Abstract
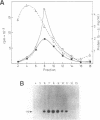
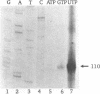
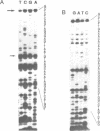
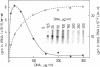
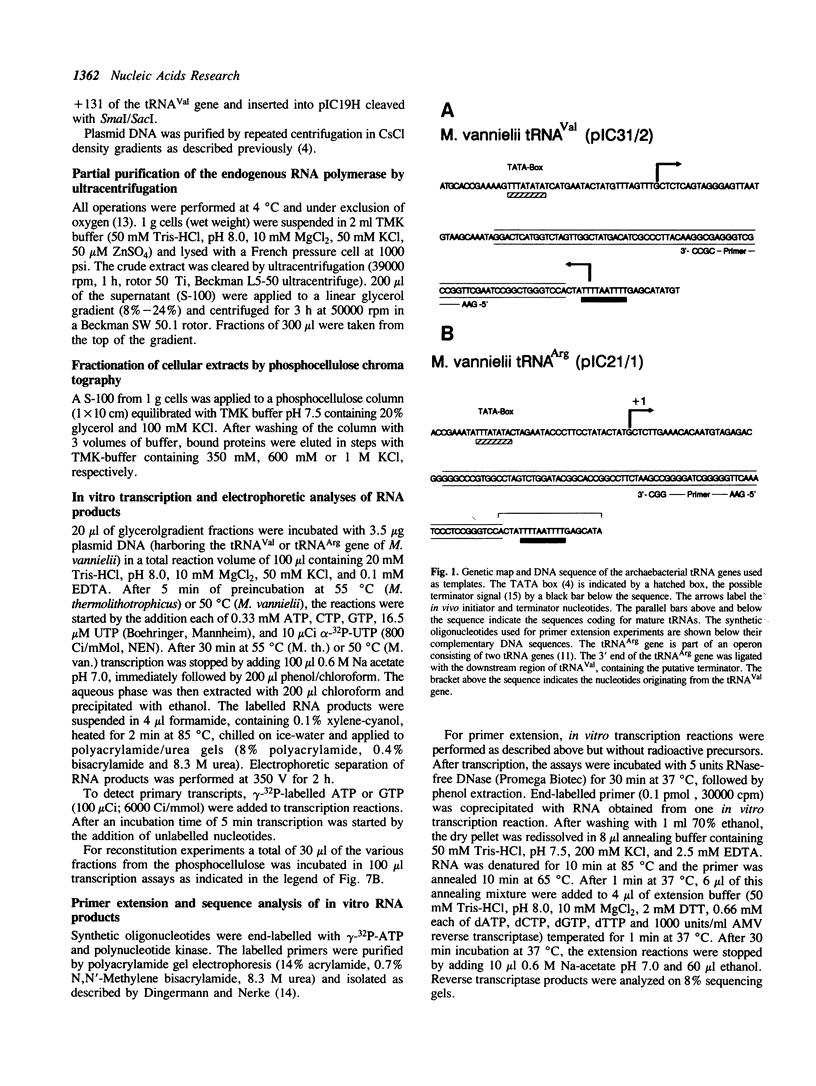
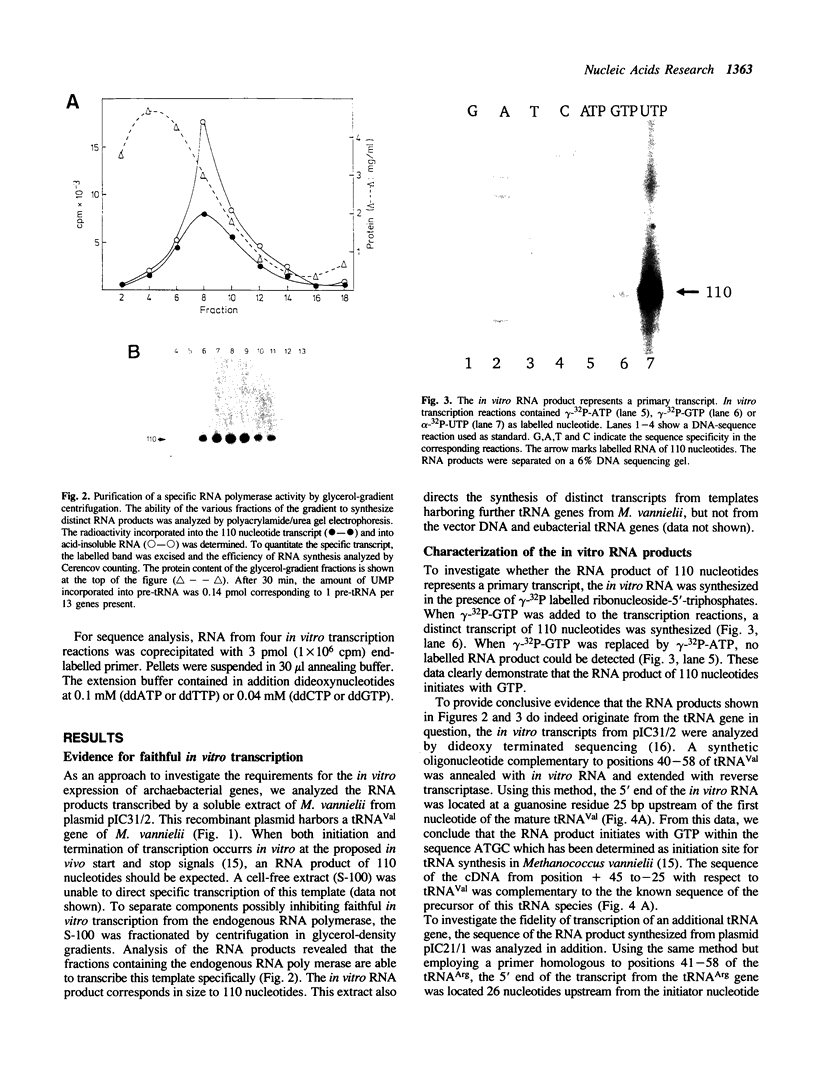
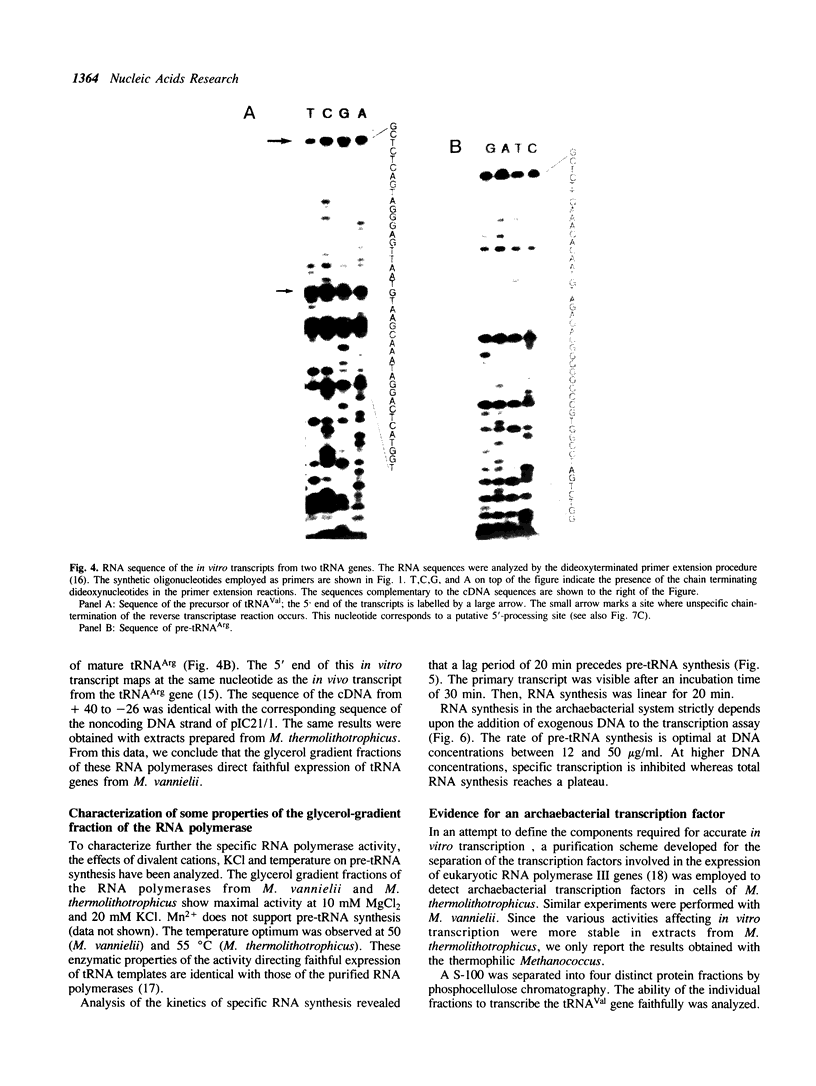
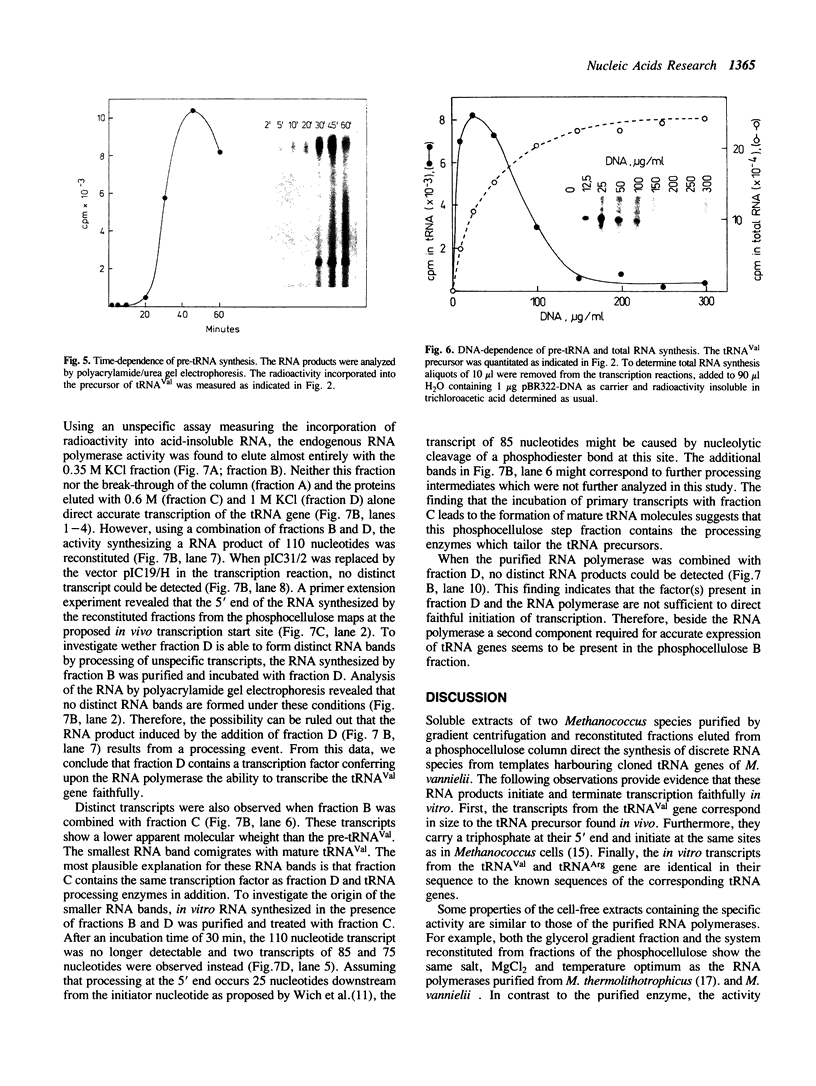
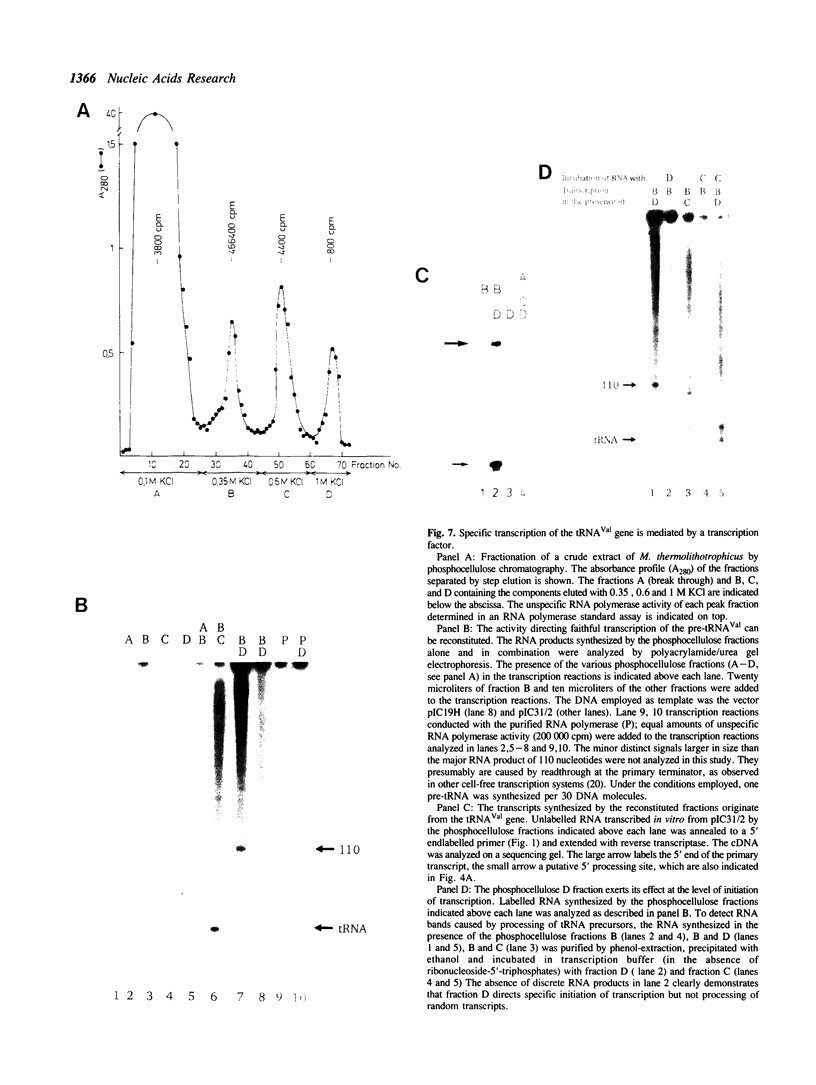
Our understanding of the mechanism of RNA biosynthesis in archaebacteria is limited, due in part to the inability of purified RNA polymerases to transcribe purified genes accurately in vitro. In the present study, we show that cell extracts of Methanococcus vannielii and Methanococcus thermolithotrophicus purified by gradient centrifugation synthesize a distinct transcript from templates harboring a cloned homologous tRNA(Val) and tRNA(Arg) gene. The in vitro transcripts initiate with GTP at the same sites as in Methanococcus cells. About 60% of the sequence of the in vitro RNA products was analyzed by dideoxyterminated primer extension and found to be identical with that of the precursors of tRNA(Val) and