Abstract
NADPH oxidase (NOX) is a multicomponent enzyme complex that generates reactive oxygen species (ROS) in response to a wide range of stimuli. ROS is involved as key secondary messengers in numerous signaling pathways, and NADPH oxidases complex has been increasingly recognized as key elements of intracellular signaling of hepatic fibrogenesis. In the liver, NADPH oxidase is functionally expressed both in the phagocytic form and in the non-phagocytic form. The non-phagocytic NADPH oxidase complex is structurally and functionally similar to the phagocytic NADPH, resulting in reduction of molecular oxygen to generate superoxide. There are six homologous NOX proteins in the non-phagocytic forms of NADPH oxidase. An emerging concept is that both phagocytic and nonphagocytic NADPH oxidase components in hepatic stellate cells (HSCs) mediate hepatic fibrosis, suggesting its potential role as a pharmacological target for anti-fibrotic therapy. The molecular components and signaling pathways of various NADPH oxidase homologues that are critical for the fibrotic activity in HSCs need to be more clearly identified.
Keywords: NADPH oxidase, Reactive oxygen species, Oxidative stress, Hepatic fibrosis, Hepatic stellate cell
INTRODUCTION
Chronic hepatic inflammation which is caused by excessive alcoholic consumption, hepatitis B or C virus, and non-alcoholic steatohepatitis results in hepatic fibrosis.1 The terminal outcome of liver fibrosis is liver cirrhosis, characterized by excessive deposition of extracellular matrix proteins and the appearance of regenerative nodules, accompanied by hepatic failure, portal hypertention and hepatocellular carcinoma. Portal hypertension may cause serious complications such as esophageal variceal bleeding, ascites, and hepatic encephalopathy that are the major causes of death in cirrhotic patients. However, there is no effective anti-fibrotic therapy to treat or reverse hepatic fibrosis and liver cirrhosis. Therefore there is an urgent need to develop anti-fibrotic agent through the research for the molecular pathogenesis of hepatic fibrogenesis.
The hepatic stellate cell (HSC) is the main fibrogenic cell type in the injured liver and it also has been identified as an important effector in liver inflammation.1 HSCs are located in the space of Disse in close proximity to hepatocytes on one side and endothelial and Kupffer cells on the other side. Quiescent HSCs are desmin-positive, perisinusoidal cells that are the primary cell in the body responsible for vitamin A storage.1 Upon activation by liver injury, quiescent HSCs transdifferentiate into myofibroblast which produce inflammatory cytokines and several extracellular matrix proteins and glycoproteins including at least five collagen types, fibronectin, laminin, entactin, tenascin, and several proteoglycans. 2
Reactive oxygen species (ROS) are involved as key secondary messengers in numerous signaling pathways including transcriptional regulation, differentiation, proliferation to oncogenic transformation, and activation of programmed cell death.3 It has previously been demonstrated that ROS significantly contributes hepatic fibrogenesis from various liver injuries including alcohol abuse, hepatitis C virus infection, iron overload and chronic cholestasis.4,5 ROS can stimulate the production of the type I collagen,6 and may act as an intracellular signaling mediator of the fibrogenic action of TGF-β.7,8 ROS may be generated by multiple sources including mitochondrial respiratory chain, cytochrome p4502E1, peroxisomes, and NADPH oxidases (NOXs) in the liver.5 Cumulating evidences indicate the critical role of NOX-mediated ROS in hepatic fibrogenesis which will be described in this review.
