Abstract
Background/Aims
It has been shown that the drug-eluting beads loaded with doxorubicin (DEBDOX) are effective for the treatment of hepatocellular carcinoma (HCC). However, the optimal safety and efficacy still remain to be established by using various bead sizes, doxorubicin doses, and the degree of stasis.The aim of this study was to determine the optimal safety and efficacy of DEBDOX in the treatment of HCC.
Methods
Analysis of a 503-patient prospective, multicenter, multinational Bead Registry Database from 2007 to 2010 identified 206 patients who had been treated for HCC with DEBDOX. Primary endpoints were to compare safety, tolerance, response rates, and overall survival based on bead size (100-300, 300-500, 500-700, and 700-900 µm), number of vials, doxorubicin dose, and degree of stasis.
Results
In total, 206 patients underwent 343 treatments. The use of all four bead sizes was similar based on Child-Pugh class and Okuda stage, with a significantly higher use (50%) of beads of size 100-300 µm in patients with portal vein thrombosis (P=0.05). Significant differences were seen for the number of median treatments, median doxorubicin dose, lobar infusion), and degree of complete stasis. The rate of adverse events was higher for larger beads than for smaller beads (28% vs. 16%; P=0.02).
Conclusions
Bead size and dose may vary according to disease distribution. Smaller beads offer the opportunity for repeated treatments, a larger cumulative dose delivery, a lesser degree of complete stasis, and fewer adverse events.
Keywords: Drug eluting beads, Doxorubicin, Hepatocellular carcinoma, Chemoembolization, Adverse events
INTRODUCTION
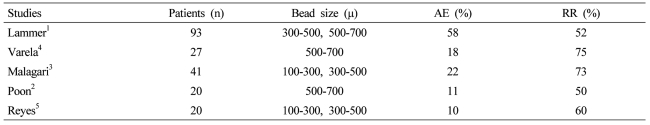
Drug-eluting beads loaded with doxorubicin (DEBDOX) have recently demonstrated to have an improved safety profile and similar efficacy when compared to conventional transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC) patients.1 Because of these initial improved outcomes there has been additional prospective reported trials demonstrating the effectiveness of DEBDOX with variable adverse event rates (Table 1).1-5 However, optimal safety and efficacy still remains unanswered through the use of various bead sizes, various doxorubicin doses delivered, and various degrees of stasis, following bead infusion with the mixed results of additional embolic agents utilized after initial DEBDOX use. Continued debate continues in regards to conventional TACE in regards to the additional use of embolic particles and the optimal dosing of chemotherapeutic agent.6-9 Recent prospective single arm and randomized phase II trials continue to demonstrate this variable use with all type of bead sizes and bead combinations being utilized with concomitant variable adverse event rates and fairly comparable response rates (Table 1).1-5
Table 1.
Prospective studies evaluating the use of drug-eluting beads loaded with doxorubicin (DEBDOX) for the treatment of hepatocellular carcinoma (HCC)
AE, adverse events; RR, response rates.
Thus, the aim of this study was to assess the optimal safety and efficacy of DEBDOX in the treatment of HCC by evaluating bead size, dose of doxorubicin and angiographic end point.
PATIENTS AND METHODS
Patients
Written informed consent was obtained from each patient included in the study and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by each institutional IRB. This prospective, multi-institutional, open, non-controlled, repeat-treatment registry of 206 patients undergoing 343 treatments for HCC was evaluated from January 2007 to February 2010. The registry satisfies the strict criteria for critical appraising of the quality of a registry study including a well described patient population, ability to generate hypotheses and answer questions, high quality data, with good quality control, independent assessment of outcomes, good clinically-relevant follow-up with minimal loss of patients to follow-ups, and comparable patient evaluation across all participating institutions.
