine group, who showed viral rebound during lamivudine therapy but no resistance was found in the clevudine group during 48-week treatment period.
Conclusions
A 48-week dosing with clevudine 30 mg daily was superior to lamivudine 100 mg daily in suppressing HBV replication, with no emergence of viral breakthrough in patients with HBeAg positive chronic hepatits B.
Keywords: Hepatitis B virus, Clevudine, Lamivudine, Clinical study, Resistance
INTRODUCTION
Worldwide and especially in Asia-Pacific region, chronic hepatitis B (CHB) virus infection is the major cause of liver-related morbidity and mortality.1-3 Indeed, it is estimated that one-quarter of chronic HBV patients will die from hepatocellular carcinoma and end-staged liver cirrhosis. The virus is transmitted via contact with blood, semen, or saliva of those with acute, chronic or active infection and perinatal transmission from an infected mother to child is the major route of infection in Southeast Asia.4 It is estimated that there are 350 million chronic HBV patients, worldwide.5 The primary aim of treatment is to achieve sustained immune control on viral replication, which is associated with long-term clinical benefits. This is defined as hepatitis B 'e' antigen (HBeAg) seroconversion in HBeAg-positive patients and sustained HBV DNA suppression to less than 10,000 copies/mL in HBeAg-negative patients.6 Though lamivudine is effective in reducing HBV viral replication, prolonged therapy leads to the development of drug-resistant mutants, which will adversely affect the natural history of chronic HBV infection.7 Previously clevudine at the dose of 30 mg daily has been shown to produce potent antiviral activity with a prolonged viral suppression after 24 weeks of treatment for chronic HBeAg-positive patients.8 The median log10 HBV DNA change from baseline was -5.10 log10 copies/mL and 68% of the treated patients had normalization of serum alanine aminotransferase (ALT) level. Sixty-five percent (65%) of patients had HBV DNA levels below the lower limit of detection (LOD) by Digene HC II assay (4,700 copies/mL), and 59% had negative HBV DNA levels (<300 copies/mL) by Amplicor PCR at the end of 24 weeks of treatment. At 6 months post-treatment, viral suppression was sustained with a median suppression of -2.02 log10 copies/mL. Furthermore, near to one-fifth of patients had serum HBV DNA below LOD by Digene HC II assay (4,700 copies/mL) and 61% had normal ALT at 6 months posttreatment. The prolonged anti-viral effect after withdrawal from clevudine is unknown.9 Therefore, based on the hypothesis that dosing with clevudine for at least 1 year may provide more prolonged and profound suppression of HBV DNA, we designed the current double-blinded study to evaluate the safety and antiviral activity for a 48-week treatment of clevudine 30 mg daily compared with lamivudine 100 mg daily in patients with HBeAg-positive chronic hepatitis B.
PATIENTS AND METHODS
1. Study design
This is a double-blinded, randomized, clinical study. It was conducted at two clinical centers in Hong Kong (Queen Mary Hospital and Alice Ho Miu Ling Nethersole Hospital, Hong Kong). Patients were randomized, based on a predetermined computer generated list, to receive either clevudine 30 mg or lamivudine 100 mg (1:1) once daily for 48 weeks.
The study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with Good Clinical Practice guidelines. Written informed consent was obtained from all study participants before being examined for eligibility criteria. The study protocol and the informed consent form were approved by the ethics committee at each study site.
Patients were monitored at baseline, week 4, 8, 12, 16, 20, 24, 32, 40 and week 48 during the dosing period. Patients underwent clinical assessments of tolerability (open-ended interview), physical exam and blood samples were tested according to the protocol.
A sub-study on hepatitis B viral dynamic was done. The sub-study involved the first 30 enrolled patients. The purpose of the sub-study was to define the nature of the antiviral effect of clevudine compared to lamivudine. A separate consent was obtained from the study patients participated in this sub-study. If the patient agreed to participate in this sub-study, they were asked to visit the clinic on day 4, 7, 10, 14, week 3 before the visit at week 4 of the main study.