NADPH oxidase homologues and components
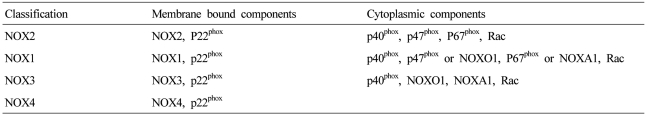
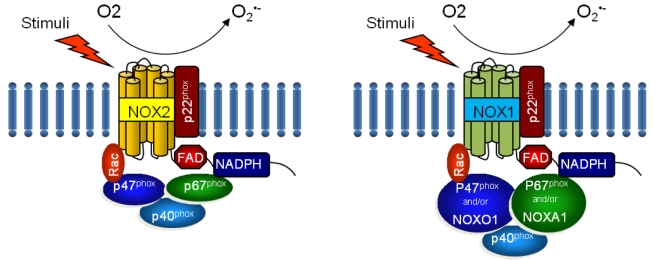
NOX is a multicomponent enzyme complex that generates ROS in response to a wide range of stimuli including TNF-α, IL-1β, leptin and angiotensin II (Ang II).9,10 NOX generates superoxide (O2·-) from molecular oxygen using NADPH as an electron donor, and superoxide converts to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Classic NOX is the phagocytic NOX found in neutrophils. The phagocytic NOX complex consists of the catalytic subunit gp91phox(renamed NOX2) together with the regulatory subunit p22phox located in the membrane. The other regulatory components p47phox, p40phox, p67phox and the small GTPase Rac are normally located in the cytoplasm.11 Upon stimulation with agonists, the cytosolic subunits translocate to the membrane-bound cytochrome complex leading to enzymatic activity. Humans have 4 additional homologous NOX proteins (NOX1, NOX3, NOX4, NOX5) and 2 related enzymes (DUOX1, DUOX2) that may function in non-phagocytes.11 Among the seven members of the NOX family, NOX1 is structurally and functionally similar to NOX2. While NOX1 is also p22phox-dependent, NOX1 may use NOXO1 (homologue of p47phox) to organize the enzyme assembly and NOXA1 (homologue of p67phox) for enzyme activation.12 In contrast, NOX4 requires only p22phox without recruitment of cytosolic regulatory components to exert its enzymatic activity.13 All NOX enzymes are able to catalyze the reduction of molecular oxygen to superoxide, but there are key differences in their activation, subunit composition, localization, and expression (Table 1, Fig. 1).14
Table 1.
The components of NADPH oxidase homologues
NOX, NADPH oxidase; NOXO, NADPH oxidase organizer; NOXA, NADPH oxidase activator; Rac, Ras-related C3 botulinum toxin substrate.
Figure 1.
Structure of phagocytic NOX2 and non-phagocytic NOX1 complex. NOX, NADPH oxidase; NOXO, NADPH oxidase organizer; NOXA, NADPH oxidase activator; Rac, Ras-related C3 botulinum toxin substrate.
NADPH oxidase-mediated ROS generation and human diseases
Chronic granulomatous disease (CGD) has been described for several decades as a human disease resulting from defects in NOX complex.15 The genetic defect of phagocytic NOX activity results in an inability to produce the superoxide anion necessary for killing bacteria and fungi by neutophils and phagocytes. The patients with CGD predispose to serious bacterial and fungal infections as well as granulomatous complications. It has been reported that at least 5 different genes involved in NOX activity may cause CGD.15 The gene mutation of NOX2 on the X chromosome account for about two thirds of the cases and present as severe form with earlier symptom manifestation. Meanwhile, mutations in p47phox, p67phox, and p22phox account for about one thirds of the cases as less severe form characterized by autosomal recessive inheritance.16
In contrast with CGD that are defective in NOX function, several human diseases are associated with excessive ROS production by an overactive NOX. NOX mediated oxidative stress may cause endothelial dysfunction, vascular smooth muscle contraction and hypertrophy that results in various vascular diseases such as hypertension, hypercholesterolemia, and atherosclerosis.14 Amyotrophic lateral sclerosis (ALS) is a progressive motor neuron degenerative disease. Although multiple factors seem to be implicated in the underlying pathogenesis, recent evidence strongly suggest that oxidative stress is an important component of disease progression of ALS.17 It was reported that oxidative damage is increased in both sporadic and familial form of ALS which is associated with mutations of SOD1.18 In a SOD1G93A transgenic mouse, an animal model of ALS, both NOX1 and NOX2 are involved in disease progression.19,20 In ALS pathogenesis, the activation of proinflammatory transcription factor NF-κB by TNF-α and IL-1β has been reported to involve NOX.17 More recently, the important role of neuronal inflammation associated with redox-active signaling endosomes (redoxosomes) has been reported.21 After TNF-α or IL-1β stimulation, dynamin-dependent co-endocytosis of the ligand-activated receptor and NOX complexes occur.22,23 As a next step, SOD1 is recruited to the redoxosome, where it binds to Rac1 and stabilizes the GTP-bound active form of Rac1.22 Oxidation of Rac1 by H2O2 uncouples SOD1 binding in a reversible fashion. The expression of ALS mutant forms of SOD1 (G93A) enhances NOX dependent superoxide production and Rac1 activation in redoxosome.24 Taken together, mutations in SOD1 leads to increased NOX dependent ROS production and hyperactivation of proinflamamtory signaling that can kill motor neurons to develop ALS, where endosomal NOX regulation by SOD1/Rac1 interaction seems to be critical.17 The possible disturbance of the above self-regulated redox signaling for NOX-derived ROS production may also be implicated in hepatic fibrogenesis. However, the detailed intracellular signaling of NOX-mediated superoxide production and subsequent conversion to H2O2 in HSCs is largely unknown.
