Abstract
Dipeptidyl peptidase-IV inhibitors improve glucose homeostasis in type 2 diabetics by inhibiting degradation of the incretin hormones. Dipeptidyl peptidase-IV inhibition also prevents the breakdown of the vasoconstrictor neuropeptide Y and, when angiotensin-converting enzyme (ACE) is inhibited, substance P. This study tested the hypothesis that dipeptidyl peptidase-IV inhibition would enhance the blood pressure response to acute ACE inhibition. Subjects with the metabolic syndrome were treated with 0 mg of enalapril (n = 9), 5 mg of enalapril (n = 8), or 10 mg enalapril (n = 7) after treatment with sitagliptin (100 mg/day for 5 days and matching placebo for 5 days) in a randomized, cross-over fashion. Sitagliptin decreased serum dipeptidyl peptidase-IV activity (13.08 ± 1.45 versus 30.28 ± 1.76 nmol/mL/min during placebo; P≤0.001) and fasting blood glucose. Enalapril decreased ACE activity in a dose-dependent manner (P<0.001). Sitagliptin lowered blood pressure during enalapril (0 mg; P=0.02) and augmented the hypotensive response to 5 mg of enalapril (P=0.05). In contrast, sitagliptin attenuated the hypotensive response to 10 mg of enalapril (P=0.02). During sitagliptin, but not during placebo, 10 mg of enalapril significantly increased heart rate and plasma norepinephrine concentrations. There was no effect of 0 or 5 mg of enalapril on heart rate or norepinephrine after treatment with either sitagliptin or placebo. Sitagliptin enhanced the dose-dependent effect of enalapril on renal blood flow. In summary, sitagliptin lowers blood pressure during placebo or submaximal ACE inhibition; sitagliptin activates the sympathetic nervous system to diminish hypotension when ACE is maximally inhibited. This study provides the first evidence for an interactive hemodynamic effect of dipeptidyl peptidase-IV and ACE inhibition in humans.
Keywords: dipeptidyl peptidase-IV inhibitors, ACE inhibitors, blood pressure, hypertension, sympathetic nervous system
Selective dipeptidyl peptidase-IV (DPP-4) inhibitors improve glycemic control in patients with type 2 diabetes by decreasing degradation of incretin hormones.1,2 The incretin hormones glucagon-like peptide-1 and glucose-dependent insulinotropic peptide augment nutrient-mediated insulin release.3 Glucagon-like peptide-1 also promotes endothelium-dependent vasodilation in the human vasculature in subjects with type 2 diabetes and stable coronary artery disease.4 In addition to preventing the degradation of glucagon-like peptide-1 and glucose-dependent insulinotropic peptide, DPP-4 inhibitors prevent the degradation of other peptides with a penultimate proline (or alanine) including substance P and the neurotransmitter neuropeptide Y. By altering the degradation of vasoactive substances, DPP-4 inhibitors have the potential to affect blood pressure. Two groups have reported a blood pressure–lowering effect of the DPP-4 inhibitor vildagliptin in clinical trials,5,6 whereas Jackson et al have reported that DPP-4 inhibition increases blood pressure in the spontaneously hypertensive rat model.7
High blood pressure is prevalent among patients with insulin resistance and type 2 diabetes. DPP-4 inhibitors could thus affect blood pressure in clinical trials by interacting with antihypertensive medications and, in particular, angiotensin-converting enzyme (ACE) inhibitors. ACE inhibition prevents the carboxy-terminus degradation of bradykinin and substance P. When ACE is inhibited, bradykinin is inactivated primarily by aminopeptidase P, but substance P is cleaved at its amino-terminus and inactivated by DPP-4. For this reason, inhibiting both DPP-4 and ACE may be expected to promote the effects of substance P.
ACE inhibitors improve morbidity and mortality in diabetic patients8–10 and are widely used for the treatment of hypertension in this patient population. Thus, understanding the interactive effect of ACE and DPP-4 inhibitor on blood pressure could have a major clinical impact. This study tested the hypothesis that pretreatment with the DPP-4 inhibitor sitagliptin would potentiate the acute decrease in blood pressure in response to the ACE inhibitor enalapril in subjects with metabolic syndrome.