2. Study population
Eligible patients were adults, 18-60 years of age, with chronic HBeAg-positive hepatitis. They all have hepatitis B surface and e antigen positive for at least 6 months, with a serum HBV DNA levels more than 3×106 copies/mL and serum ALT levels between 1.0 and 10 times the upper limit of normal (ULN). Exclusion criteria included co-infection with hepatitis C, or the human immunodeficiency virus; evidence of cirrhosis or hepatocellular carcinoma; previous exposure to any nucleoside analog that is active against HBV; use of interferon alpha within 6 months before enrollment. Patients who were taking any traditional Chinese medication, or had been taking any traditional Chinese medication within the last 2 weeks prior to screening were excluded. Breastfeeding or pregnant women or women of childbearing age unwilling to use barrier contraceptive methods were also excluded.
The sample size was calculated to detect 30% of statistically significant differences in the proportion of patients with serum HBV DNA below 300 copies/mL at week 48 between the clevudine treatment group and the lamivudine group, assuming that lamivudine and clevudine responses were 36% and 67%, respectively. The sample size was estimated using the Z-test with an alpha level of 0.05 and 80% power for comparing lamivudine group with clevudine treatment group. The ratio of patient numbers in each group (lamivudine 100 mg daily versus clevudine 30 mg daily) was 1:1. According to this assumption, 40 patients per group was required. Taking a small drop-out rate of around 10 percent, a total of 92 patients were enrolled, i.e., 46 patients per group.
3. Efficacy endpoints
The primary efficacy end point was the proportion of patients with negative HBV DNA (<300 copies/ml) by Amplicor PCR at week 48. Serum HBV DNA levels were measured at a central laboratory using the COBAS Amplicor PCR assay (Roche Molecular Systems, Branchburg, NJ, USA) with a LOD of 300 copies/mL.
Secondary end points included the reduction in serum HBV DNA, as defined as median log10 decrease from baseline at week 48, HBeAg loss, HBeAg seroconversion (HBeAg loss and appearance of anti-HBe), and serum ALT normalization. A viral breakthrough is defined as increase in serum HBV DNA by 1 log (10 fold) above nadir in patients with viral load initially suppressed by at least 2 logs from baseline.
The viral dynamic over the first 12 weeks was summarized by treatment groups for the sub-study on 30 subjects. Serial serum HBV DNA samples were analyzed by a viral load function previously applied to the clearance kinetics of HBV from serum during a lamivudine and famciclovir viral dynamics study.10 The efficacy (ε) of inhibition of viral production, the free virus clearance rate constant (µ), and the infected cell loss rate constant (a) were determined by fitting the viral load function to the data using non-linear regression.
4. Safety analysis
The safety analysis included data from all 92 eligible patients who received at least one dose of study medication after randomization. Safety evaluations included analysis of adverse events, serious adverse events, and deaths. Exacerbation of hepatitis B was defined as ALT and/or AST elevations (a) >20×ULN or (b) >10×ULN and a 10-fold change from the lowest on-study value. Exacerbation of hepatitis B was considered as a serious adverse event.
5. Analysis of genotypic resistance
The analysis of the HBV DNA polymerase domain (amino acids 119-247) by dideoxy sequencing was performed at baseline and at the end of 48 weeks dosing period for all of the patients with positive HBV DNA by Amplicor PCR assay. For the detection of lamivudine-related YMDD mutations at rt180 and rt204, restriction fragment length polymorphism (RFLP) assay was performed additionally at baseline and at the end of 48 weeks dosing period as described.11 A genotypic resistance is defined as increase in serum HBV DNA by 1 log (10 fold) above nadir in patients with viral load initially suppressed by at least 2 logs from baseline and genotypic mutation.
6. Statistical analysis
The results were analyzed on the basis of intention-to-treat and all 92 eligible patients who received at least one dose of study medication after randomization were included in the safety analysis. Patients discontinuing the study after receiving the first study drug dose were included in the efficacy analysis until the time of their discontinuation.
The Statistical Analysis System (SAS) Release 9.2 was used for the statistical analysis. Statistical significance was performed with an alpha level of 0.05. A two-sided Chi-Square test was used to compare the difference of the proportion of patients with negative HBV DNA at week 48 between the two groups, ALT normalization, serology response and incidence of adverse events (AEs). Two-sample Wilcoxon rank sums test were used for viral dynamic analysis.