Angiotensin II induces hepatic fibrosis via NADPH oxidase activation in hepatic stellate cells
Key elements of the renin angiotensin system such as angiotensin converting enzyme and angiotensin type I receptor (AT1R) are present in normal liver tissue, and there is major up-regulation of the system in the bile duct-ligated liver.25 Several studies demonstrated that inhibition of Angiotensin II (Ang II) synthesis and/or the blockade of AT1R markedly decrease inflammation and hepatic firosis in experimental models of chronic liver injury.26-28 In parallel, the study investigating the fibrogenic response in WT and AT1R-/- mouse demonstrated that hepatic collagen accumulation was lower in AT1R-/- mice compared to WT mice after bile duct ligation (BDL).29 p47phox-/- mice have been widely used as a genetic model to study NOX inhibition.30-32 Bataller et al. demonstrated that p47phox-/- mice showed attenuated liver injury and fibrosis compared with WT counterparts after BDL.33
As shown previously in the kidney,34 Ang II exerts its activity for the regulation of intracellular pathways using NOX as a mediator in HSCs.33,35 Several experiments demonstrate that Ang II is able to induce ROS production in human activated HSCs. Ang II induces a marked increase in ROS formation in dichlorodihydroflorescein diacetate (DCFDA)-loaded activated human HSCs. Ang II-induced ROS formation in HSCs is inhibited when HSCs are treated with either a NOX inhibitor diphenylene iodonium (DPI)36 or with the AT1R antagonist, losartan.28 Furthermore, Ang II induces the phosphorylation of p47phox through AT1R in activated HSCs. In particular, Ang II leads to an increase of intracellular ROS in HSCs isolated from wild type (WT) mice, but not in p47phox-/- HSCs. Also, Ang II induces DNA synthesis and cell migration in WT HSCs, as well as phosphorylation of ERK and c-Jun, while these effects are blunted in p47phox-/- HSCs.33 Taken together, Ang II directly acts on HSCs through the activation of the NOX complex and the subsequent production of ROS. In addition, NOX is a crucial mediator of the proliferative action of PDGF and profibrogenic action of leptin.37,38 Leptin-induced NOX acts downstream of JAK activation but is independent of STAT3.38 These reports in conjunction with previous studies on Ang II, place NOX in the center of the fibrogenic signaling response in HSCs and demonstrate its potential pharmacological target for antifibrotic therapies.