tRNA(Arg). This finding indicates that this RNA polymerase fraction both initiates and terminates transcription faithfully in vitro. After purification of a cell-free extract (S-100) of M. thermolithotrophicus by phosphocellulose chromatography, the endogenous RNA polymerase has lost its ability to transcribe the tRNA(Val) gene accurately. The activity directing specific expression of this template was reconstituted by the addition of a protein-fraction devoid of RNA polymerase activity. Thus, a transcription factor appears to be required for accurate cell-free expression of tRNA genes from M. vannielii.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Brown J. W., Thomm M., Beckler G. S., Frey G., Stetter K. O., Reeve J. N. An archaebacterial RNA polymerase binding site and transcription initiation of the hisA gene in Methanococcus vannielii. Nucleic Acids Res. 1988 Jan 11;16(1):135–150. doi: 10.1093/nar/16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Dingermann T., Nerke K. Primer extension analysis of tRNA gene transcripts synthesized in vitro and in vivo. Anal Biochem. 1987 May 1;162(2):466–475. doi: 10.1016/0003-2697(87)90422-2. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Jahn D., Wingender E., Seifart K. H. Transcription complexes for various class III genes differ in parameters of formation and stability towards salt. J Mol Biol. 1987 Jan 20;193(2):303–313. doi: 10.1016/0022-2836(87)90221-x. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Ng S. Y., Parker C. S., Roeder R. G. Transcription of cloned Xenopus 5S RNA genes by X. laevis RNA polymerase III in reconstituted systems. Proc Natl Acad Sci U S A. 1979 Jan;76(1):136–140. doi: 10.1073/pnas.76.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J., Matsui T., Roeder R. G. Multiple factors are required for the accurate transcription of purified genes by RNA polymerase III. J Biol Chem. 1980 Dec 25;255(24):11986–11991. [PubMed] [Google Scholar]
- Thomm M., Sherf B. A., Reeve J. N. RNA polymerase-binding and transcription initiation sites upstream of the methyl reductase operon of Methanococcus vannielii. J Bacteriol. 1988 Apr;170(4):1958–1961. doi: 10.1128/jb.170.4.1958-1961.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomm M., Stetter K. O. Transcription in methanogens. Evidence for specific in vitro transcription of the purified DNA-dependent RNA polymerase of Methanococcus thermolithotrophicus. Eur J Biochem. 1985 Jun 3;149(2):345–351. doi: 10.1111/j.1432-1033.1985.tb08932.x. [DOI] [PubMed] [Google Scholar]
- Thomm M., Wich G. An archaebacterial promoter element for stable RNA genes with homology to the TATA box of higher eukaryotes. Nucleic Acids Res. 1988 Jan 11;16(1):151–163. doi: 10.1093/nar/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomm M., Wich G., Brown J. W., Frey G., Sherf B. A., Beckler G. S. An archaebacterial promoter sequence assigned by RNA polymerase binding experiments. Can J Microbiol. 1989 Jan;35(1):30–35. doi: 10.1139/m89-005. [DOI] [PubMed] [Google Scholar]
- Wich G., Hummel H., Jarsch M., Bär U., Böck A. Transcription signals for stable RNA genes in Methanococcus. Nucleic Acids Res. 1986 Mar 25;14(6):2459–2479. doi: 10.1093/nar/14.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]