Abstract
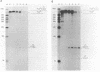
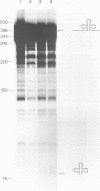
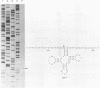
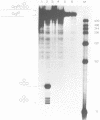
A lysate of purified mitochondria of the higher plant Oenothera processes in vitro synthesized tRNA precursors to the mature tRNA size. In vitro synthesized transcripts containing genuine plant mitochondrial tRNAs and analogous RNAs from mitochondrial loci with plastid derived tRNA sequences are accurately processed by an RNAase P-like activity to yield the mature 5'-terminus. A four nucleotide deletion in the anticodon stem-loop structure, however, prevents processing. The results show that in vitro transcripts containing tRNAs from sequence fragments of plastid origin integrated in plant mitochondrial genomes can be processed correctly in plant mitochondria, if tRNA sequences and structures are intact.
Full text
PDF
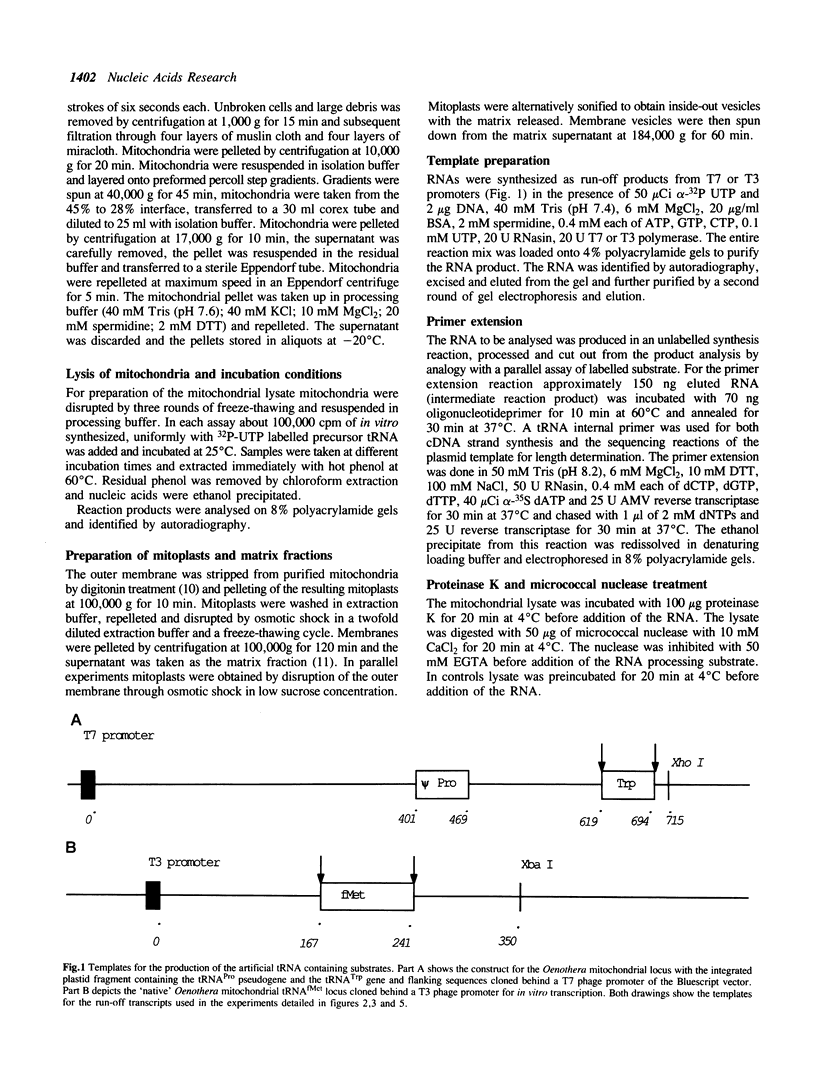
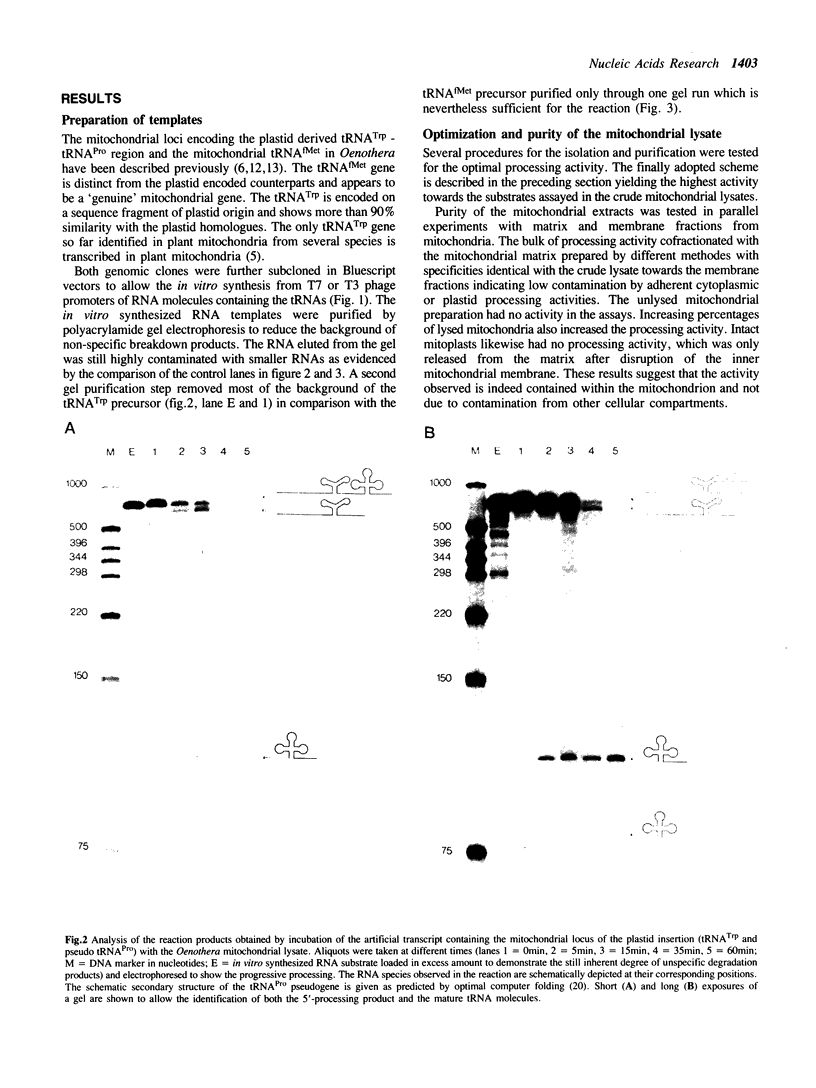
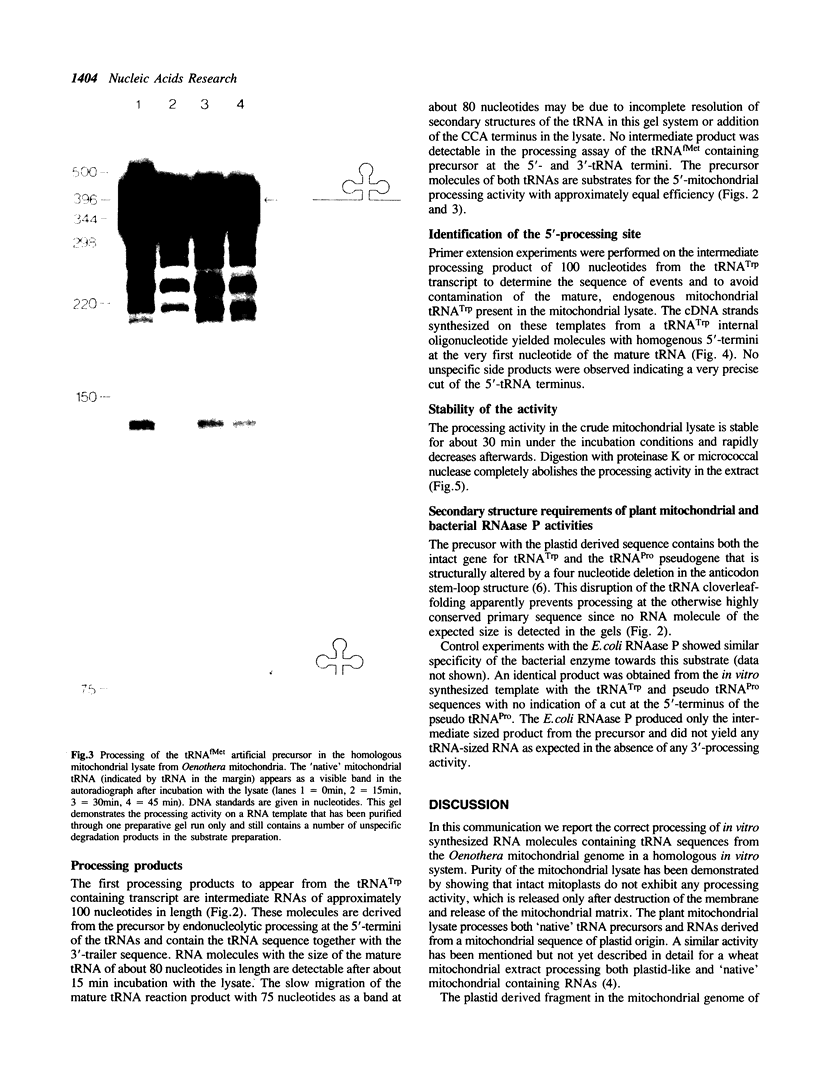
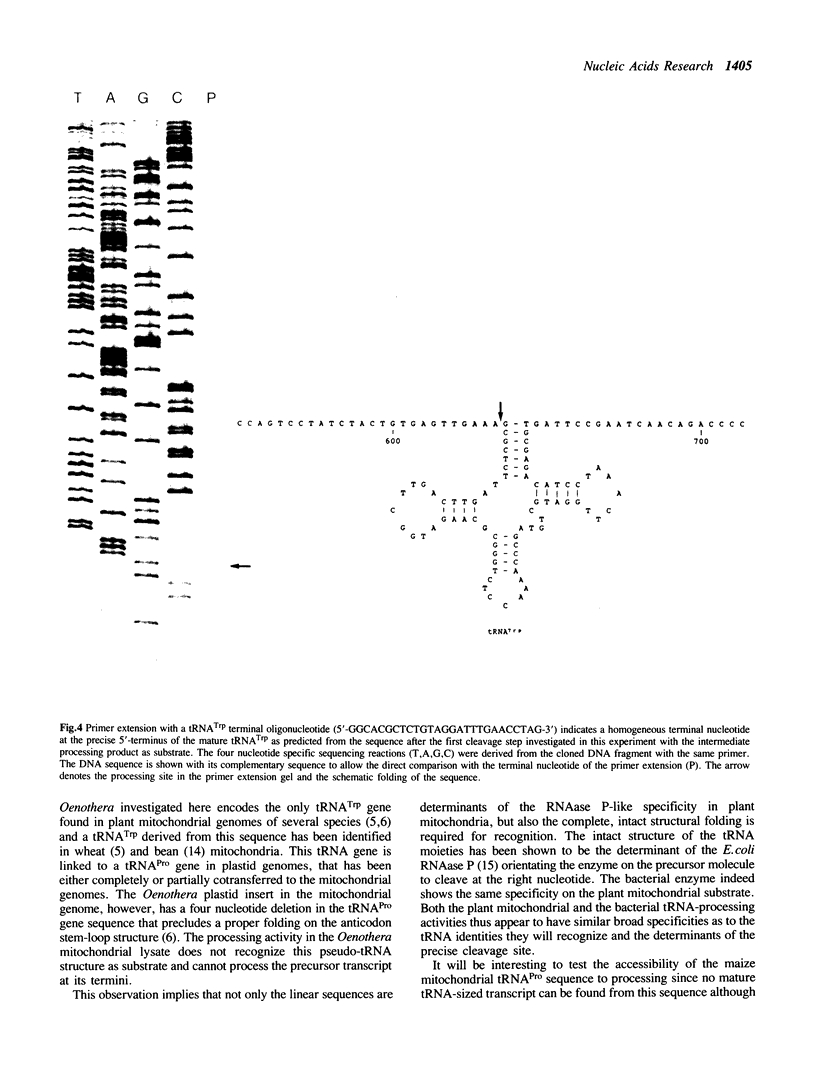