Patients aged 18 years or older with HCC unsuitable for resection or percutaneous ablation, without portal invasion, were eligible for the evaluation. A confirmed diagnosis of HCC according to European Association for the Study of the Liver (EASL), an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and preserved liver function were satisfied. Patients were excluded if they had another primary tumor, advanced liver disease (defined as bilirubin levels >3 mg/dL, aspartate aminotransferase (AST) or alanine aminotransferase (ALT)>5×upper normal limit or >250 U/L), advanced tumoral disease (vascular invasion, or contraindications for doxorubicin administration). Limited, focal extrahepatic disease that was felt not to be life limiting was allowed in this evaluation. Standard pre-therapy evaluation of patients with HCC included at least a three-phase computed tomography (CT) of the abdomen and pelvis or a dynamic magnetic resonance imaging (MRI) depending on the institution and the availability of the technology for use.
Patients were assessed for 30 days after each treatment for any treatment related adverse experiences, and monitored for two years to assess survival. All adverse events were recorded using the standards and terminology set forth by the Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, Version 3.0. Defined grade were: Grade 1-mild adverse events, Grade 2-moderate adverse event, Grade 3-severe adverse event, Grade 4-life- threatening or disabling adverse event, and Grade 5-death related to adverse event. Follow-up assessments included a tri-phase CT scan of the liver within at least two months of completion of treatment with the evaluation of the enhancement pattern of the target lesion and tumor response rates measured according to modified RECIST criteria.
Treatment with DEBDOX and outcomes
Diagnostic angiography was performed by an interventional radiologist and consisted of selective celiac and superior mesenteric arteriogram to evaluate the hepatic arterial anatomy. For tumors near the periphery of the liver, evaluation of potential extrahepatic supply to the tumors such as the inferior phrenic, gastroepiploic and internal mammary arteries was performed. Once the degree of hepatic tumor perfusion was evaluated, the next step was to limit any type of extrahepatic perfusion of the chemotherapeutic treatment. The most common branches that will lead to extrahepatic disposition of treatment are the right gastric and the gastroduodenal arteries, which are either controlled prior to infusion using coil embolization, or distal catheter placement. In addition, particular attention is paid to identification of the cystic artery to ensure that the catheter tip is past this point. This avoids extrahepatic infusion of embolic material into the gallbladder and maximizes therapy control.
The bead size used for each patient was up to the treating interventional radiologist, since at the time of this study no optimal bead size had been described. Similarly there are also, similar elution characteristics in all bead sizes, and thus the type of bead was not standardized.
Patients originally followed a plan of either two or three treatment cycles based on the extent of liver involvement with a repeat CT scan every three months from the initial first treatment cycle to evaluate response as well as planned re-treatment. For patients with bilobar disease, a planned minimum of four treatments (100-150 mg each treatment, depending on the extent of tumor burden and the extent of hepatic parenchymal reserve) were loaded into two bead vials of similar size to those described above. The plan included at least two treatments per lobe every three to four weeks depending on toxicity, also as above. These patients also had a planned repeat CT scan three months from the first dose to evaluate tumor response. For example, if patients present with bilobar disease, they would receive a first bead treatment to the right lobe, then three weeks after a second bead treatment to left lobe, then three weeks later a third bead treatment to right lobe, and then again three weeks later to left lobe.
Image guided precision chemoembolization, peri-procedural medications including pain medications, antibiotic prophylaxis and corticosteroids and proton pump inhibitors were all performed at the physician's discretion.
The primary end point for this study was 12 months response rate and overall survival in the management of HCC.
All bead therapies were performed with the DC/LC™ bead microsphere (Drug Eluting Bead [DEB]; www.biocompatibles.com, Biocompatibles UK, Surrey, UK). The saline suspension in the DC/LC™ bead microsphere was removed and the beads were mixed with doxorubicin solution at a dose of 75 mg per 2 mL at least 4 h before the procedure depending on the dose that was planned to be delivered. The mixing of DEBDOX was performed with non-ionic contrast (approximately 50/50 dilution) prior to injection. Minimum recommended volume of loaded bead to contrast mixture is approximately 10.0 mL to ensure smooth catheter delivery. After appropriate mixing and removal of the un-eluted supernatant, a microcatheter is then placed intra-arterially. Placement is again based on the extent of liver disease, as described above. Any of the four size beads could be used for these infusions, according to physician evaluation. DEBDOX is injected slowly to avoid reflux of embolic material. Additional embolic material is not usually recommended after appropriate bead infusion, but was up to the physician's discretion. A Lobar infusion was defined as catheter placement at the time of bead infusion just pass the hepatic artery bifurcation, or if because of a late cystic arterial take off, then a posterior sectoral infusion followed by an anterio sector infusion such that a whole lobe of the liver was treated at one session. A segmental infusion was defined as at least two segments of the liver treated with beads and a sub-segmental infusion was defined as a single segment treated. The degree of stasis was defined as none - no change in hepatic arterial blood from before bead infusion, partial - defined as loss of peripheral tumor vessels, near - near complete loss of intra-tumoral vascularity, and complete - no evidence of hepatic arterial flow to tumor and segmental liver.
