Abstract
Background/Aims
The Barcelona Clinic Liver Cancer (BCLC) staging system is logical for the staging and treatment of hepatocellular carcinoma (HCC) because it was based on survival data. This study evaluated the applicability of the BCLC staging system and reasons for divergence from BCLC-recommended treatments in Korean HCC patients.
Methods
One hundred and sixty consecutive HCC patients were prospectively enrolled. Treatments were generally recommended according to the guideline of the American Association for the Study of Liver Diseases, but patients were also informed about alternative treatments. The final decision was made with patient agreement, and was based on the doctor's preferences when a patient was unable to reach a decision.
Results
There were 2 (1%), 101 (64%), 20 (12.5%), 34 (21.5%), and 3 (1%) patients with very early-, early-, intermediate-, advanced-, and terminal-stage disease, respectively. Only 64 patients (40%) were treated according to BCLC recommendations. The treatment deviated from BCLC recommendations in 68% (69/101) and 79% (27/34) of patients with early and advanced stage, respectively. The main causes of deviation were refusal to undergo surgery, the presence of an indeterminate malignancy nodule, the absence of a suitable donor, or financial problems.
Conclusions
Donor shortage, financial problems, the relatively limited efficacy of molecular targeting agents, and the presence of an indeterminate nodule were the main causes of deviation from BCLC recommendations. Even after excluding cases in which decisions were made by patient preference, only 66% of the HCC patients were treated according to BCLC recommendations. Treatment guidelines that reflect the Korean situation are mandatory for HCC patients.
Keywords: Hepatocellular carcinoma, Barcelona Clinic Liver Cancer (BCLC), Neoplasm staging, Treatment, Korea
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common malignant tumor and the second leading cause of cancer death in Korea. Recently, the principles of HCC therapy have changed rapidly due to the increased detection of early HCC and the therapeutic modality advancements made, and as a result, various staging systems and treatment options are currently used. HCC is unique because in addition to cancer stage underlying liver function substantially affects prognosis. Furthermore, no global consensus has been reached regarding the application of the several HCC staging systems, which include the Tumor Node Metastasis (TNM) staging system,1 the Okuda staging system,2 the Barcelona Clinic Liver Cancer (BCLC) staging system,3,4 the Cancer of Liver Italian Program (CLIP) staging system,5 Group d'Etude et de Traitement du Carcinome Hepatocellulaire (GRETCH) scoring system,6 the Japanese Integrated Staging (JIS) score,7 and Tokyo scoring system.8
The BCLC staging system was proposed and has been validated by several groups in the United States and Europe to offer the best means of stage classification and treatment guidance for HCC. Accordingly, the BCLC staging system is viewed as being necessary for clinical trials and the reporting of clinical data. However, although this staging system was developed using an evidence-based approach, its application can be problematic because of differences between countries in terms of available medical resources, the lack of a broad consensus on its cost-effectiveness, the priority of surgical treatment, or because the tumor stage can be indeterminate as the BCLC system is based on clinical and radiological staging.
Therefore, the aims of this prospective study were to evaluate whether the BCLC staging system and its treatment guidelines are applicable in the majority of Korean HCC patients, and to identify causes of discrepancies.
MATERIALS AND METHODS
Patients
Between December 2007 and December 2008, 244 patients with at least one hepatic mass who were admitted to one hepatologist at the Asan Medical Center were prospectively enrolled. HCC was diagnosed using the noninvasive diagnostic criteria of the American Association for the Study of Liver Diseases (AASLD) proposed in 2005 or by histopathological examination. Of these 244 patients, 160 patients were finally diagnosed to have HCC, and these patients constituted the study cohort.