RESULTS
1. Study population
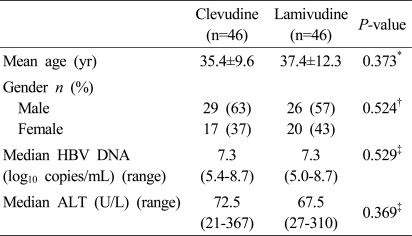
A total of 92 eligible patients were enrolled at two sites and received at least one dose of clevudine 30 mg daily (n=46) or lamivudine 100 mg daily (n=46) in a blinded fashion. The baseline characteristics of the two treatment groups were shown in Table 1.
Table 1.
Patient characteristics at baseline
HBV, hepatitis B virus; ALT, alanine aminotransferase.
*Two sample t-test, †Chi-square test, ‡Wilcoxon rank-sum test.
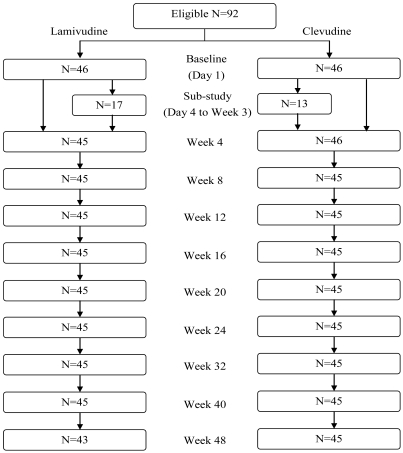
The number of withdrawal was 4 at week 48 for efficacy analysis (1 patient from clevudine group and 3 patients from lamivudine group) (Fig. 1). Hence, 88 patients (45 in the clevudine group and 43 in the lamivudine group) completed the 48-week treatment period. One patient discontinued the study in the clevudine group due to withdrawal of consent and 3 patients discontinued the study in the lamivudine group due to withdrawal of consent, lamivudine resistance, and death.
Figure 1.
Flow chart of the present study.
2. Virologic and serologic endpoints
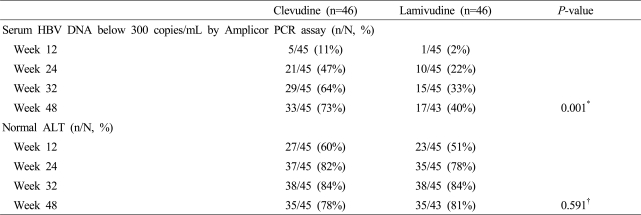
Clevudine treatment for 48 weeks produced potent viral suppression compared to lamivudine treatment. At week 48, 33 of 45 of patients (73%) in the clevudine group had undetectable serum HBV DNA levels by Amplicor PCR assay (less than 300 copies/mL), while 17 of 43 of patients (40%) in the lamivudine group had undetectable serum HBV DNA levels (p=0.001) (Table 2).
Table 2.
Summary of viral and biochemical responses in the clevudine and lamivudine groups
*McNemar's test, †Chi-Square test.
Median serum HBV DNA reductions from baseline at week 48 were 4.27 and 3.17 log10 copies/mL in the clevudine and lamivudine groups, respectively (p<0.0001). At week 48, rate of HBeAg loss (18% in clevudine group versus 14% in lamivudine group) and HBeAg seroconversion (18% in clevudine group and 12% in lamivudine group) was comparable in both groups. No viral breakthrough was observed in the clevudine group, while 24% of patients in the lamivudine group showed viral breakthrough.
3. The Viral dynamic over the first 12 weeks
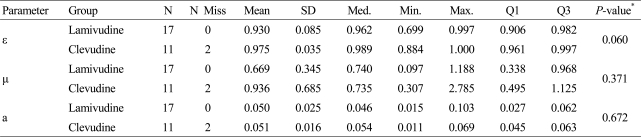
A sub-study with the first 30 enrolled patients was performed. For each individual patient, the viral dynamics parameters (ε, µ, and a) were estimated by fitting the viral load function to the data using non-linear regression.
Table 3 summarizes the estimated parameters by treatment groups. The p-values of the Wilcoxon Rank-Sum test were 0.060, 0.371 and 0.672 for ε, µ and a, respectively. The differences of the median of the estimates between the groups were not statistically significant.
Table 3.
Estimates of viral dynamics parameters
R002 was excluded because of numerical issue (Estimates were biased) and R027 was excluded for missing data at week 8 and 12.
*Two-Sample Wilcoxon Rank Sums Test.
4. Biochemical endpoints
The proportion of patients who had normalization of serum ALT was 78% in the clevudine group and 81% in the lamivudine group at week 48 (p=0.591) (Table 2).