Data was censored at the last recorded patient contact if an endpoint was not reached. Recurrence was also evaluated using positron emission tomography (PET) scan. A recurrence was the re-occurrence of viable tumor by radiologic CT criteria of a vascular mass. In the event of subsequent hepatic therapy for recurrence of disease only the first procedure was used for the purposes of this study.
Statistical analysis
Chi-square, Student's t-test, and Mann-Whitney's U-test for nominal, continuous, and ordinal variables were used to evaluate the association of independent variables to complications. Proportional hazards analysis was performed on all variables found significant by univariate analysis. Relative risk (RR) with 95% confidence intervals was calculated as a measure of association. Patients with diffuse disease (>10 lesions) were compared to patients without diffuse disease. Differences of P<0.05 were considered significant. Statistical analysis was performed using JMP software (JMP, SAS Institute Inc., Cary, NC, USA).
RESULTS
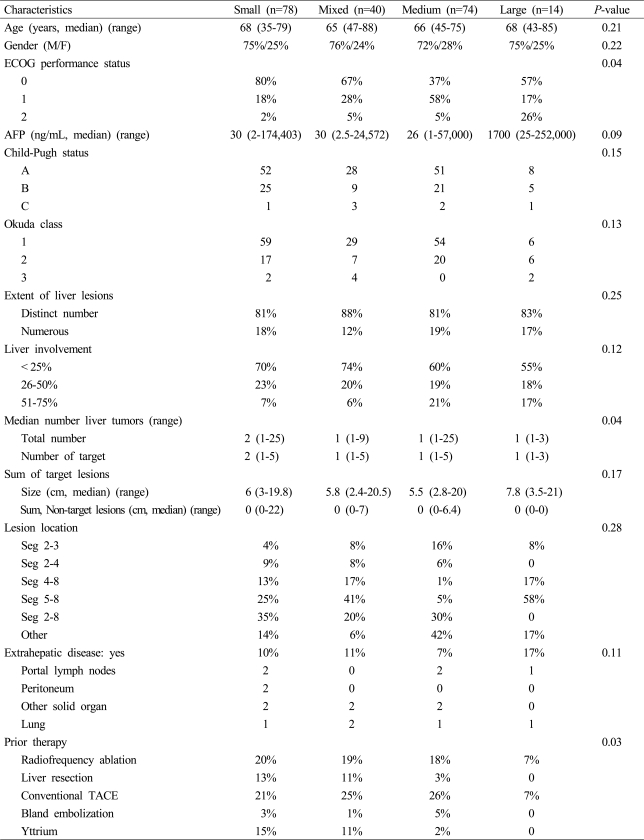
A total of 206 patients were included in this review with a majority of patients being treated either with a small bead combination (100-300 µ beads alone) or a medium bead combination (one vial 300-500 µ and one vial 500-700 µ) followed by a mixed bead combination (one vial of 100-300 µ and one vial of 300-500 µ) with the smallest number being patients treated with large beads alone (500-700 µ only). All four groups above were similar in age as well as male-tofemale distribution as demonstrated in Table 2. A majority of small and mixed bead treated patients had a better overall performance status with 80% of the small bead and 67% of the mixed bead patients having ECOG of 0 (P=0.04). The alphfetoprotein levels for all four groups were similar median values with also similar range from as low as 2 to as high as 252,000 ng/mL. The percentage of patients in both early and late stage Child's-Pugh status as well as early and late stage Okuda class was also similar across all four groups. The extent of disease was also similar in all four groups with a greater percentage of patients having less than 25% liver involvement in the small and mixed bead treated patients (P=0.12). Patients treated with small beads were more likely to have a median number of tumors of two with the remaining three groups having a median number of one (P=0.04). The sum of target lesions as well as the sum of all non-target lesions was also similar across all four groups. Small percentages of all four groups did have patients with extra-hepatic disease including portal lymph nodes, peritoneal metastasis, other solid organ metastasis or a lung metastasis. Interestingly, a majority of patient treated with small beads had been treated either with hepatic resection, radiofrequency ablation, prior conventional TACE and even Yttrium-90 prior to their exposure with small beads with DEBDOX (P=0.03).
