Neuroinflammation is a prominent pathologic feature in the spinal cord of patients with amyotrophic lateral sclerosis (ALS), and is characterized by glial activation and infiltrating T cells.1 A similar inflammatory response is present in spinal cords of ALS mice1 and is preceded by evidence of a “dying back phenomenon” which includes motor axon degeneration and alterations of the neuromuscular junction.2 The presence of monocytes/macrophages surrounding the degenerating peripheral nerve fibers is an early event that occurs prior to the onset of clinical signs of motor weakness,3 and thus raises the question whether the peripheral nerve inflammatory response initiates, or is in response to, the neurodegenerative process. To address this question, we evaluated the time course of denervation and accompanying inflammatory responses in the lumbar spinal cord–sciatic nerve–gastrocnemius and the cervical spinal cord–phrenic nerve–diaphragm motor units of ALS mice, and found that denervation occurred prior to inflammation; also, both denervation and inflammation occurred earlier in the sciatic nerve motor unit than in the phrenic nerve motor unit. Therefore, peripheral nerve inflammation is probably not the cause of denervation, but rather a response to the neurodegenerative process.
Methods.
Peripheral nerves (phrenic and sciatic), muscle (diaphragm and gastrocnemius), and spinal cords from ALS (mSOD1G93A) and wild-type (WT) mice on a B6/SJL background were evaluated by quantitative RT-PCR (qRT-PCR) at 10, 20, 55, and 77 days and end-stage disease (n = 3 for each time point) and fluorescent immunohistochemistry. Denervation was evaluated by qRT-PCR for the temporal changes in the mRNA levels of γ (fetal) and ε (adult) acetylcholine receptor (AChR) subunits. All experimental procedures involving animals were approved by The Methodist Research Institute's Institutional Animal Care and Use Committee in compliance with NIH guidelines. Data were analyzed using two-tailed Student t test and group means were plotted ± SEM; p < 0.05 was considered statistically significant. Differences between groups were analyzed using a 2-way analysis of variance.
Results.
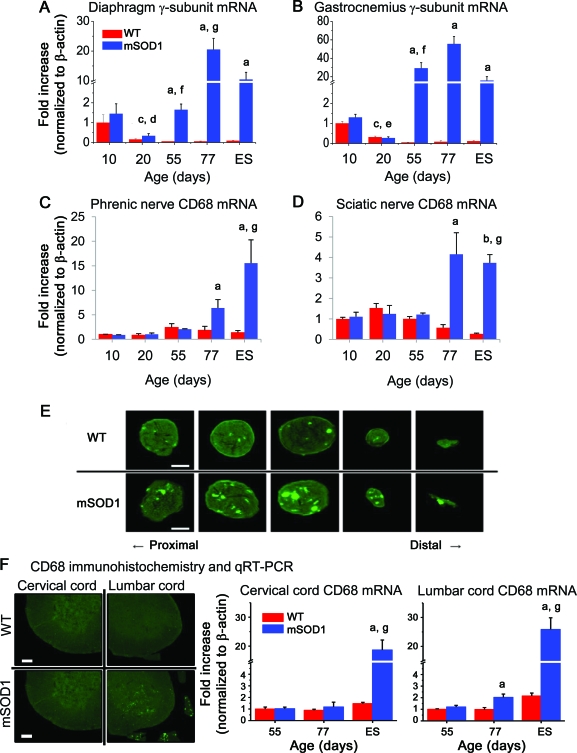
As evidence of denervation, the reappearance of the AChR fetal γ subunit was first noted in diaphragm and gastrocnemius muscles at 55 days (p = 0.033 for diaphragm and p = 0.041 for gastrocnemius, compared with their WT counterparts; figure, A and B) whereas the adult ε subunit remained unchanged in both muscles (not shown). The expression of CD68 mRNA, an inflammatory marker of phagocytic macrophages, was increased in mSOD1 mice muscle after 77 days compared with muscle from WT mice (p = 0.048 for diaphragm and p = 0.041 for gastrocnemius, figure, C and D); the mRNA levels for CCL2 mirrored the temporal expression levels of CD68 (not shown). Immunohistochemistry for CD68 confirmed that at 77 days inflammation was increased in phrenic nerves of mSOD1 mice compared with WT mice and was markedly increased in distal than proximal segments (figure, E) and although the sciatic nerves also showed increased CD68 signals, the proximal and distal segments were comparable; consistent with the qRT-PCR data, CD68 signal was not increased in mSOD1 mice at 55 days of age (not shown). The corresponding lumbar spinal cord level of the sciatic nerve showed increased CD68 signal in mSOD1 mice compared with WT mice at 77 days (p = 0.041, figure, F); CD68 mRNA was not increased in the corresponding cervical spinal cord level of the phrenic nerve in mSOD1 mice at this time point.