5. The Analysis of genotypic resistance
Comparative analysis of genomic sequence of HBV isolated from the serum of the patients at baseline and at the time of viral rebound showed no resistance in the clevudine groups, while 11 (24%) patients treated with lamivudine was found to have lamivudine-related resistance at the end of 48 weeks of treatment.
6. Safety and tolerability
The drug compliance was 99% during the study period. In the lamivudine group, there were two discontinuations of study due to adverse events (one with exacerbation of hepatitis B due to lamivudine resistance and another due to liver failure led by lamivudine resistance).
During the treatment period, the incidence of adverse events was similar in the two groups (clevudine group: 39%, lamivudine group: 33%). The most frequent adverse events (occurring in ≥ 5% of patients), were flu-like symptoms (22%), headache and epigastric pain in the clevudine group. In the lamivudine group, the most frequent adverse events were flu-like symptoms (17%), increases in ALT and AST level. The incidence of serious adverse events (SAEs) during treatment was 7% (3/46) in the clevudine group and 9% (4/46) in the lamivudine group. From the 3 SAEs reported in the clevudine group, 2 SAEs were exacerbation of hepatitis B which resolved without any treatment and 1 SAE was myopathy reported at week 43 with grade 3 of creatinine phosphate kinase (CPK) value which was resolved after discontinuation of clevudine treatment. From the 4 SAEs reported in the lamivudine group, 1 SAE was hospitalization due to back pain and 3 SAEs were exacerbation of hepatitis B. One patient died in the lamivudine group due to liver failure led by lamivudine resistance. The incidence of ≥ grade 3 laboratory abnormality was similar in both groups except red blood cell, mean cell volume and CPK. The incidence of ≥ grade 3 laboratory abnormality was 6.7% and 0% in the clevudine and lamivudine groups, respectively.
DISCUSSION
In this randomized double-blinded study, clevudine 30 mg daily was shown to be a highly potent antiviral activity in HBeAg-positive chronic hepatitis B patients compared to lamivudine. Clevudine suppressed HBV DNA by a median of 4.27 log10 copies/mL, while the reduction serum HBV DNA was only 3.17 log10 copies/mL in the lamivudine group. The proportion of patients with negative HBV DNA (i.e. <300 copies/ml) at week 48 which is the primary endpoint was 73% in the clevudine group and 40% in the lamivudine group which shows the statistically significant difference.
Although direct head-to-head comparisons are not available, this degree of HBV DNA reduction by clevudine at week 48 (73% with negative HBV DNA by Amplicor PCR) compared favorably to adefovir 10 mg daily (21%), entecavir 0.5 mg daily (67%) and telbivudine 600 mg daily (60%).12-14
From previous study, we have learned that early viral suppression may predict long-term efficacy during antiviral therapy. Similar to an earlier studies,7,8 clevudine is again shown to be superior to lamivudine in early viral suppression, with the proportion of patients with negative HBV DNA being 47% in the clevudine group and 22% in the lamivudine group at week 24. In addition, by measuring serum HBV DNA levels with a more sensitive assay, further analysis was performed to estimate viral clearance kinetics, in the sub-study.10 However, the median of the estimates for viral dynamics between the groups were not statistically significant and this could be related to the small number of patients being studied. In our current study, the proportion of patients with normalization of serum ALT at week 48 was similar in both groups, 78% and 81% in the clevudine and lamivudine groups, respectively. Also, the rate of HBeAg loss and HBeAg seroconversion were similar in the two groups. This is comparable to the previous 24 weeks clevudine study.
Lamivudine has been one of the most widely used nucleoside analogues for CHB, largely due to its ability to significantly reduce HBV replication, favorable tolerability profile and relatively low cost. However, suppression of HBV DNA is not sustained in all patients and after only 6 months of continuous treatment, substitutions at position 204 in the tyrosine-methionine-aspartate-aspartate (YMDD) locus of HBV DNA polymerase can be detected. This YMDD mutation is the main cause of resistance to lamivudine, the incidence of which increases with treatment duration-approaching 70% after 4-5 years of continuous drug exposure.15 YMDD mutant HBV is associated with a diminished clinical and virologic responses that may lead to a significant number of patients experiencing deterioration in liver function, and withdrawal of lamivudine therapy in patients who develop YMDD mutations can be associated with ALT flares. Hence, it is of great importance to have other nucleoside analogues with lower rate of viral resistance. In our current study, by analyzing the genomic sequence of HBV isolated from the serum of the patients treated with clevudine showed no viral resistance, while 24% of patients in the lamivudine group showed lamivudine-related resistance (rtL180M, rtM204I/V). In the latter patients, there was associated viral rebound during 48-week treatment period. Further evaluation is necessary to understand the resistance rate with long-term treatment of clevudine.