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Baer M. F., Bartkiewicz M., Gold H., Guerrier-Takada C., Kirsebom L. A., Lumelsky N., Peck K. Catalysis by the RNA subunit of RNase P--a minireview. Gene. 1989 Oct 15;82(1):63–64. doi: 10.1016/0378-1119(89)90030-9. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brennicke A. Mitochondrial DNA from Oenothera berteriana: PURIFICATION AND PROPERTIES. Plant Physiol. 1980 Jun;65(6):1207–1210. doi: 10.1104/pp.65.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce P. B., Gray M. W. Chloroplast-like transfer RNA genes expressed in wheat mitochondria. Nucleic Acids Res. 1989 Jul 25;17(14):5461–5476. doi: 10.1093/nar/17.14.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus G. M., Henrich J. P., Kelly W. G., Schmitz S. A., Castora F. J. Purification and characterization of a type I DNA topoisomerase from calf thymus mitochondria. Biochemistry. 1987 Sep 22;26(19):6195–6203. doi: 10.1021/bi00393a036. [DOI] [PubMed] [Google Scholar]
- Leon P., Walbot V., Bedinger P. Molecular analysis of the linear 2.3 kb plasmid of maize mitochondria: apparent capture of tRNA genes. Nucleic Acids Res. 1989 Jun 12;17(11):4089–4099. doi: 10.1093/nar/17.