Abstract
Background/Aims
Transarterial chemoembolization (TACE) has long been used as a palliative therapy for unresectable hepatocellular carcinoma (HCC). High-dose hepatic arterial infusion chemotherapy (HAIC) has showed favorable outcomes in patients with intractable, advanced HCC. The aim of this study was to compare the effectiveness and safety of high-dose HAIC and conventional TACE using doxorubicin for advanced HCC.
Methods
The high-dose HAIC group comprised 36 patients who were enrolled prospectively from six institutions. The enrollment criteria were good liver function, main portal vein invasion (including vascular shunt), infiltrative type, bilobar involvement, and/or refractory to prior conventional treatment (TACE, radiofrequency ablation, or percutaneous ethanol injection), and documented progressive disease. Patients received 5-fluorouracil (500 mg/m2 on days 1~3) and cisplatin (60 mg/m2 on day 2 every 4 weeks) via an implantable port system. In the TACE group, 31 patients with characteristics similar to those in the high-dose HAIC group were recruited retrospectively from a single center. Patients underwent a transarterial infusion of doxorubicin every 4~8 weeks.
Results
Overall, 6 patients (8.9%) achieved a partial response and 20 patients (29.8%) had stable disease. The objective response rate (complete response+partial response) was significantly better in the high-dose HAIC group than in the TACE group (16.7% vs. 0%, P=0.030). Overall survival was longer in the high-dose HAIC group than in the TACE group (median survival, 193 vs. 119 days; P=0.026). There were no serious adverse effects in the high-dose HAIC group, while hepatic complications occurred more often in the TACE group.
Conclusions
High-dose HAIC appears to improve the tumor response and survival outcome compared to conventional TACE using doxorubicin in patients with intractable, advanced HCC.
Keywords: Carcinoma, Hepatocellular, Hepatic arterial infusion chemotherapy, Transarterial chemoembolization, Doxorubicin
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death worldwide.1 While treatment options including surgical resection, transplantation, and ablation can increase the chance of achieving complete remission, these strategies are not suitable for most HCC patients due to underlying cirrhosis or the advanced stage of the disease at the time of diagnosis. Still, many patients are diagnosed at an unresectable, advanced stage, though diagnosis of HCC at an early stage has been increased by virtue of surveillance of populations at high risk for the development of HCC.2 The prognosis of patients with unresectable HCC is poor; if left untreated, the median survival is less than 6 months.3,4 Transarterial chemotherapy with or without embolization is widely used as a palliative therapy for unresectable, advanced HCC. Few studies have reported on patients with massive HCC (i.e., >10 cm in diameter); most reports on the treatment response of unresectable, advanced HCC have analyzed tumors 5~7 cm in size.5,6 Currently, repetitive hepatic arterial infusion chemotherapy (HAIC) with chemotherapeutic agents delivered via an implantable port system has been reported to be a beneficial therapeutic option for patients with advanced HCC.7-12 Woo et al. reported that both high-dose and low-dose HAIC regimens were effective and safe, and high-dose HAIC manifested a better tumor response and a tendency to improve survival compared to low-dose HAIC in a multicenter, prospective clinical trial conducted with intractable, advanced HCC patients with main portal vein invasion or bilobar involvement.13 However, the comparative efficacy and safety of high-dose HAIC and transarterial chemoembolization (TACE) using doxorubicin, which is generally used as a palliative treatment, is unclear. The present study was undertaken to compare these regimens in terms of tumor response, survival and adverse effects in intractable, advanced HCC.