Methods
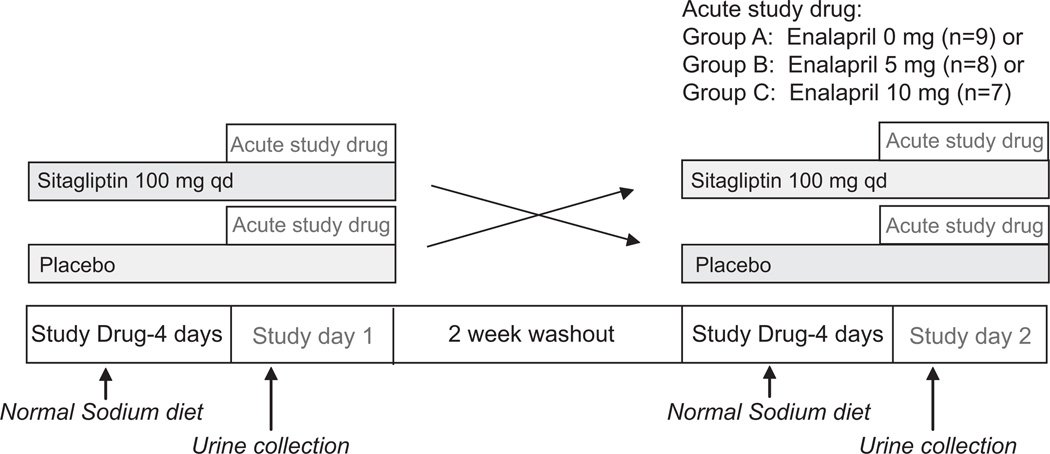
Subjects with the metabolic syndrome (National Cholesterol Education Program criteria)11 participated in a prospective, double-blind, randomized, placebo-controlled, parallel group cross-over study (Figure 1). The study was performed according to the Declaration of Helsinki after being approved by the Vanderbilt University institutional review board, and all patients gave written informed consent.
Figure 1.
Study protocol.
Antihypertensive medications were withdrawn 3 weeks before the study. Each subject was studied 2 times per research protocol (Figure 1). Subjects were randomized to receive sitagliptin (100 mg/day) or placebo for 5 days before each of 2 study days in a cross-over fashion. Subjects were further randomized in parallel to receive an acute dose of placebo (group A, called enalapril 0 mg throughout the remainder of the article to avoid confusion with the placebo for sitagliptin), enalapril 5 mg (group B), or enalapril 10 mg (group C) on each study day. Subjects were provided a normal sodium diet (160 mmol/day) for 3 days before each study day, a 24-hour urine specimen for measurement of sodium excretion the day before each study day was collected, and subjects fasted overnight.
At 7:30 am each study day, blood pressure and heart rate were measured 3 times, 2 minutes apart. Subjects were given an 8 mg/kg loading dose of para-aminohippurate followed by continuous infusion of 12 mg/min for the duration of the study to measure renal plasma flow (RPF). One hour after initiation of para-aminohippurate, subjects were given their oral study medications (time 0). Blood was obtained via indwelling venous catheter just before study drug administration and hourly thereafter for 8 hours. Subjects fasted for the duration of the study day.
The primary end point was blood pressure. Blood pressure and heart rate were measured every 5 minutes during the study using an automated blood pressure cuff (Dinamap; Critikon); the 6 preceding measurements were averaged each hour. Secondary measures were ACE activity, DPP-4 activity, glucose, insulin, RPF, aldosterone, and catecholamines. RPF was calculated based on steady-state para-aminohippurate concentrations as described previously.12 Although biomarkers were measured hourly for the 8-hour study, we present the values at baseline and maximal response (6 hours) in the response table for simplicity.
Statistical Methods
Data are presented as means ±SD. General linear model repeated-measures ANOVA was used to evaluate the effect of sitagliptin (within-subject variable) or ACE inhibitor dose group (between-subject variable) on blood pressure, heart rate, and other parameters over time. Tertile of body mass index, gender, or hypertension were included as a between-subject variable as appropriate. Post hoc analyses were conducted using a paired t test. A 2-sided P value <0.05 was considered significant. Statistical analyses were performed using SPSS software v.17.0 (SPSS Inc.).