The safety evaluation was performed for all patients who had taken at least one dose of study medication after randomization. Incidence of adverse event during the 48-week treatment period was similar between clevudine and lamivudine groups. During 48-week treatment period, SAEs were reported from 3 patients in the clevudine group and 4 patients in the lamivudine group. From the 3 SAEs reported in the clevudine group, 2 SAEs were exacerbation of hepatitis B which were resolved without special treatment and 1 SAE was myopathy which was resolved after stopping of clevudine therapy. From the 4 SAEs reported in the lamivudine group, 1 SAE was hospitalization due to back pain and 3 SAEs were exacerbation of hepatitis. From them, one patient died due to liver failure led by lamivudine resistance. The incidence of ≥grade 3 lab abnormality was similar in both groups except red blood cell, mean cell volume and creatinine phosphate kinase parameters.
Myopathy was reported during clevudine therapy of 8-13 months.16 During 48-week treatment period in this study, there was one SAE of myopathy reported at week 43 with grade 3 of CPK value in the clevudine group, which was resolved after discontinuation of clevudine treatment.
In conclusion, 48-week dosing with clevudine 30 mg daily showed superior viral suppression to lamivudine 100 mg daily in HBeAg-positive chronic hepatitis B patients.
Acknowledgements
Financial support: This study was sponsored by Bukwang Pharmaceutical Co., Ltd.
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- anti-HBe
hepatitis B e antibody
- DNA
deoxyribonucleic acid
- HBeAg
hepatitis B e antigen
- HBV
hepatitis B virus
- SAE
serious adverse event
- ULN
upper limit of normal
References
- 1.Zuckerman AJ. The association of hepatitis B with primary hepatocellular carcinoma. J Infect. 1983;7(Suppl. 1):73–77. doi: 10.1016/s0163-4453(83)96764-6. [DOI] [PubMed] [Google Scholar]
- 2.Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- 3.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 4.Yao JL. Perinatal transmission of hepatitis B virus infection and vaccination in China. Gut. 1996;38(suppl 2):S37–S38. doi: 10.1136/gut.38.suppl_2.s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korenman J, Baker B, Waggoner J, Everhart JE, Di Biscreglie AM, Hoofnagle JH. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med. 1991;114:629–634. doi: 10.7326/0003-4819-114-8-629. [DOI] [PubMed] [Google Scholar]
- 6.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates; results after 3 years of therapy. Hepatology. 2001;33:1527–1532. doi: 10.1053/jhep.2001.25084. [DOI] [PubMed] [Google Scholar]
- 8.Yoo BC, Kim JH, Chung YH, Lee KS, Paik SW, Ryu SH, et al. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2007;45:1172–1178. doi: 10.1002/hep.21629. [DOI] [PubMed] [Google Scholar]
- 9.Menne S, Ronecker CA, Korba BE, Gerin JL, Tennant BC, Cote PJ. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-β-L-arabinofuranosyl)-uracil (L-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J Virol. 2002;76:5305–5314. doi: 10.1128/JVI.76.11.5305-5314.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau GKK, Tsiang M, Hou JL, Yuen S, Carman WF, Zhang L, et al. Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology. 2000;32:394–399. doi: 10.1053/jhep.2000.9143. [DOI] [PubMed] [Google Scholar]
- 11.Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711–1716. doi: 10.1002/hep.510270634. [DOI] [PubMed] [Google Scholar]
- 12.FDA NDA 21-449; Hepsera-statistical review and evaluation. 2010. U S Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) 2010. FDA web site (online), < http://www.fda.gov/Drugs/default.htm>.
- 13.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A Comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 14.Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 15.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003;125:1714–1722. doi: 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Seok JI, Lee DK, Lee CH, Park MS, Kim SY, Kim HS, et al. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology. 2009;49:2080–2086. doi: 10.1002/hep.22959. [DOI] [PubMed] [Google Scholar]