METHODS
Patients
In the HAIC group, patients with intractable, advanced HCC including major portal vein invasion or bilobar involvement were enrolled in a multicenter, prospective study from January 2006 to January 2008, with the intent of evaluating efficacy and safety.13 In the TACE group, data were retrospectively collected from patients in a single center meeting the same inclusion criteria between January 2003 and December 2007. The diagnosis of HCC was made either histologically or by typical radiologic findings of HCC on two dynamic imaging examinations, or based upon one dynamic technique with an elevated serum alpha-fetoprotein (AFP) levels (> 400 ng/mL). Intractable, advanced HCC was defined as HCC with main portal invasion, diffuse bilobar involvement and/or refractory to surgical resection or nonsurgical intervention [TACE, radiofrequency (RF) ablation, or percutaneous ethanol injection (PEI)].11
Patients with HCC who met the following criteria were eligible for enrollment: age between 18 and 70 years, Child-Pugh score of 5 or 6, Eastern Cooperative Oncology Group (ECOG) performance status of 0~1, serum creatinine level ≤1.5 mg/dL, aminotransferase < 200 IU/mL, absolute neutrophil count ≥ 1,500/mm3, platelet count ≥75,000/mm3 and hemoglobin ≥ 10 g/dL. Patients were excluded if they had extrahepatic primary malignancy or metastasis and/or intractable comorbid medical illness. Informed consent was give to all patients. The prospective study was conducted according to the current declaration of Helsinki, and both prospective and retrospective studies were approved by the institutional ethics committee of each participating institution.
Treatment methods
In the HAIC group, the regimen consisted of 5-fluorouracil (5-FU; 500 mg/m2) for 5 hours on days 1~3 and cisplatin (60 mg/m2) for 2 hours on day 2. Each session was conducted every 4 weeks via an implantable port system. In the TACE group, patients underwent a transarterial infusion of doxorubicin (10~60 mg) in a mixture of 5~10 mL of lipiodol (Guerbet, Anulnaysous- Boid, France), and was partly accompanied by embolization using gelfoam in selected cases. Unless contraindicated, treatment sessions were repeated every 4~8 weeks.
Intravenous hydration was carried to prevent nephrotoxicity and prophylactic anti-emetics comprised of 5-hydroxytryptamine-3 antagonists were given to all patients in both groups. Treatment cycles were repeated until disease progressed or unacceptable toxicity was evident, or the patient refused treatment. The dose of chemotherapy or interval of treatment cycles was adjusted according to hepatic dysfunction or other significant toxicity at every treatment cycle.
Study assessment
The primary efficacy was objective response rate [complete response (CR)+partial response (PR)] and the secondary efficacy was overall survival (OS). Pretreatment evaluation included medical history, physical examination, laboratory tests (complete blood count, blood chemistry, virus serology, serum AFP), chest and abdominal X-rays, and computed tomography (CT) or magnetic resonance imaging (MRI). During treatment, toxicity evaluation, laboratory tests, chest and abdominal X-rays were carried out repeatedly before each treatment cycle. CT scans were performed every two cycles or to evaluate tumor response or document disease progression.
Tumor responses were classified according to the World Health Organization criteria with the European Association for the Study of the Liver modifications. The objective response was defined as the sum of the CR and PR. Patients who achieved CR, PR, and stable disease (SD) were included in disease control group.14 OS was calculated as the time from initiation of treatment until death or last follow-up visit. Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTCAE; ver. 3.0).15
Statistical analyses
Treatment response in both groups was analyzed using the Chi-square test or Fisher's exact test. The chi-square test or Fisher's exact test, where appropriate, and the Mann-Whitney U test were used for the analysis of other variables. The cumulative survival rates were estimated using the Kaplan-Meier method, and the differences were compared using the log-rank test. A Cox proportional-hazards model was used to identify independent predictors of survival. A P-value less than 0.05 was considered significant. All data was analyzed using SPSS version 14.0 software (SPSS, Chicago, IL, USA).
RESULTS
Patient characteristics
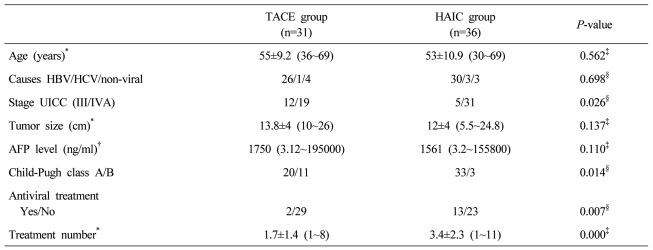
A total of 67 patients were analyzed. Of these, 31 patients were included in the TACE group, and 36 in the HAIC group. The baseline characteristics of the patients between two groups are summarized in Table 1. There was no significant difference between the two groups in terms of age, cause of HCC, tumor size and serum AFP. Patients with stage IVa, antiviral therapy, and multiple treatment cycles were more common in the HAIC group, while patients with Child-Pugh 7 were more common in TACE group.