Results
Baseline Patient Characteristics
Baseline characteristics appear in Table 1 and Supplemental Table I (available online at http://hyper.ahajournals.org). For acute study drug, 9 subjects received 0 mg enalapril, 8 subjects received 5 mg enalapril, and 7 subjects received 10 mg enalapril (Figure 1; study protocol). There were no adverse effects of the combination of sitagliptin and enalapril in this acute study.
Table 1.
Subject Characteristics
| Parameter | Value |
|---|---|
| Age, years | 42.83±2.23 |
| Race, black:white:Hispanic | 5:17:2 |
| Gender, male:female | 12:12 |
| Hypertension, yes:no | 12:12 |
| Impaired fasting glucose, yes:no | 11:13 |
| Impaired glucose tolerance, yes:no | 15:9 |
| Metabolic syndrome, yes:no | 24:0 |
| Type 2 DM, yes:no | 4:20 |
| Obstructive sleep apnea, yes:no | 5:19 |
| Family history of DM, yes:no | 12:12 |
| Family history of HTN, yes:no | 13:11 |
| BMI, kg/m2 | 35.64±0.89 |
| % Fat | 37.66±2.04 |
| Fasting plasma glucose, mg/dL | 100.08±2.11 |
| Serum triglycerides, mg/dL | 131.08±12.00 |
| Serum HDL, mg/dL | 42.33±3.23 |
| Systolic blood pressure, mm Hg | 134.13±2.94 |
| Diastolic blood pressure, mm Hg | 87.14±1.87 |
| Mean arterial pressure, mm Hg | 102.85±10.36 |
| Heart rate, beats per minute | 77.46±2.44 |
| Waist girth, cm | 117.54±1.82 |
Values are given as means±SD or as proportions.
DM indicates diabetes mellitus; HTN, hypertension; BMI, body mass index.
Effect of Treatment on DPP-4 Activity and ACE Activity
Sitagliptin pretreatment decreased DPP-4 activity compared with placebo (Table 2). ACE activity decreased in a dose-dependent manner 6 hours after treatment with 5 mg and 10 mg of enalapril (Table 2). Baseline ACE activity trended higher in the enalapril 5 mg group (P=0.08). Although there was a trend toward lower baseline ACE activity during sitagliptin compared with placebo (giving a significant sitagliptin×enalapril dose ×time term), sitagliptin did not affect ACE activity after enalapril. Likewise, there was no effect of enalapril on DPP-4 activity.
Table 2.
Influence of Sitagliptin and Enalapril on ACE and DPP-4 Activity, Endocrine Parameters, and Renal Hemodynamics
| ACE Activity, DPP-4 Activity, Endocrine Parameters, and Renal Hemodynamics |
Placebo | Sitagliptin |
P Value: Sitagliptin vs Placebo |
P Value: Time |
P Value: Enalapril Dose×Time |
P Value: Sitagliptin×Enalapril Dose×Time |
||
|---|---|---|---|---|---|---|---|---|
| 0 Hours | 6 Hours | 0 Hours | 6 Hours | |||||
| ACE activity, U/L | 0.15 | <0.001 | <0.001 | 0.04 | ||||
| Enalapril 0 mg | 37.1±13.0 | 39.3±10.9 | 34.9±11.0 | 34.9±9.5 | ||||
| Enalapril 5 mg | 46.0±18.8 | 19.8±5.2*† | 45.5±15.5 | 16.6±6.5*† | ||||
| Enalapril 10 mg | 38.3±11.3 | 9.6±5.6*†‡ | 33.3±12.1 | 10.1±7.0*† | ||||
| DPP-4 activity, U/L | <0.001 | 0.02 | 0.55 | 0.49 | ||||
| Enalapril 0 mg | 33.5±10.2 | 26.7±12.5 | 13.0±6.9§ | 10.6±4.9§ | ||||
| Enalapril 5 mg | 26.8±6.3 | 29.9±9.0 | 9.5±3.6§ | 12.0±3.4§ | ||||
| Enalapril 10 mg | 30.1±8.2 | 31.1±12.1 | 17.2±8.8§‡ | 13.3±4.5§ | ||||