Methods
The determination of BCLC stage
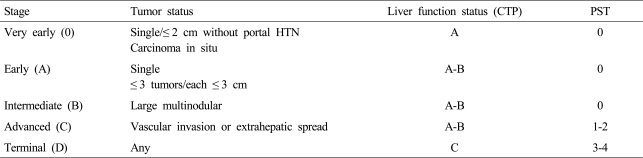
To determine BCLC stages, we investigated tumor size and number, liver function test, prothrombin time, performance status, and cancer-related symptoms. Portal hypertension was defined by the presence of esophagogastric varices by esophagogastroduodenal endoscopy, ascites, or splenomegaly and a platelet count <100,000/mm3. All patients underwent four-phase dynamic computed tomography (CT), chest CT, and a bone scan for staging. In cases with another intrahepatic nodule not typical of HCC by dynamic CT, evaluations were made by dynamic magnetic resonance imaging (MRI) or biopsy, if the additional nodule was deemed to be capable of affecting the choice of treatment modality. In cases with an extrahepatic lesion larger than 1 cm, evaluations were conducted by positron emission tomography (PET)-CT or biopsy, and when biopsy, dynamic MRI, or PET-CT failed to show HCC or typical findings of HCC (arterial enhancement and portal/delayed washout by dynamic MRI, or hot uptake by PET-CT), nodules were classified 'indeterminate' and followed. Initial tumor staging was performed based on the assumption that these nodules were not HCC. According to the status of HCC at initial diagnosis, HCC patients were classified as 0 (very early), A (early), B (intermediate), C (advanced), or D (terminal) using BCLC staging system (Table 1). Final HCC stages were determined after a follow-up examination. If the indeterminate nodules grew or then showed typical findings of HCC, they were presumed to be HCC and tumor stages were subsequently modified.
Table 1.
The BCLC staging classification for hepatocellular carcinoma
Determination of treatment options
After determining BCLC stage, the hepatologist in charge, a surgeon, and radiologists discussed whether the treatment option recommended by the BCLC staging system was technically feasible. When the recommended treatment could not be applied because of technical problems or the presence of comorbidity, the cases concerned were regarded to represent deviations from the recommended BCLC treatment. Patients were then informed of the standard treatment option as recommended by the BCLC, and of its advantages and disadvantages, including treatment outcomes, treatment-related morbidities, and cost. The patients were also informed of the details of alternative treatments. More specifically, we explained the details of: surgical resection and radiofrequency ablation (RFA) for stage 0; surgical resection, liver transplantation (LT), and RFA for stage A; transcatheter arterial chemoembolization (TACE) for stage B; and of sorafenib or some other targeing agent, systemic chemotherapy, TACE, TACE plus radiation therapy (RT) for stage C. Patients who had a single HCC in BCLC A were recommended surgical resection if they were non-cirrhotic or had cirrhosis but had well preserved liver function, normal bilirubin and hepatic vein pressure gradient <10 mmHg. If patients with HCC corresponding to the Milan criteria showed increased portal pressure or bilirubin, they were recommended LT. However, if patients with a single HCC or up to three nodules lesser than 3 cm in diameter had portal hypertension or increased bilirubin, they were recommended RFA. Child-Turcotte-Pugh (CTP) B patients in BCLC stage C were informed of sorafenib, but it was not recommended as a standard therapy because previous studies included a small number of patients in CTP B class.9,10 Patients with an indeterminate nodule, were informed of the possibility that the nodule could be HCC. Treatment was recommended based on the assumption that these lesions were benign. Nevertheless, patients were also informed of the advantages and disadvantages of each treatment option with respect to potential nodule benignity or malignancy. Final treatment decisions were principally made by patients. However, decisions were made by the doctor in charge if a patient could not decide or hesitated to accept the recommended treatment, which usually occurred when there was a financial problem. For patients with 1st or 2nd portal vein (PV) branch invasion with a liver function of CTP A and a tumor size of less than half total liver volume that refused or hesitated to use sorafenib or another molecular targeting agent under clinical trial, TACE with RT was recommended based on the treatment outcomes of our retrospective studies.11,12
We investigated treatment deviations from BCLC recommendations and analyzed the causes of deviations. This study was approved by the Investigation and Ethics Committee for Human Research at Asan Medical Center.
RESULTS
Patient characteristics
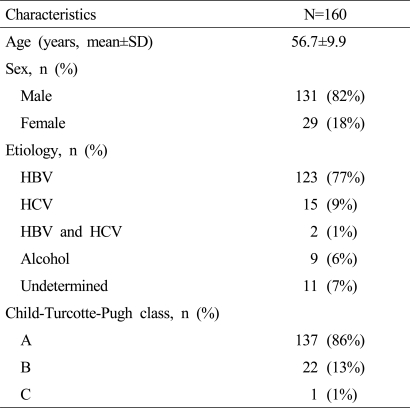
The baseline characteristics of the 160 patients are shown in Table 2. Of these, 131 (82%) were men of mean age 56.7±9.9 years. The etiologies of underlying liver disease were; hepatitis B virus (77%), hepatitis C virus (9%), co-infection with hepatitis B and C virus (1%), and alcohol (6%). The numbers of patients in CTP classes A, B, and C were 137 (86%), 22 (13%), and 1 (1%), respectively.