Table 1.
Baseline characteristics of the patients in the two groups
*Expressed as mean±SD (range), †Expressed as median (range), ‡Mann-Whitney U test, §Fisher's exact test.
TACE, Transarterial chemoembolization; HAIC, Hepatic arterial infusion chemotherapy; HBV, hepatitis B virus; HCV, hepatitis C virus; UICC, Union for International Cancer Control; AFP, alpha fetoprotein.
Treatment response
The median dose of cisplatin and 5-FU at each cycle was 100 mg (range 60~120 mg) and 880 mg (range 500~1,000 mg) in the HAIC group. The median dose of doxorubicin at each cycle was 45 mg (range 10~60 mg) in the TACE group. In the TACE group, 5 out of 19 patients with stage IVa had main portal vein invasion, and embolization was not performed in these patients except for one patient, whom embolization of arterial branches was carried out. Evaluation of tumor response was possible in 61 out of 67 patients, the remaining six patients were withdrawn from the study due to loss to follow-up or transfer to another hospital. There was no CR in both groups. PR, SD, and progressive disease (PD) were 16.7%, 33.3%, and 44.4%, respectively, in the HAIC group and 0%, 25.8%, and 61.2%, respectively, in the TACE group. The objective response rate (CR+PR) of the HAIC group was significantly higher (16.7%) than that of the TACE group (0%) (P=0.030). The disease control rate (CR+PR+SD) was also better in the HAIC group (50%) than in the TACE group (25.8%) (P=0.033) (Table 2).
Table 2.
Tumor response according to the treatment modality
*Fisher's exact test.
TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Survival and prognostic factors
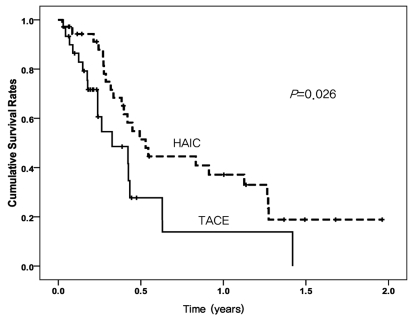
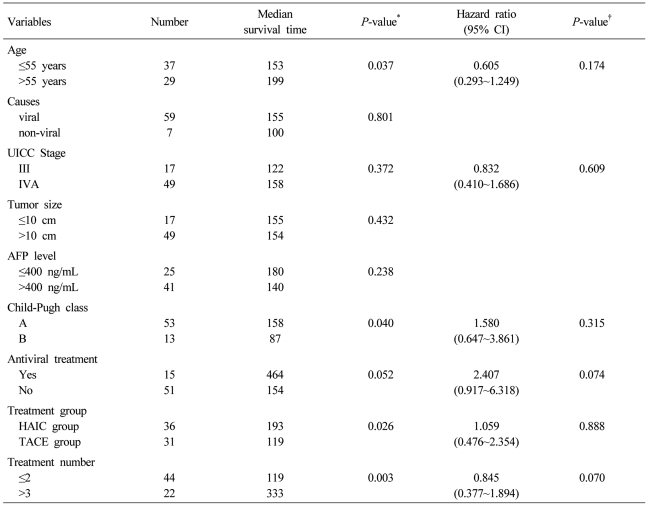
Median follow-up period was 125.5 days (range, 9~716 days). The median OS of 193 days (95% CI 133~252 days) in the HAIC group was longer than that of 119 days (95% CI 41.9~196 days) in the TACE group (P=0.026) (Fig. 1). Univariate analysis identified prognostic factors related to survival: age (P=0.037), Child-Pugh class (P=0.040), treatment group (P=0.026), and number of treatments (P=0.003). Of these, no factor was revealed as an independent factor for survival on multivariate analysis (Table 3).
Figure 1.
Cumulative survival curves of the high-dose hepatic arterial infusion chemotherapy (HAIC) group (dashed line) and the conventional transarterial chemoembolization (TACE) group (solid line). Overall survival was significantly longer in the high-dose HAIC group than in the conventional TACE group (median survival, 193 vs. 119 days; log-rank test, P=0.026).
Table 3.