| 24-Hour urine Na+ excretion, mEq | 123.6±73.0 | 146.1±61.4 | 0.08 | NA | NA | NA | ||
| Renal plasma flow, mL/min/1.73 mol/L2 | 0.34 | 0.04 | 0.007 | 0.04 | ||||
| Enalapril 0 mg | 529.1±148.6 | 522.3±225.7 | 574.3±155.5 | 606.8±154.6 | ||||
| Enalapril 5 mg | 656.6±171.1 | 647.7±225.5 | 654.3±233.3 | 743.6±277.3 | ||||
| Enalapril 10 mg | 618.3±205.2 | 685.68±197.0 | 621.1±161.8 | 840.3±378.5 | ||||
| Plasma aldosterone, pg/mL | 0.89 | <0.001 | 0.02 | 0.27 | ||||
| Enalapril 0 mg | 154.7±39.4 | 137.1±29.5 | 136.4±43.7 | 146.5±62.6 | ||||
| Enalapril 5 mg | 149.6±81.5 | 95.3±21.3† | 144.8±78.9 | 70.4±48.9† | ||||
| Enalapril 10 mg | 108.1±25.8 | 68.3±35.3† | 130.1±60.2 | 91.3±22.8¶ | ||||
| Plasma glucose, mg/dL | 0.05 | <0.001 | 0.64 | 0.04 | ||||
| Enalapril 0 mg | 102.9±10.6 | 87.0±7.1 | 97.9±7.7§ | 89.0±4.4 | ||||
| Enalapril 5 mg | 101.0±8.0 | 91.4±5.0 | 96.6±5.5 | 90.7±6.6 | ||||
| Enalapril 10 mg | 98.3±8.5 | 89.0±6.7 | 93.0±4.2 | 88.4±5.5 | ||||
| Plasma insulin, µU/mL | 0.57 | 0.04 | 0.51 | 0.94 | ||||
| Enalapril 0 mg | 20.4±13.5 | 20.2±19.5 | 18.8±9.6 | 16.1±10.8 | ||||
| Enalapril 5 mg | 15.5±16.1 | 11.3±10.8 | 17.7±21.7 | 10.2±6.9 | ||||
| Enalapril 10 mg | 19.3±8.3 | 13.2±5.5 | 27.5±36.7 | 16.6±9.3 | ||||
Data are presented as means±SD of the means.
For post hoc comparisons: *P<0.001 vs time 0;
P<0.01 vs 0 mg of enalapril;
P<0.05 vs 5 mg of enalapril;
P<0.01 vs placebo;
P<0.05 vs 0 mg of enalapril.
Influence of Treatment on 24-Hour Urine Sodium Excretion, RPF, and Aldosterone
Twenty-four hour urine sodium excretion was statistically similar during sitagliptin and placebo. Sitagliptin alone did not affect RPF (Table 2). ACE inhibition increased renal blood flow, and there was an interactive effect of sitagliptin and enalapril on renal blood flow (Table 2). There was a significant dose-dependent increase in RPF in response to enalapril during sitagliptin (maximal change in RPF over time, 169.8 ± 204.4, 280.6 ± 244.7, and 456.3 ± 282.4 mL/min/1.73 mol/L2 after 0 mg, 5 mg, and 10 mg, respectively; P=0.02); this was not significant during placebo (maximal change in RPF over time 222.7 ± 126.8, 260.6 ± 237.6, and 375.8 ± 107.4 mL/min/1.73 mol/L2 after 0 mg, 5 mg, and 10 mg, respectively).
Enalapril decreased aldosterone concentrations in a dose-dependent manner (Table 2). Sitagliptin did not alter the aldosterone response to acute ACE inhibition.
Influence of Sitagliptin on Glucose and Insulin Concentrations
Glucose concentrations were significantly lower at baseline with sitagliptin (mean baseline for all 3 groups 96.3 ± 6.5 mg/dL versus 101.3 ± 9.0 mg/dL with placebo; P=0.04). Glucose concentrations decreased significantly over time during the study days (from 101.3 ± 9.0 to 89.0 ± 6.3 mg/dL with placebo and from 96.3 ± 6.3 to 89.5 ± 5.3 mg/dL with sitagliptin; P<0.001; Table 2). There was no effect of enalapril dose on glucose (Table 2). Although insulin concentrations decreased significantly during the study, there was no effect of sitagliptin on insulin concentrations (Table 2).