Table 2.
Baseline characteristics of the study population
HBV, hepatitis B virus; HCV, hepatitis C virus.
Classification of BCLC stage
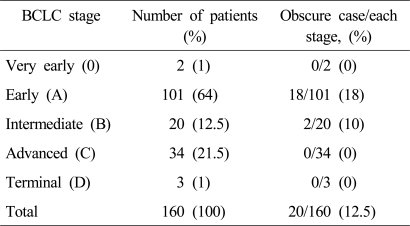
Two (1%), 101 (64%), 20 (12.5%), 34 (21.5%) and 3 patients (2%) were classified initially to the very early (0), early (A), intermediate (B), advanced (C), and terminal (D) BCLC stages, respectively.
Twenty patients (12.5%) had at least one indeterminate lesion at initial work-up: 18 patients (18%) of BCLC stage A and 2 patients (10%) of stage B. No patient of BCLC 0, BCLC C, or BCLC D stages had an indeterminate lesion (Table 3).
Table 3.
BCLC staging of the study population
Reasons for an obscure stage: the presence of an indeterminate intrahepatic nodule in 14 (70%) and of an extrahepatic nodule in 6 (30%).
The indeterminate lesions of the 18 patients of BCLC A were 12 dysplastic nodules in the liver, 2 arterio-portal (AP) shunts, 2 indeterminate nodules in lung, and 2 lymph node enlargements of less than 2 cm. Of these 18 patients, 4 patients with an extrahepatic lesions were candidates for LT. However, all refused to undergo LT fearing extrahepatic HCC recurrence after LT. In the remaining 14 patients, treatment was recommended on the assumption that the indeterminate nodules were benign lesions, but 9 patients refused surgical resection. In a follow-up test, one suspected AP shunt turned out to be HCC. However, 17 (94%) of the indeterminate lesions in BCLC A were finally diagnosed as benign. Of the two patients of BCLC B stage with an indeterminate lesion, one had a lesion in the lung, and in the other had a suspected lesion in the right hepatic vein. The nodule in the lung proved to be a metastatic lesion during follow-up, whereas the lesion in the right hepatic vein was finally revealed to be benign.
Treatment in practice according to BCLC stage
All patients with BCLC 0 (2/2), B (20/20), or D (3/3) followed the treatment recommendations of the BCLC staging system without deviation. However, 68% patients (69/101) with BCLC A and 79% patients (27/34) with BCLC C did not receive BCLC recommendations.
Of the 101 with BCLC A, surgical resection was indicated in 55, LT in 34, and RFA in 11, according to BCLC recommendations.
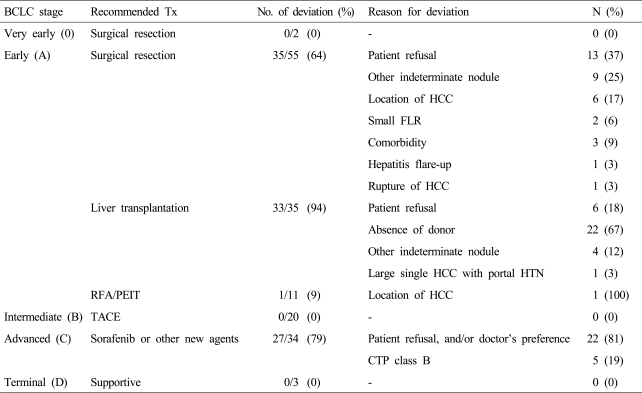
Thirty-five (64%) of 55 patients in whom surgical resection was indicated received treatments other than surgical resection, namely, RFA (25/35) and TACE (10/35). The causes of these deviations were patient refusal (37%), the presence of an intrahepatic indeterminate nodule (25%), and a difficult location for surgical resection (17%). Other reasons were insufficient remnant liver volume (6%) and a high surgical risk due to comorbidity (9%) (Table 4).
Table 4.
Reasons for divergence from BCLC recommendations
CTP, Child-Turcotte-Pugh; FLR, future liver remnant volume; HCC, hepatocellular carcinoma; HTN, hypertension; PEIT, percutaneous ethanol injection therapy; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization; Tx, treatment.