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manam S., Van Tuyle G. C. Separation and characterization of 5'- and 3'-tRNA processing nucleases from rat liver mitochondria. J Biol Chem. 1987 Jul 25;262(21):10272–10279. [PubMed] [Google Scholar]
- Marechal L., Runeberg-Roos P., Grienenberger J. M., Colin J., Weil J. H., Lejeune B., Quetier F., Lonsdale D. M. Homology in the region containing a tRNA(Trp) gene and a (complete or partial) tRNA(Pro) gene in wheat mitochondrial and chloroplast genomes. Curr Genet. 1987;12(2):91–98. doi: 10.1007/BF00434662. [DOI] [PubMed] [Google Scholar]
- Maréchal L., Guillemaut P., Grienenberger J. M., Jeannin G., Weil J. H. Sequence and codon recognition of bean mitochondria and chloroplast tRNAsTrp: evidence for a high degree of homology. Nucleic Acids Res. 1985 Jun 25;13(12):4411–4416. doi: 10.1093/nar/13.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C. Characterization of the yeast mitochondrial locus necessary for tRNA biosynthesis: DNA sequence analysis and identification of a new transcript. Cell. 1983 Oct;34(3):911–917. doi: 10.1016/0092-8674(83)90548-2. [DOI] [PubMed] [Google Scholar]
