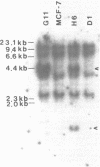
Abstract
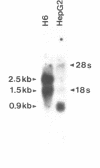
We have studied the effect of selenium on the expression of a cellular glutathione peroxidase, GSHPx-1, in transfected MCF-7 cells and in doxorubicin-resistant (Adrr) MCF-7 cells. A GSHPx-1 cDNA with a Rous Sarcoma virus promoter was transfected into a human mammary carcinoma cell line, MCF-7, which has very low endogenous cytosolic glutathione (GSH) peroxidase activity and no detectable message. The transfectant with the highest GSH peroxidase activity among the isolates, MCF-7H6, was characterized. Adrr MCF-7 cells, a subline of MCF-7 cells, also has elevated GSH peroxidase activity. GSH peroxidase expressed by MCF-7H6 and Adrr MCF-7 cells is similar to the endogenous GSHPx-1 based on molecular weight, immunoreactivity, and metabolic labeling with 75Se. MCF-7H6 and Adrr MCF-7 cells grown in Se-deficient media had 2.6 +/- 2.4 (mean +/- S.D.) and 4.2 +/- 3.6 units/mg protein of GSH peroxidase specific activity, respectively. Se supplementation increased GSH peroxidase activity in a concentration- and time-dependent fashion. Enzymatic activity reached a level of 164 +/- 62 in MCF-7H6 cells and 114 +/- 27 in Adrr MCF-7 cells within 5 days of growth in media supplemented with 30 nM Se. Northern analysis revealed that Se-deficient MCF-7H6 cells expressed 2.1 +/- 0.4-fold less GSHPx-1 mRNA than their Se-sufficient counterparts. Similarly, Se-deficient Adrr MCF-7 cells expressed 3.3 +/- 1.8-fold less GSHPx-1 mRNA than their Se-supplemented counterparts after the quantity of mRNA was normalized with beta-actin. These studies suggest that modulation of GSH peroxidase activity by Se in both MCF-7H6 transfectants expressing pRSV-GSHPx-1 and Adrr MCF-7 cells expressing endogenous GSHPx-1 occurs largely at the translational level, and to a lesser degree at the level of mRNA, possibly by stabilizing GSHPx-1 mRNA since the transfected cDNA in MCF-7H6 cells has only 5 nucleotides 5' to the AUG initiation codon.
Full text
PDF


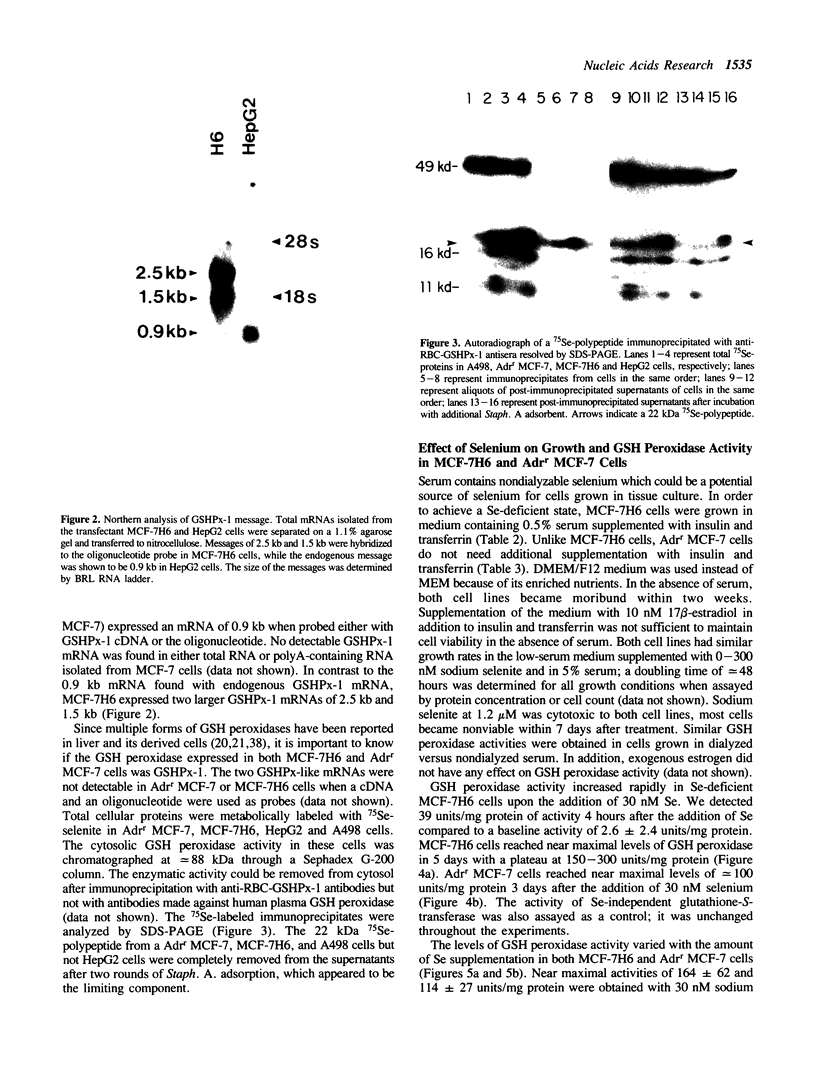
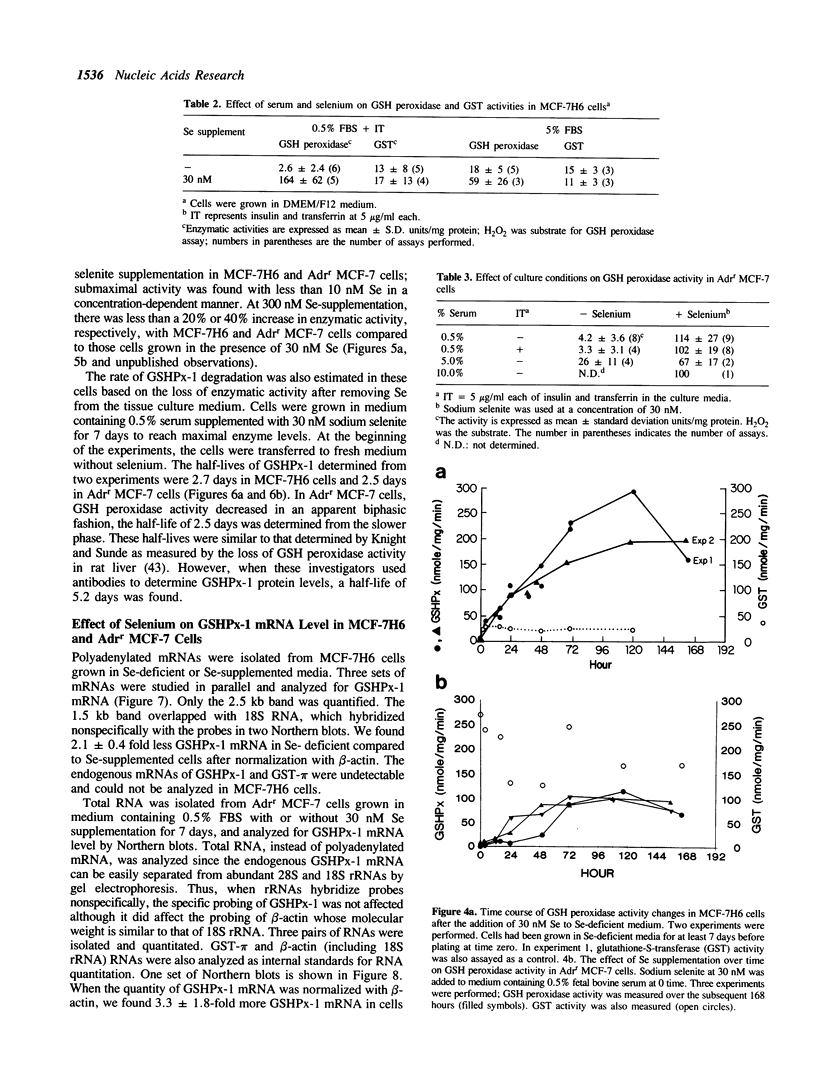
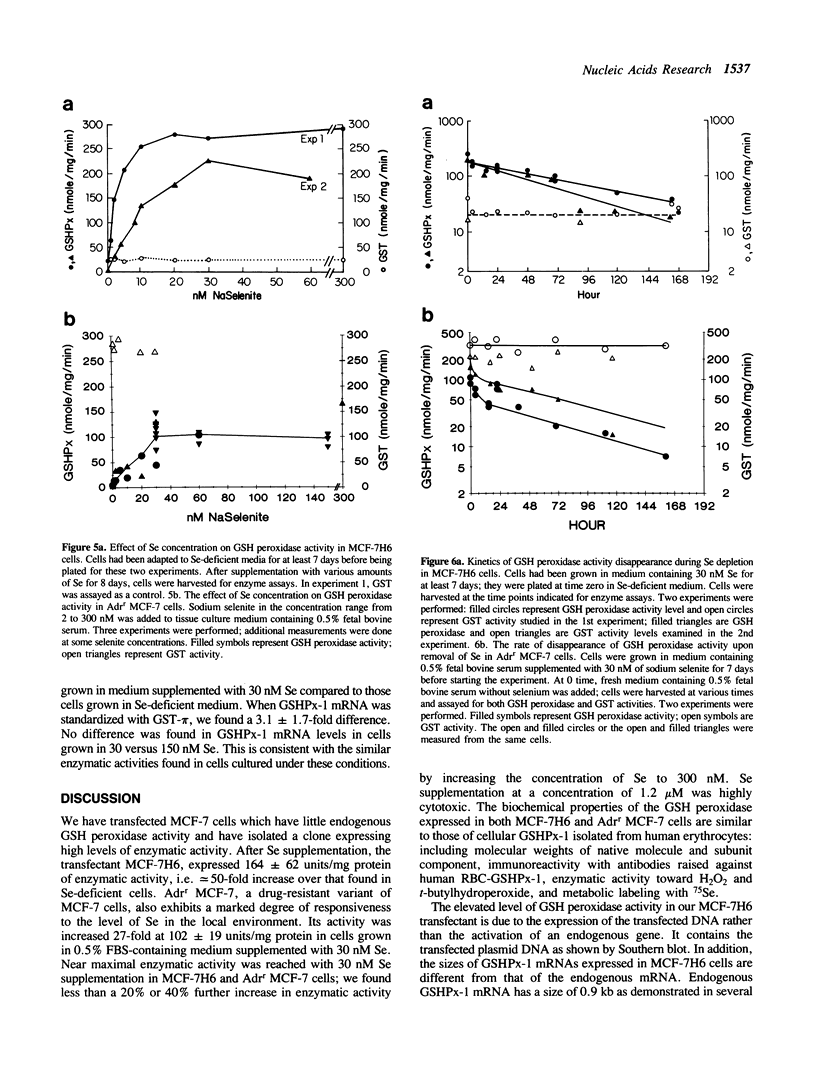
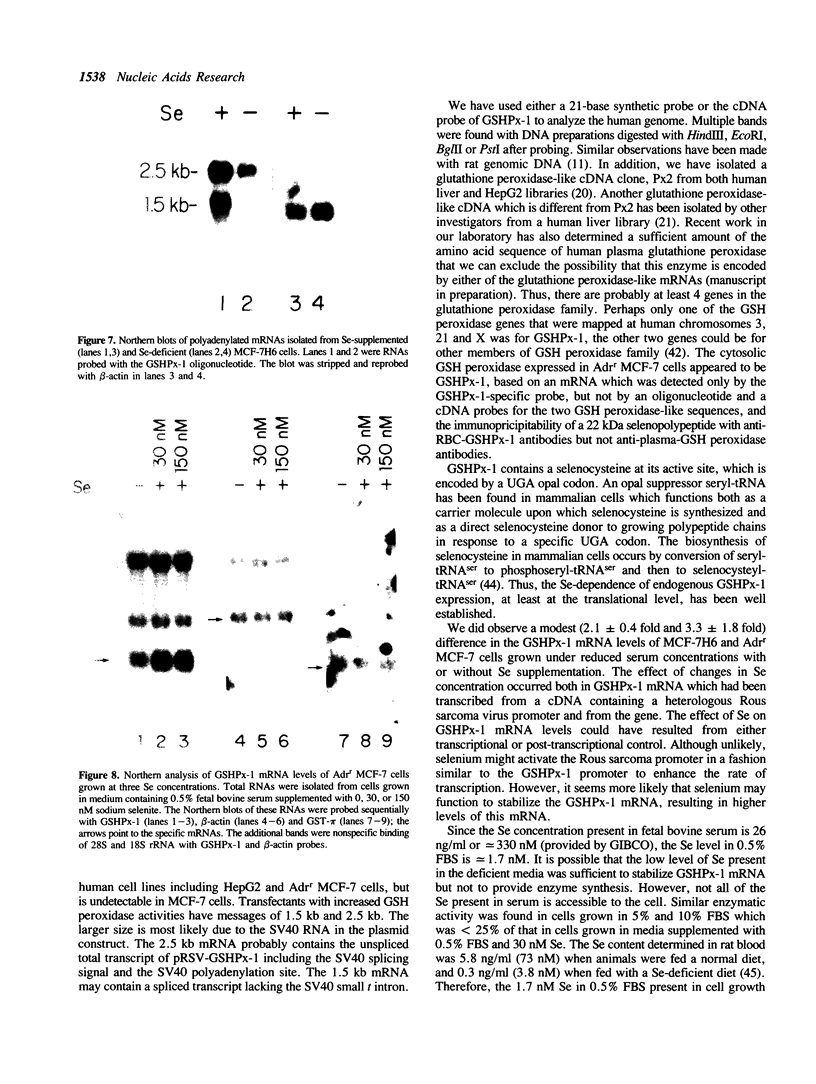