Of 33 (94%) of 35 patients in whom LT was indicated 23 underwent RFA and 10 underwent TACE. The causes of these deviations were patient refusal due to a financial problem (18%), the absence of a donor (67%), the presence of an extra-hepatic indeterminate nodule (12%), and the size of HCC over than Milan criteria (3%). One patient (9%) of 11 in whom RFA was indicated underwent TACE due to the tumor location.
Of the 34 patients with BCLC C, 7 (21%) were treated with sorafenib or another molecular targeting agent under clinical trials. However, the majority (27/34, 79%) including 5 patients in CTP B were treated with an alternative treatment option because they refused or hesitated the use of sorafenib (Table 4). The reasons for refusal were high cost and a lower expected survival benefit than expectation. These 27 patients were treated with TACE and/or RT (23/27), systemic chemotherapy (2/27), or supportive therapy (2/27).
DISCUSSION
Unlike other solid tumors, the staging of HCC must reflect underlying liver function and the degree of tumor progression. In this regard, the BCLC staging system has the advantages that its recommended treatment options are based on HCC stage and that it can predict survival. Many studies in Western countries have validated the usefulness of this staging system.13,14 However, it has not been validated in Asia where available medical resources, cultural backgrounds and socio-economic status are quite different.
In this prospective study, we undertook to determine the proportion of HCC patients that followed BCLC treatment guidelines.
Several factors affect the choice of treatment options. First, since the BCLC staging system is based on clinical and radiological findings, and thus, it is not possible to determine exact stage in some patients. This is especially problematic for the BCLC staging system because its recommended treatment options are dependent on tumor stage. Furthermore, in the present study, 12.5% of HCC patients had an indeterminate intra- or extra-hepatic lesion and most of these lesions were found in early stage patients. Although 90% of these lesions ultimately turned out to be benign, the presence of such lesions still can affect the choice of invasive treatment options, such as LT or surgical resection, since a lack of arterial enhancement on dynamic imaging studies or hot PET-CT uptake does not necessarily exclude the possibility of HCC. However, no recommendations have been issued regarding the treatment or the follow up of patients with indeterminate lesions.
Second, LT is not a practically available treatment option in Korea, especially in early stage patients in CTP A or B with evidence of portal hypertension. In fact, only two patients of 34 potential candidates for LT underwent LT. In Korea, the donation rate is very low to accommodate LT candidates. Although 3,501 candidates for LT have been registered at the Korean Network for Organ Sharing (KONOS) at the end of 2009, only 233 cadaveric donor LTs were performed in 2008.
Considering that cases with an urgent transplantation status (KONOS status 1 and 2a) accounted for 50% in cadaveric donor LTs,15 the likelihood of cadaveric donor LT is very low in HCC patients.16 Furthermore, HCC patients cannot have priority for cadaveric donor LT due to a relatively good liver function, according to the KONOS guidelines. Of course, living donor LT is actively pursued and accounts for about 80% of all LTs performed in Korea. Nevertheless, candidates for LT annually accumulated to between 400 and 500. A donor shortage problem also exists in the West, but the number of cadaveric donors per million of the population ranges between 10 and 35, whereas in Asia it is usually less than five. In Korea, the donation rate is extremely low at fewer than two donors per million.17 The lack of living-related liver donors and financial problems are also important reasons why LT is not performed. Another important reason is that patients, especially those with CTP A, usually do not want family members to undergo major hepatectomy. Therefore, the ideal candidates for surgical resection or RFA in the BCLC staging system should be modified in Asian countries where LT cannot be widely used.
Third, in addition to overall survival, invasiveness and treatment related morbidities are important factors that influence treatment choice. The AASLD guidelines say that ablation as a first-line treatment option is controversial, and that local ablation is recommended for patients that cannot undergo resection or that it used as a bridge to transplantation. However, in our series, the most common reason for deviation from surgical resection was patient refusal (37%) due to a fear of surgery. The presence of an indeterminate nodule also significantly influenced patient preferences regarding relatively non-invasive treatment options (25%). Collectively, only 32% of our early stage patients (32/100) chose the BCLC recommended treatment. The strict indications of the BCLC for a specific curative treatment option cannot be applied to Korean patients with BCLC A, and therefore, recommendation of possible curative treatment options such as surgical resection, RFA or LT for patients with BCLC stage A is a more practical option in Korea.