Table 2.
Clinical characteristics of HCC patients treated with DEBDOX according to bead size used
DEBDOX, drug-eluting beads loaded with doxorubicin; HCC, hepatocellular carcinoma; ECOG, Eastern Cooperative Oncology Group; AFP, alphafetoprotein; TACE, transarterial chemoembolization.
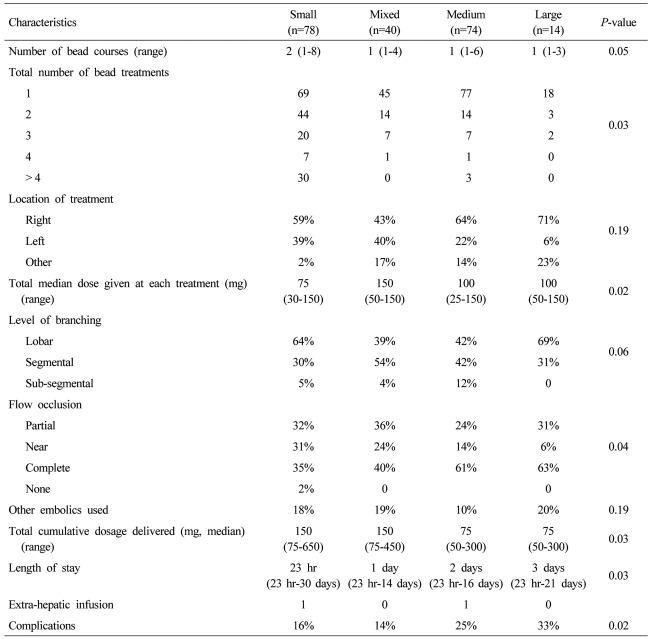
Of the 206 patients, a total of 343 DEBDOX treatments were performed. Table 3 showed the DEBDOX treatments and outcomes of the patients. Patients treated with small beads were more likely to have more number of bead treatments with a median of two and a range of one to eight with the remaining three groups having a median of one bead treatment and a range of one to four in a majority of all of the remaining three groups (P=0.05). The total dose given at each perspective bead treatment was similar across all three groups with a slightly higher dose in the medium and large bead treated patients. A majority of all patients were treated either in a lobar or segmental approach with a small percentage treated in a sub-segmental-type of approach. Patients who were treated with medium and large bead combinations had a statistically significant greater incidence of complete stasis following their bead treatment (P=0.04). There was as similar use across all four groups of additional embolic, that being either unloaded bead or other types of particles utilized following initial bead treatment in order to achieve a greater degree of stasis. The total cumulative dose given to the entire liver of a patient was significantly higher in the small and mixed bead groups with a median dose of 150 mg respectively and a higher range of dose delivered with as high as 650 mg total in the small bead patient and 450 mg total in the mixed bead patient (P=0.03). The length of stay was also statistically significantly higher in the medium and large bead patients with them having a median length of stay of two to three days in comparison to the small bead patient of 23 hours (P=0.03). The degree of adverse events was also statistically significantly higher in the large and medium group of patients with 33% and 25%, respectively (P=0.02)
Table 3.
DEBDOX treatment and outcomes in HCC patients
DEBDOX, drug-eluting beads loaded with doxorubicin; HCC, hepatocellular carcinoma.