Influence of Sitagliptin on Hemodynamic Effects of Acute ACE Inhibition
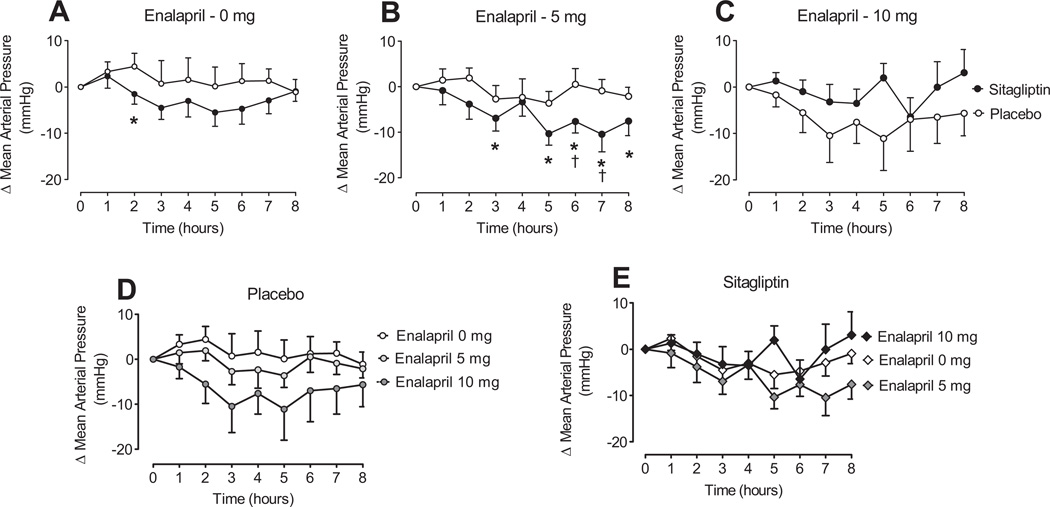
Sitagliptin did not affect baseline blood pressure (mean arterial blood pressure [MAP] 97.1 ± 12.8 mm Hg during sitagliptin versus 95.3 ± 12.7 mm Hg during placebo). There was an interactive effect of sitagliptin and ACE inhibitor dose on change in MAP (P=0.008 for sitagliptin×enalapril dose; Figure 2). Sitagliptin enhanced the decrease in MAP in response to 0 mg of enalapril (P=0.02; Figure 2A) and 5 mg of enalapril (P=0.05; Figure 2B). In contrast, sitagliptin prevented the decrease in MAP with 10 mg of enalapril (P=0.02 for sitagliptin effect; Figure 2C). The net effect was that there was a dose-dependent effect of enalapril on blood pressure during placebo (mean change in MAP 2.7 ± 2.1, −0.9 ± 2.5, and −7.9 ± 2.4 mm Hg during 0 mg, 5 mg, and 10 mg of enalapril, respectively; P=0.02 for dose effect) but not during sitagliptin (mean change in MAP −2.3 ± 2.0, −5.7 ± 2.2, and −0.9 ± 2.3 mm Hg during 0 mg, 5 mg, and 10 mg enalapril; P=0.38).
Figure 2.
Change in MAP in response to 0 mg of enalapril (A), 5 mg of enalapril (B), or 10 mg of enalapril (C) after 5-day treatment with sitagliptin (100 mg/day) or placebo. For ANOVA, see Results: Influence of Sitagliptin on Hemodynamic Effects of Acute ACE Inhibition. *P<0.05 vs baseline; †P<0.05 vs placebo.