Another major discrepancy from recommended treatment occurred in patients with BCLC C. Although sorafenib has proven benefits in BCLC C,9,10 most patients (81%) did not agree to the use of sorafenib. Some patients left the choice to the hepatologist in charge, but did not agree to the use of sorafenib usually because of a financial problem or a lower survival benefit than expected. Most of these patients were treated by TACE with/without RT based on our previous findings11 and a guideline issued by the Korean Liver Cancer Study Group. Although no prospective randomized trial has been conducted, several retrospective analyses have reported a median survival of over 18 months in Korean patients with 1st or 2nd branch PV invasion treated by superselective TACE or TACE plus RT.11,12,18,19 Therefore, it is debatable issue among Korean physicians as to whether these patients should be treated with sorafenib initially despite a lack of evidence with other treatment options. It is also controversial as to whether sorafenib should be recommended as a first-line standard treatment in CTP B patients since previous randomized trials included only small numbers of CTP B class patients.9,10 However, other treatment options are not usually feasible for these patients.
In the present study, the patient with a single HCC larger than 5 cm in diameter and evidence of portal hypertension was suggested LT then it would have been contrary to the Milan criteria. Even if the University of California at San Francisco criteria had been applied, LT would not have been recommended because the HCC was greater than 6.5 cm. Similarly, no recommendation is made by the BCLC staging system regarding the classification and treatment of HCC patients with a ruptured tumor or major bile duct invasion. Accordingly, the BCLC staging system cannot be applied to all HCC patients in Korea where the prevalence of HCC is high and many high-risk patients are not received surveillance program.
Summarizing, although the BCLC staging system has been validated in the West, provides recommended treatment options according to HCC stage, and can predict survival, it is difficult to apply to HCC patients in Korea. Even after excluding treatment divergences due to patient preferences, only 66% of patients were treated in accord with the BCLC guidelines in our cohort. The main reasons for these discrepancies were an extreme shortage of liver donors and the financial problems posed by LT, a preference for a less invasive treatment, high cost, and the low anticipated survival benefit of sorafenib. Furthermore, we recommend that future studies be conducted to determine recommended treatment options in patients with an indeterminate intra- or extra-hepatic lesion and in the few patients in whom stage cannot be determined by the BCLC staging system. Accordingly, treatment guidelines are required for HCC patients that accurately reflect the particular situations in Korea.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- BCLC
Barcelona Clinic Liver Cancer
- CLIP
Cancer of Liver Italian Program
- CT
computed tomography
- CTP
Child-Turcotte-Pugh
- GRETCH
Group d'Etude et de Traitement du Carcinome Hepatocellulaire
- HCC
hepatocellular carcinoma
- JIS
Japan Integrated Staging Score
- KONOS
Korean network for organ sharing
- LT
liver transplantation
- MRI
magnetic resonance imaging
- TNM
Tumor Node Metastasis
- PET
positron emission tomography
- PV
portal vein
- PT
radiation therapy
- TACE
transcatheter arterial chemoembolization
- RFA
radiofrequency ablation
References
- 1.Greene F, Page D, Fleming I, editors. American Joint Committee on cancer staging manual. 6th ed. New York: Springer; 2002. Liver including intrahepatic bile ducts; pp. 131–144. [Google Scholar]
- 2.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 5.The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 6.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 8.Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–425. doi: 10.1136/gut.2003.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806–814. doi: 10.1111/j.1440-1746.2008.05728.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 13.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 14.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, et al. Living donor exchange program for adult living donor liver transplantation: preliminary experience at the Asan medical center, Korea. J Korean Soc Transplant. 2008;22:92–96. [Google Scholar]
- 16.Park SJ, Lim YS, Hwang S, Heo NY, Lee HC, Suh DJ, et al. Emergency adult-to-adult living-donor liver transplantation for acute liver failure in a hepatitis B virus endemic area. Hepatology. 2010;51:903–911. doi: 10.1002/hep.23369. [DOI] [PubMed] [Google Scholar]
- 17.de Villa VH, Lo CM, Chen CL. Ethics and rationale of living-donor liver transplantation in Asia. Transplantation. 2003;75(3 Suppl):S2–S5. doi: 10.1097/01.TP.0000046532.44975.57. [DOI] [PubMed] [Google Scholar]
- 18.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 19.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]