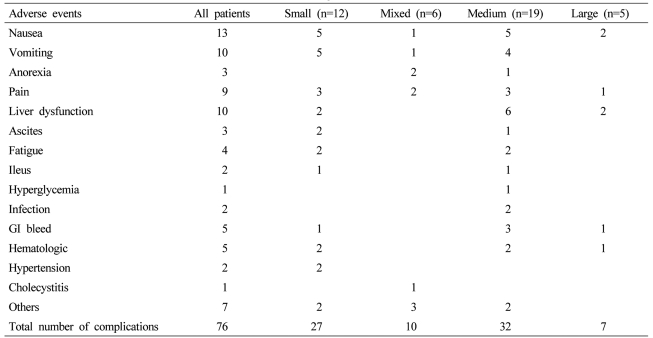
The types and severity of complications were fairly similar across all four groups (Table 4), however, there was a larger number of patients in the medium group who developed liver dysfunction and gastrointestinal bleeding that did lead to post-bead death in the 30-day post-treatment follow-up (P=0.04). The overall procedure-related mortality in the 205 patients treated was 6.8% with a majority of all of these mortalities occurring in patients who were treated with either a medium or large bead size (Table 5).
Table 4.
Adverse events related to DEBDOX treatment according to bead size used
DEBDOX, drug-eluting beads loaded with doxorubicin.
Table 5.
Grade of adverse events related to DEBDOX treatment according to bead size used
DEBDOX, drug-eluting beads loaded with doxorubicin.
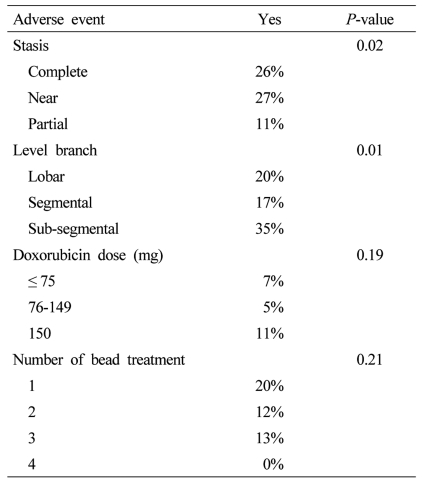
In a review of other predictive factors for adverse event rates, there was a statistically significant increase in overall adverse event rates in patients who underwent the angiographic endpoint of either a complete stasis or a near stasis with nearly double adverse event rates in patients who had partial stasis (Table 6). Similarly there was also a statistically significant increase in adverse advent rates in patients who were treated in a sub-segmental DEBDOX infusion in relation to patients who received only lobar or segmental infusion, however, there were no significant differences in adverse event rates related to the doxorubicin dose delivered with similar adverse event rates in patients who received less than equal to 75 mg (7% adverse event rate), 76-150 mg (5% adverse event rate) or 150 mg (11% adverse event rates). Similarly, regardless if a patient was undergoing their first bead treatment or their, fourth bead treatment, there was also none statistically significant difference in overall adverse event rates, regardless of the number of bead treatments that the patient was undergoing.
Table 6.
Factors associated with the incidence of adverse events
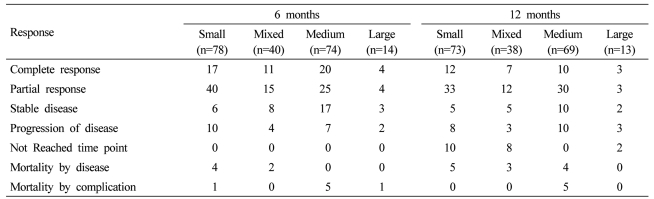
In a review of 6 month and 12 months response rates there were similar response rates in all four groups (Table 7), with small bead demonstrating 6 months of 73% and 12 month of 61%, Mixed was 88% and 50%, Medium was 60% and 57%, and Large was 57% and 46% respectively. After a median follow up of 12 months, similar overall survival was seen in all groups with small bead median of 16 months, mixed 13 months, medium 14 months, and large 11 months (P=0.2).
Table 7.