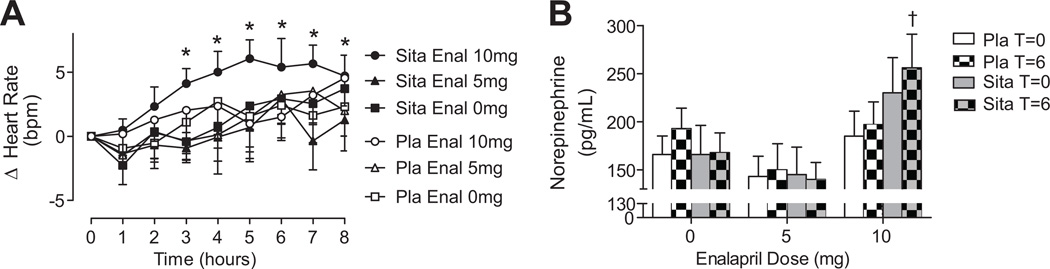
There was no effect of sitagliptin on baseline heart rate. There was a significant interactive effect of sitagliptin and enalapril (P=0.03 for sita×enalapril dose×time) on heart rate. Heart rate increased significantly in response to 10 mg of enalapril during sitagliptin but not during placebo (Figure 3A). Heart rate did not change in response to 0 mg or 5 mg of enalapril during either placebo or sitagliptin.
Figure 3.
A, Change in heart rate in response to enalapril after 5-day treatment with sitagliptin (100 mg/day) or placebo. B, Effect of enalapril on plasma norepinephrine concentrations after 5-day treatment with sitagliptin or placebo. For ANOVA, see Results: Influence of Sitagliptin on Hemodynamic Effects of Acute ACE Inhibition. *P<0.05 vs baseline; †P<0.05 vs placebo.
There was a significant interactive effect of sitagliptin and enalapril (P=0.02 for the interaction) on plasma norepinephrine concentrations. During sitagliptin, plasma norepinephrine increased significantly in response to 10 mg of enalapril but not in response to 0 mg or 5 mg of enalapril. There was no effect of any dose of enalapril on norepinephrine concentrations during placebo (Figure 3B).
Discussion
Using the DPP-4 inhibitor sitagliptin, this study tested the hypothesis that DPP-4 inhibition would augment the blood pressure–lowering effect of acute ACE inhibition in subjects with the metabolic syndrome. We found that during placebo (0 mg of enalapril) and low-dose ACE inhibition (5 mg of enalapril), sitagliptin lowered blood pressure. However, this trend was reversed during higher-dose acute ACE inhibition (10 mg of enalapril). Further, sitagliptin increased heart rate in subjects treated with the higher dose of enalapril. During sitagliptin, norepinephrine concentrations also increased significantly after the higher dose of enalapril. These data suggest that activation of the sympathetic nervous system counteracts the antihypertensive effect of maximal ACE inhibition during sitagliptin.
DPP-4 inactivates substance P when ACE is inhibited. Substance P acts as a vasodilator but also increases sympathetic outflow.13 In addition to glucagon-like peptide-1, glucose-dependent insulinotropic peptide, and substance P, DPP-4 also cleaves neuropeptide Y1–36 (NPY1–36), converting it to neuropeptide Y3–36 (NPY3–36). NPY1–36 is a Y1-receptor agonist released from sympathetic nerves. NPY1–36 causes vasoconstriction, whereas Y3–36 is a selective Y2-receptor agonist without effect on vascular tone.14 Thus, during high-dose ACE inhibition and DPP-4 inhibition, activation of the sympathetic nervous system by substance P and decreased degradation of NPY1–36 may offset decreased degradation of vasodilatory peptides.
Consistent with our findings in humans, Jackson et al reported that treatment of spontaneously hypertensive rats with sitagliptin reduces blood pressure in the absence of ACE inhibitor but increases blood pressure during high-dose ACE inhibition.7 This effect was blocked by a ganglionic blocker.7 Moreover, Boschmann et al reported that vildagliptin augmented plasma norepinephrine concentrations in human subjects before and after a mixed-meal test.15 Interestingly, although subjects in the Boschmann study were not on β-adrenoreceptor blockers, other antihypertensive medications were allowed at stable doses; thus, presence of ACE inhibitors was permitted, although not specifically reported. Together, these 3 studies suggest that during maximal ACE inhibition, sitagliptin enhances activation of the sympathetic nervous system.
In addition to causing activation of the sympathetic nervous system, sitagliptin has been reported to increase the interactive effect of sympathetic activation and angiotensin II on renal perfusion pressure during constant flow in isolated kidneys from hypertensive rats.14 In contrast, in our study, sitagliptin enhanced the renal vasodilatory effect of enalapril, reflecting the fact that the formation of angiotensin II was blocked.