Figure. Denervation precedes inflammation in peripheral nerves of amyotrophic lateral sclerosis (ALS) mice.
(A, B) Quantitative RT-PCR (qRT-PCR) demonstrated the reappearance of the acetylcholine receptor (AChR) fetal γ subunit mRNA in diaphragm and gastrocnemius muscles occurs at 55 days of age in ALS mice. (C, D) Increased expression of CD68 mRNA was first noted at 77 days of age in phrenic and sciatic nerves of ALS mice. (E) Immunohistochemistry for CD68 corroborated the qRT-PCR result that at 77 days of age inflammation was increased in phrenic nerve of ALS mice. (F) Immunohistochemistry and qRT-PCR for CD68 demonstrates that inflammation was evident earlier in the lumbar spinal cord compared with the cervical spinal cord of ALS mice. ap < 0.05 vs wild-type (WT), bp < 0.01 vs WT, cp < 0.01 vs 10-day-old WT mice, dp < 0.05 vs 10-day-old mSOD1 mice, ep < 0.01 vs 10-day-old mSOD1 mice, fp < 0.05 vs 20-day-old mSOD1 mice, and gp < 0.05 vs 55-day-old mSOD1 mice. In panel E, scale bars = 50 μm. In panel F, scale bars = 200 μm.
Discussion.
Our results confirmed the accumulation of CD68+ macrophages and microglia within peripheral nerves and spinal cords of presymptomatic ALS mice, respectively.4 These inflammatory cells infiltrated only after demonstrable denervation, and thus are unlikely to have initiated denervation. The increased expression of CCL2 concomitant with the appearance of CD68+ macrophages suggests that CCL2, possibly secreted by Schwann cells, may regulate infiltration of these cells in these peripheral nerves5; however, the function of these infiltrating CD68+ macrophages cannot be inferred from this study. Nevertheless, the increased inflammatory response in phrenic nerve with minimal motoneuron injury in the cervical spinal cords of ALS mice suggests a phagocytic and possibly neuroprotective role for the CD68+ macrophage infiltration rather than a neurotoxic function mediated by spinal cord microglia. This interpretation is in accord with the demonstration that the innate immune response in peripheral nerves is separate and distinct from spinal cord immune activation in ALS mice; in the ALS spinal cord, the immune response may indeed contribute to the pathoprogression of motoneuron degeneration.6 Thus, peripheral nerve inflammation is most likely in response to the neurodegenerative process; the inflammation does not initiate this process. Furthermore, the more extensive and earlier involvement of lumbar spinal cord–sciatic nerve–gastrocnemius motor unit compared with cervical cord–phrenic nerve–diaphragm motor unit is in accord with the demonstration that fast fatigable motor units are the most vulnerable in ALS mice.7
Acknowledgments
Acknowledgment: The authors thank W. Zhao, B. Liao, A. Huang, J. Wang, X. Wang, S. Wen, M. Chen, and D. Cridebring for their technical assistance.
Footnotes
Author contributions: experimental team: O.K. and D.R.B.; writing team: O.K., D.R.B., J.S.H., and S.H.A.; all authors approved the manuscript; statistical analyses: O.K.; data analyses: O.K., D.R.B., J.S.H., and S.H.A.
Disclosure: Dr. Kano receives research support from The Uehara Memorial Foundation and Kanae Foundation. Dr. Beers and Dr. Henkel report no disclosures. Dr. Appel serves on a scientific advisory board for Neuraltus Pharmaceuticals, Inc.; has received a speaker honorarium from Avanir Pharmaceuticals; receives research support from the NIH and the Muscular Dystrophy Association; and has served as an expert consultant in a medico-legal case.
References
- 1. Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol 2010; 31: 7– 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 2004; 185: 232– 240 [DOI] [PubMed] [Google Scholar]
- 3. Lincecum JM, Vieira FG, Wang MZ, et al. From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet 2010; 42: 392– 399 [DOI] [PubMed] [Google Scholar]
- 4. Chiu IM, Phatnanib H, Kuligowskia, et al. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci USA 2009; 106: 20960– 20965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 2002; 22: 6696– 6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain 2011; 134: 1293– 1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci 2009; 12: 627– 636 [DOI] [PubMed] [Google Scholar]