The molecular mechanism of NOX activation by Ang II is still incompletely understood. In vascular smooth muscle cell, Ang II-stimulated ROS generation is biphasic.39 The fast response is dependent on protein kinase C (PKC) which phosphorylates p47phox then phosphorylated p47phox translocates to membrane where it activates NOX catalytic subunit NOX1 and/or NOX2. The slow response requires Rac activation by upstream c-Src, EGF receptor transactivation, phosphatidylinositol-3-kinase activation.39 Ang II may stimulate PKC-dependent NOX activity through three different phospholipases (PL) such as PLC, PLD, and PLA2.14 The prolonged production of NOX-mediated ROS requires upregulation of NOX catalytic subunits and components.40
Both phagocytic and non-phagocytic NADPH oxidases in hepatic stellate cells mediate hepatic firosis
In the liver, NOX is functionally expressed both in the phagocytic form and in the non-phagocytic form.41 Through analysis of mRNA expression of NOX components in isolated liver cell tractions from wild type C57BL/6 mice, we demonstrated that phagocytic NOX components such as NOX2, p40phox, p47phox, and p67phox are mainly expressed in Kupffer cells, whereas nonphagocytic NOX components including NOX1, NOXO1, and NOXA1 are expressed in HSCs and endothelial cells.42
Although NOX plays an important role in HSCs activation, the exact structure of this complex is still unknown. All components and functionalities of the phagocytic NOX are already well established, but the respective interactions between the non-phagocytic NOX components are still under investigation. p47phox is well established component as playing a central role in the activity of NOX in HSC.33 Another integral component required for the activation of NOX is Rac1. Rac1 is a member of the Rho family of small GTPase proteins that regulates cell proliferation and dynamic reorganization of the actin cytoskeleton.43
We reported that both NOX1 and NOX2 proteins are upregulated in the fibrotic liver. In particular, non-phagocytic NOX components as well as phagocytic NOX are upregulated in activated HSCs compared to quiescent HSCs.42 Wild-type HSCs express the mRNAs for both phagocytic NOX2 and non-phagocytic NOX including NOX1 and NOX4. HSCs also express other NOX components including p47phox, p67phox, p40phox, NOXO1, NOXA1, and Rac1. Both phagocytic and non-phagocytic NOX catalytic subunits including NOX1, NOX2, and NOX4 are upregulated in activated HSCs compared to quiescent HSCs. Indeed, the mRNA for the cytoplasmic factor p47phox and the cell membrane proteins NOX2 and NOX1 are detected at very low levels in quiescent HSCs by real time RT-PCR, while they are highly expressed following HSC activation in culture and in cells freshly isolated from patients with liver firosis.33,42 These data indicate that after HSCs are activated in vivo, there is activation of both phagocytic and non-phagocytic NOX components.
Regarding the contributory role of phagocytic NOX2 and non-phagocytic NOX1 in hepatic fibrosis, we observed that mice deficient for either NOX1 or NOX2 displayed significantly less hepatic fibrosis compared to WT mice after CCl4 injections or BDL.42 Through experiments of hepatic fibrosis induction in NOX1 or NOX2 bone marrow chimeric mice, we demonstrated that both NOX1 and NOX2 in endogenous liver cells including HSCs mediate hepatic fibrosis.42 Recently other investigator also reported that NOX1 mediates hepatic fibrosis by mechanism that NOX1-derived ROS oxidize and inactivate phosphatase and tensin homologue (PTEN) resulting in protein kinase B (Akt)/forkhead box O(FOXO) 4 activation and promoting HSC proliferation.44 Thus, non-phagocytic NOX as well as phagocytic NOX represents a fundamental mediator in experimental liver fibrosis.
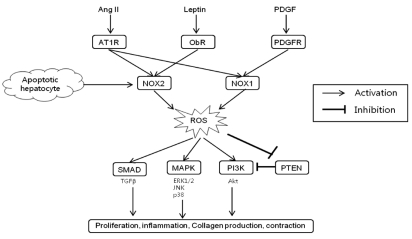
In addition to receptor-mediated agonists such as Ang II, NOX may be activated in HSCs by the engulfment of apoptotic bodies from dying hepatocytes leading to the up-regulation of collagen α1(I) in HSCs.45 The engulfment of apoptotic bodies not only acts on macrophages stimulating production of TGFβ1,46 but also on HSCs promoting their activation. In support of this model, apoptotic bodies activate NOX in HSCs, thereby up-regulating HSCs activation and firosis.47 Specifically, apoptotic bodies in HSCs increase ROS production in an NOX-dependent manner. The increased production of ROS and collagen in response to apoptotic bodies is blocked by DPI, a NOX inhibitor. Jiang et al. reported that phagocytosis of apoptotic hepatocytes directly induce HSC activation and collagen production via NOX2.48 It can be presumed that NOX2 expression in HSCs may reflect the phagocytic function of HSCs.47 These results are again consistent with an important role of NOX in HSCs activation. The profibrogenic role of NOX-mediated ROS in HSCs is schematically summarized in Figure 2.
Figure 2.