Response rates for all of the evaluated patients
DISCUSSION
DEBDOX has been proven in multiple studies to demonstrate enhanced anti-tumor effects with similar or increased survival and decrease in overall side effects related to other hepatic arterial therapies. Response rates in all series have ranged from as low as 50% at 6 months, to as high as 70% at 6 months based on various reports. Varela et al10 reported response rates of 60-70% and similarly reported only minor overall adverse event rate of 18%, even with the use of a single vial of 500-700 µ beads but did report 2 patients with liver abscess (7.4%) and 1 death (3.7%). The degree of stasis and degree of doxorubicin dose delivered for this specific patient is not revealed in this manuscript and thus the true source for this adverse event rate is not able to be delineated.
A similar smaller Phase II study by Poon et al11 also used the same bead size and did demonstrate an a small adverse event rate of 11% and did not report any liver abscess or death, thus demonstrating the potential safety of beads up to 500-700 µ in size.
However, in contrast, the recent randomized phase II Precision V Trial, which utilized a combination bead size of 300-500 µ followed by 500-700 µ beads showed a dramatic increase of overall adverse event rate of 58% with this combination bead size.12 However, other predictive factors of adverse events being type of segmental infusion as well as degree of stasis were not evaluated in this randomized phase II trial, thus delineating if this is related to the bead size alone or in combination, the bead size, the angiographic technique and the end point is not able to be truly delineated in this reported trial.
In an evaluation of a smaller bead size combination by Malagari et al13 who utilized a 100-300 µ and then 300-500 µ bead size, adverse event rates were dramatically less with an overall of 22%. The explanation for the use of 100-300 µ and 300-500 µ are specifically explained in this trial and are related to lesion size (<6 cm or ≥6 cm or the presence or absence of arteriovenous shunts). Given that this was an overall survival comparison of DEBDOX to bland embolization, the evaluation of the bead size, degree of stasis, and degree of doxorubicin and drug delivered, was not a primary end point and thus was not reported in this manuscript. They did, however, report a liver abscess rate of 4.8% and a liver failure rate, also of 4.8%, similar to the previously reported trials.
All of the previous studies present an overall adverse event rates, but do not answer what is the optimal bead size, optimal doxorubicin dose and angiographic techniques. Herein the results of this prospective multi institutional registry does demonstrate a statistically significant increase in overall adverse event rates as well as severe adverse event rates, being death in patients who are receiving a larger bead combination of either 300-500 µ, 500-700 µ, or 500-700 µ alone. The predominant predictors of this adverse event rate do appear to be generated because of a higher incidence of complete stasis as well as a preponderance for a segmental or sub-segmental bead infusion. Lastly, this group of larger bead size was also more commonly being treated with additional embolic agents following DEBDOX treatment, thus potentiating the overall anoxic event and leading to a greater incidence of complications relative to hepatic ischemia. In contrast, even though there was a greater incidence of either complete or near stasis we do not see an enhanced response rate at either 6 or 12 months in patients treated with a larger bead size. Thus, calling into the question as to whether efficacy is improved by either relative hypoxia and repeated at doxorubicin drug delivery versus complete anoxia at the time of initial chemoembolization therapy.14-16 This data does not allow us to answer that question but does demonstrate significant better tolerance and improvement in overall survival in patients who were able to undergo repeated DEBDOX based treatment. This improvement in overall survival appears to be related to a greater tolerance of the patient and the subsequent tolerance of the diseased liver to then undergo repeated bead treatments because of a reduction in overall adverse event rates. Given the complexity of treating HCC is a bi-modal type of disease, that being treatment of both the cancer as well as the underlying hepatic parenchyma, it is reasonable to assume that a greater tolerance of treatment that is able to be able to retreat at set intervals would portend to an improvement in overall outcomes because of a reduction in toxicity. This logic is well established in the medical oncology literature in the fact that patients who have significant toxicity and are reliant on significant dose reduction of systemic chemotherapy or dose delays of systemic chemotherapy do not achieve the similar benefits of patients who are able to be treated at full dose and on schedule. It is thus acceptable to make that correlation with the use of DEBDOX based treatment in that patients who are unable to undergo repeated treatments at appropriate intervals with reduced toxicity will thus have an improved overall outcome versus patients who are only able to be undertreated with significant toxicity.