- Morales M. J., Wise C. A., Hollingsworth M. J., Martin N. C. Characterization of yeast mitochondrial RNase P: an intact RNA subunit is not essential for activity in vitro. Nucleic Acids Res. 1989 Sep 12;17(17):6865–6881. doi: 10.1093/nar/17.17.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaré A., Lonsdale D., Weil J. H., Grienenberger J. M. Sequence analysis of the tRNA(Tyr) and tRNA(Lys) genes and evidence for the transcription of a chloroplast-like tRNA(Met) in maize mitochondria. Curr Genet. 1989 Sep;16(3):195–201. doi: 10.1007/BF00391477. [DOI] [PubMed] [Google Scholar]
- Schuster W., Grienenberger J. M., Weil J. H., Brennicke A. Comparison of tRNA(Trp) and tRNA(Pro) gene sequences in the chloroplast and mitochondrial genomes of oenothera. Nucleic Acids Res. 1988 Aug 11;16(15):7737–7737. doi: 10.1093/nar/16.15.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintz H., Grienenberger J. M., Weil J. H., Lonsdale D. M. Location and nucleotide sequence of two tRNA genes and a tRNA pseudo-gene in the maize mitochondrial genome: evidence for the transcription of a chloroplast gene in mitochondria. Curr Genet. 1988 Mar;13(3):247–254. doi: 10.1007/BF00387771. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]