Enalapril decreased ACE activity in a dose-dependent manner, and sitagliptin did not alter ACE activity during enalapril, indicating that the interactive effect of sitagliptin and enalapril on blood pressure could not be attributed to attenuation of ACE inhibition by sitagliptin. Likewise, enalapril did not alter DPP-4 activity or glucose. Although there was an apparent interactive effect of enalapril×sitagliptin×time, this reflected slight differences in baseline glucose among the 3 experimental groups. Baseline glucose concentrations were lower during sitagliptin versus placebo, consistent with the known hypoglycemic effects of DPP-4 inhibition.1 A lack of effect of sitagliptin on insulin concentrations is consistent with previous studies showing increased proinsulin to insulin ratio as well as improved homeostasis model assessment-β measure but no difference in fasting insulin concentrations.1
This study has limitations. First, we studied the effect of sitagliptin on the hypotensive response to acute ACE inhibition. The effect of DPP-4 inhibition on the hypotensive response to chronic ACE inhibition requires additional study. Second, we studied a limited dose range of ACE inhibition with the once-daily dosing and only 5 and 10 mg. Third, studying subjects on different ACE doses in parallel rather than in cross-over fashion may limit generalizability of the findings. Finally, we studied subjects with the metabolic syndrome in the fasting state and therefore may have missed an interactive effect of DPP-4 and ACE inhibition on postprandial glucose. We studied subjects with the metabolic syndrome to avoid any confounding effect of other antidiabetic agents. We studied subjects in the fasting state to avoid effects of eating on blood pressure. Future studies are needed in patients with type 2 diabetes.
Perspective
Diabetes and hypertension commonly coexist in the same patient. ACE inhibitors reduce mortality in diabetes and are used in combination with antidiabetic agents. This study examined the effect of DPP-4 inhibition on the acute antihypertensive effect of the ACE inhibitor enalapril. DPP-4 inhibition lowered blood pressure during placebo and low-dose ACE inhibition, but this effect was lost during higher-dose ACE inhibition. Further, DPP-4 inhibition stimulated the sympathetic nervous system during maximal ACE inhibition. These findings, compatible with observations in the hypertensive rat, provide the first evidence for an interactive effect of DPP-4 and ACE inhibition in humans. Additional studies are needed to examine the interactive effect of these 2 drug classes when administered chronically.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by National Institutes of Health grants R01HL060906, R01HL079184, T32HL076133, and UL1RR024975. A.M. was also supported by a Lilly Endocrine Scholars Award.
Footnotes
Disclosures
N.J.B. has served as a consultant for Merck and Company, Novartis, and Boehringer-Ingelheim. She also receives research funding from Forest Pharmaceuticals and Shire HGT.
References
- 1.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 2.Miller S, St Onge EL. Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40:1336–1343. doi: 10.1345/aph.1G665. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 4.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 6.Nathwani A, Lebeaut A, Byiers S, Gimpelewicz C, Chang I. Reduction in blood pressure in patients treated with vildagliptin as monotherapy or in combination with metformin for type 2 diabetes. Diabetes. 2006;55:A113–A113. [Google Scholar]
- 7.Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase iv inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol. 2008;35:29–34. doi: 10.1111/j.1440-1681.2007.04737.x. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28:2261–2266. doi: 10.2337/diacare.28.9.2261. [DOI] [PubMed] [Google Scholar]
- 9.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Schnurr E, Lahme W, Kuppers H. Measurement of renal clearance of inulin and PAH in the steady state without urine collection. Clin Nephrol. 1980;13:26–29. [PubMed] [Google Scholar]
- 13.Dzurik MV, Diedrich A, Black B, Paranjape SY, Raj SR, Byrne DW, Robertson D. Endogenous substance P modulates human cardiovascular regulation at rest and during orthostatic load. J Appl Physiol. 2007;102:2092–2097. doi: 10.1152/japplphysiol.00969.2006. [DOI] [PubMed] [Google Scholar]
- 14.Jackson EK, Mi Z. Sitagliptin augments sympathetic enhancement of the renovascular effects of angiotensin II in genetic hypertension. Hypertension. 2008;51:1637–1642. doi: 10.1161/HYPERTENSIONAHA.108.112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, Sweep FC, Luft FC, He Y, Foley JE, Jordan J. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:846–852. doi: 10.1210/jc.2008-1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.