Univariate and multivariate analyses of predictive factors for overall survival
*Log-rank test, †Cox proportional hazard analysis.
CI, Confidence interval; UICC, Union for International Cancer Control, AFP; alpha fetoprotein, HAIC; hepatic arterial infusion chemotherapy, TACE; transarterial chemoembolization.
Adverse events
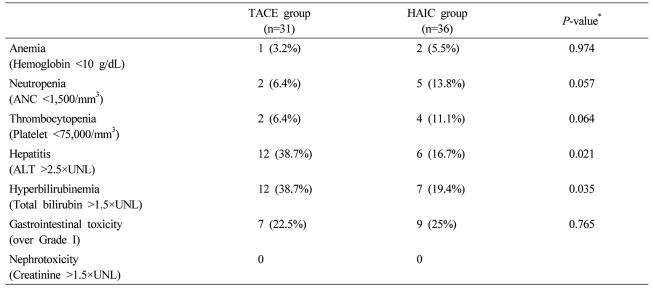
Fever, abdominal discomfort, and nausea were common. Gastrointestinal/hepatic symptoms and neutropenia/thrombocytopenia were the most serious. Neutropenia and thrombocytopenia were more common in the HAIC group, while the frequency of hepatitis and hyperbilirubinemia was significantly higher in the TACE group (Table 4). Most adverse events were controlled by medical treatment or dose modification of chemotherapeutic agents. However, four patients in the HAIC group and 18 in the TACE group had stopped further treatments due to progressive deterioration of liver function.
Table 4.
Adverse events in each treatment group
*χ2 or Fisher's exact test.
TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; ANC, absolute neutrophil count; ALT, alanine transaminase; UNL, upper normal limit.
DISCUSSION
The treatment strategies for patients with intractable, advanced HCC including main portal vein invasion, diffuse bilobar involvement, or progressive tumor refractory to surgical resection or nonsurgical intervention are very limited. When hepatic function is preserved, conventional TACE using doxorubicin is commonly used as a palliative treatment for patients with unresectable, advanced HCC.6,16 Recently, HAIC using an implantable port system has been reported to be a useful palliative treatment for patients with advanced HCC.7-12 The present study was conducted to compare the efficacy and safety of high dose HAIC, which showed favorable tumor response and survival with that of conventional TACE that is widely used palliatively. In the HAIC group, the objective response rate (CR+PR) was significantly improved compared to the TACE group (16.7% vs. 0%, P= 0.030), and median OS was also longer than in the TACE group (193 days vs. 119 days, P=0.026). Combination of two relatively high dose chemotherapeutic agents showed favorable results compared to chemotherapy with monoagent.
While earlier studies analyzed patients with HCC of size less than 10 cm and no portal vein invasion, this study included patients with advanced HCC of modified UICC stage III or IVa, larger than 10 cm, infiltrative type and/or portal vein invasion. In the TACE group, embolization was not performed in 5 out of 19 patients with stage IVa due to main portal vein invasion. Therefore, treatment response and OS of the TACE group in this study were poorer than in previous reports.6,17 In the TACE group, only single session of TACE was performed in 18 patients as a result of hepatic dysfunction as the consequence of disease progression (8 patients) or toxicity due to chemotherapeutic agents (10 patients). This might lower the effectiveness of the TACE. Moreover, the prognosis is poor in hepatitis B virus (HBV)-associated HCC.18 HBV, the major cause of HCC in this study, might contribute to the poor OS than previously reported.6,18
Concerning adverse events, neutropenia and thrombocytopenia were more likely to occur in the HAIC group, although their occurrence was not statistically significant. A possible explanation is toxicity of chemotherapeutic agents as a consequence of frequent treatment cycle (4 weeks) and more treatments in the HAIC group. Hepatitis and hyperbilirubinemia were significantly frequent in the TACE group of patients. Tumor progression in the HAIC group was less common than in the TACE group owing to a greater number of treatments, absence of embolization and good treatment response. Furthermore, high-dose chemotherapeutic agents are given over 3 days, therefore, patients might tolerate the treatment with little hepatic toxicity, making more treatments possible.