These results are confirmed by previous reports evaluating conventional TACE in which Leung et al17 found that gallbladder embolization and dose administered were associated with an increased risk of post embolic syndrome and an extended hospitalization, with odds ratios of 2.8 and 3.0, and 3.0 and 4.6, respectively. Previous embolization was associated with a decreased risk of both post embolic syndrome and extended hospitalization, with odds ratios of 0.5 and 0.4, respectively. They concluded that clinically relevant predictors of the severity of post embolic syndrome and length of post procedural hospitalization may exist through avoiding embolization of the gallbladder, and that re-embolization of previously treated vessels is associated with decreased toxicity and may assist in selecting patients for treatment on an outpatient basis. However, in a review of Patel et al, no definitive predictors were found.18
The limitations of this study are also, potentially, the benefits in that this evaluation was not formed on an established prospective protocol. However, the data present is real clinical practice in patients who are initiating DEBDOX based treatment in their interventional oncology practices. We believe that this, obviously, demonstrates a more clinically relevant and more clinically practical use of this device and believe that this type of prospective evaluation and review will allow additional new users and established DEBDOX users at the present to consider technical changes. However, we acknowledge that since this is not a randomized trial of all four bead sizes there is inherent bias that can be conveyed. We believe the similar groups based on disease presentation overcome some of this bias, but a randomized trial would be the optimal study to confirm these observational results.
Thus, in conclusion, based on this review of optimal bead size, optimal doxorubicin dosing, as well as angiographic technique, the smallest bead size being 100-300 µ offers the ability of repeat retreatment at appropriate intervals. This then allows for a larger cumulative dose delivery, less degree of complete stasis, fewer adverse event rates, and reduction in the severity of adverse event rates.
Abbreviations
- AE
adverse events
- RR
response rates
- DEBDOX
drug-eluting beads loaded with doxorubicin
- HCC
hepatocellular carcinoma
- ECOG
Eastern Cooperative Oncology Group
- AFP
alphafetoprotein
- TACE
transarterial chemoembolization
Footnotes
Dr. Martin is a consultant for Biocompatibles.
References
- 1.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541–551. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 4.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Reyes DK, Vossen JA, Kamel IR, Azad NS, Wahlin TA, Torbenson MS, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J. 2009;15:526–532. doi: 10.1097/PPO.0b013e3181c5214b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maluccio M, Covey AM, Gandhi R, Gonen M, Getrajdman GI, Brody LA, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol. 2005;16:955–961. doi: 10.1097/01.RVI.0000161377.33557.20. [DOI] [PubMed] [Google Scholar]
- 7.Maluccio MA, Covey AM, Porat LB, Schubert J, Brody LA, Sofocleous CT, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19:862–869. doi: 10.1016/j.jvir.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Brown KT, Nevins AB, Getrajdman GI, Brody LA, Kurtz RC, Fong Y, et al. Particle embolization for hepatocellular carcinoma. J Vasc Interv Radiol. 1998;9:822–828. doi: 10.1016/s1051-0443(98)70398-7. [DOI] [PubMed] [Google Scholar]
- 9.Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, et al. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma-a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol. 1992;31(Suppl):S1–S6. doi: 10.1007/BF00687096. [DOI] [PubMed] [Google Scholar]
- 10.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541–551. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 14.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 15.Mamori S, Asakura T, Ohkawa K, Tajiri H. Survivin expression in early hepatocellular carcinoma and post-treatment with anti-cancer drug under hypoxic culture condition. World J Gastroenterol. 2007;13:5306–5311. doi: 10.3748/wjg.v13.i40.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 17.Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 18.Patel NH, Hahn D, Rapp S, Bergan K, Coldwell DM. Hepatic artery embolization: factors predisposing to postembolization pain and nausea. J Vasc Interv Radiol. 2000;11:453–460. doi: 10.1016/s1051-0443(07)61377-3. [DOI] [PubMed] [Google Scholar]