Profibrogenic role of NADPH oxidase-derived ROS in hepatic stellate cells. Ang II, angiotensin II; PDGF, platelet derived growth factor; AT1R; angiotensin type 1 receptor; ObR, leptin receptor; PDGFR, PDGF receptor; NOX, NADPH oxidase; ROS, reactive oxygen species; TGF, transforming growth factor; MAPK, mitogen activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homologue; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; Akt, protein kinase B.
CONCLUSION
ROS has diverse effects with respect to different kinds, concentration, and cell types. Although higher concentration of ROS can be cytotoxic, lower concentration of ROS serves as second messengers during cellular responses to a variety of physiologic stimuli. Emerging evidences suggest that non-phagocytic NOX as well as phagocytic NOX in hepatic stellate cells mediate hepatic fibrosis. Because NOX2 activity is important in host defense, targeting specific components of non-phagocytic NOX could be a reasonable approach to develop antifibrotic therapies that would not suppress NOX2-mediated host defences. The molecular mechanism of redox signaling associated with various NOX homologues in hepatic fibrogenesis is still largely unknown. A better understanding of contributory role of various NOX homologues in the development of hepatic fibrosis may enable to develop novel therapeutic to prevent or treat hepatic fibrosis.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No.2011-0029328) (to PYH).
Abbreviations
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- ATR
angiotensin type I receptor
- BDL
bile duct ligation
- CGD
chronic granulomatous disease
- HSC
hepatic stellate cell
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX
NADPH oxidase
- PKC
protein kinase C
- PL
phospholipase
- RAS
Renin angiotensin system
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- WT
wild-type
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 4.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 5.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 6.Nieto N, Greenwel P, Friedman SL, Zhang F, Dannenberg AJ, Cederbaum AI. Ethanol and arachidonic acid increase alpha 2(I) collagen expression in rat hepatic stellate cells overexpressing cytochrome P450 2E1. Role of H2O2 and cyclooxygenase-2. J Biol Chem. 2000;275:20136–20145. doi: 10.1074/jbc.M001422200. [DOI] [PubMed] [Google Scholar]
- 7.Maher JJ, Tzagarakis C, Giménez A. Malondialdehyde stimulates collagen production by hepatic lipocytes only upon activation in primary culture. Alcohol Alcohol. 1994;29:605–610. [PubMed] [Google Scholar]
- 8.De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-beta signaling in activated rat hepatic stellate cells. J Biol Chem. 1999;274:33881–33887. doi: 10.1074/jbc.274.48.33881. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 10.De Minicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology. 2006;131:272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 13.Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kang EM, Marciano BE, DeRavin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127:1319–1326. doi: 10.1016/j.jaci.2011.03.028. quiz 1327-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter BJ, Anklesaria P, Choi S, Engelhardt JF. Redox modifier genes and pathways in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1569–1586. doi: 10.1089/ars.2008.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, et al. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, et al. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, et al. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 24.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 26.Paizis G, Gilbert RE, Cooper ME, Murthi P, Schembri JM, Wu LL, et al. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol. 2001;35:376–385. doi: 10.1016/s0168-8278(01)00146-5. [DOI] [PubMed] [Google Scholar]
- 27.Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, et al. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4) World J Gastroenterol. 2000;6:540–545. doi: 10.3748/wjg.v6.i4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, et al. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol. 2002;37:773–780. doi: 10.1016/s0168-8278(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Bataller R, Dulyx J, Coffman TM, Ginès P, Rippe RA, et al. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol. 2005;43:317–323. doi: 10.1016/j.jhep.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, et al. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox-/- and gp91phox-/- mice. J Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 32.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 35.Bataller R, Sancho-Bru P, Ginès P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal. 2005;7:1346–1355. doi: 10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]
- 36.Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, et al. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–G1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- 37.Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, et al. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272–1281. doi: 10.1002/hep.20719. [DOI] [PubMed] [Google Scholar]
- 38.De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA. Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology. 2008;48:2016–2026. doi: 10.1002/hep.22560. [DOI] [PubMed] [Google Scholar]
- 39.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 40.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, et al. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 42.Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–1741. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267–1277. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 44.Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011 May 26; doi: 10.1002/hep.24465. doi: 10.1002/hep.24465. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 46.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, et al. Phagocytosis of apoptotic bodies by hepatic stellatecells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443. doi: 10.1002/hep.21093. [DOI] [PubMed] [Google Scholar]
- 48.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]