The limitation of the current study is that a randomized and prospective comparison was not made. Therefore, the disparity in terms of tumor stage, antiviral treatments, and Child-Pugh class was apparent between the two groups. However, these factors did not independently influence OS on multivariate analysis. Even if the treatment response and OS were favorable in the HAIC group on the univariate analysis, treatment group was not an independent predictive factor for OS. This might be caused by the baseline distinction between two groups. However, it is meaningful that treatment response in the HAIC group was better than the TACE group in spite of more patients with advanced stage while preserved hepatic function and antiviral treatments.
To date, there are no standard guidelines for treatment of intractable, advanced HCC, although various therapeutic approaches for these patients have been attempted. In conclusion, high dose HAIC might be effective and safe compared to conventional TACE using doxorubicin in intractable, advanced HCC, and has a potential to improve survival outcome. Further prospective, comparative randomized controlled trials are needed to confirm the beneficial effect of high dose HAIC in intractable, advanced HCC.
Acknowledgements
This study was supported by a National R & D Program grant for cancer control, Ministry of Health, Welfare and Family Affairs, Republic of Korea (R0620390-1).
Abbreviations
- AFP
alpha fetoprotein
- HAIC
hepatic arterial infusion chemotherapy
- TACE
transarterial chemoembolization
- HCC
hepatocellular carcinoma
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Levy AE, Kowdley KV. Unresectable hepatocellular carcinoma: the need for an individualized multidisciplinary approach. J Clin Gastroenterol. 2001;33:180–182. doi: 10.1097/00004836-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 4.Jang JW, Bae SH, Choi JY, Oh HJ, Kim MS, Lee SY, et al. A combination therapy with transarterial chemo-lipiodolization and systemic chemo-infusion for large extensive hepatocellular carcinoma invading portal vein in comparison with conservative management. Cancer Chemother Pharmacol. 2007;59:9–15. doi: 10.1007/s00280-006-0239-0. [DOI] [PubMed] [Google Scholar]
- 5.Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 7.Tanioka H, Tsuji A, Morita S, Horimi T, Takamatsu M, Shirasaka T, et al. Combination chemotherapy with continuous 5-fluorouracil and low-dose cisplatin infusion for advanced hepatocellular carcinoma. Anticancer Res. 2003;23:1891–1897. [PubMed] [Google Scholar]
- 8.Seno H, Ito K, Kojima K, Nakajima N, Chiba T. Efficacy of an implanted drug delivery system for advanced hepatocellular carcinoma using 5-fluorouracil, epirubicin and mitomycin C. J Gastroenterol Hepatol. 1999;14:811–816. doi: 10.1046/j.1440-1746.1999.01956.x. [DOI] [PubMed] [Google Scholar]
- 9.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 10.Murata K, Shiraki K, Kawakita T, Yamamoto N, Okano H, Nakamura M, et al. Low-dose chemotherapy of cisplatin and 5-fluorouracil or doxorubicin via implanted fusion port for unresectable hepatocellular carcinoma. Anticancer Res. 2003;23:1719–1722. [PubMed] [Google Scholar]
- 11.Park JY, Ahn SH, Yoon YJ, Kim JK, Lee HW, Lee do Y, et al. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:129–137. doi: 10.1002/cncr.22759. [DOI] [PubMed] [Google Scholar]
- 12.Atiq OT, Kemeny N, Niedzwiecki D, Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992;69:920–924. doi: 10.1002/1097-0142(19920215)69:4<920::aid-cncr2820690414>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Woo HY, Bae SH, Park JY, Han KH, Chun HJ, Choi BG, et al. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010;65:373–382. doi: 10.1007/s00280-009-1126-2. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. European Association for the Study of the Liver. Clinical management of hepatocellular carcinoma Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Common Terminology Criteria for Adverse Events, Version 3.0. National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) 2006. [Accessed 2010]. NCI web site (online), < http://ctep.info.nih.gov/protocol Development/electronic_applications/docs/ctcaev3.pdf>.
- 16.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 17.Molinari M, Kachura JR, Dixon E, Rajan DK, Hayeems EB, Asch MR, et al. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol) 2006;18:684–692. doi: 10.1016/j.clon.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors' status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233–238. doi: 10.1053/jhep.2000.9603. [DOI] [PubMed] [Google Scholar]