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar N., Whitin J. C., Allen P. Z., Wagner D. D., Liegey P., Cohen H. J. Plasma selenium-dependent glutathione peroxidase. Cell of origin and secretion. J Biol Chem. 1989 Sep 25;264(27):15850–15855. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dunn D. K., Howells D. D., Richardson J. P., Goldfarb P. S. A human cDNA sequence for a novel glutathione peroxidase-related selenopeptide, GPRP. Nucleic Acids Res. 1989 Aug 11;17(15):6390–6390. doi: 10.1093/nar/17.15.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K. E., Burk R. F., Lane J. M. Effect of selenium depletion and repletion on plasma glutathione and glutathione-dependent enzymes in the rat. J Nutr. 1987 Jan;117(1):99–104. doi: 10.1093/jn/117.1.99. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Shaw P. H., Barth R. K., Hastie N. D. A genetic locus closely linked to a protease inhibitor gene complex controls the level of multiple RNA transcripts. Mol Cell Biol. 1985 Aug;5(8):2114–2122. doi: 10.1128/mcb.5.8.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Knight S. A., Sunde R. A. The effect of progressive selenium deficiency on anti-glutathione peroxidase antibody reactive protein in rat liver. J Nutr. 1987 Apr;117(4):732–738. doi: 10.1093/jn/117.4.732. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Levander O. A. A global view of human selenium nutrition. Annu Rev Nutr. 1987;7:227–250. doi: 10.1146/annurev.nu.07.070187.001303. [DOI] [PubMed] [Google Scholar]
- Li N. Q., Reddy P. S., Thyagaraju K., Reddy A. P., Hsu B. L., Scholz R. W., Tu C. P., Reddy C. C. Elevation of rat liver mRNA for selenium-dependent glutathione peroxidase by selenium deficiency. J Biol Chem. 1990 Jan 5;265(1):108–113. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Mitchell A., Lee B. J., Mullenbach G., Hatfield D. Gene for selenium-dependent glutathione peroxidase maps to human chromosomes 3, 21 and X. Biofactors. 1988 Dec;1(4):285–292. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Hashimoto A. Purification and properties of suppressor seryl-tRNA: ATP phosphotransferase from bovine liver. FEBS Lett. 1984 Apr 24;169(2):319–322. doi: 10.1016/0014-5793(84)80342-7. [DOI] [PubMed] [Google Scholar]
- Mullenbach G. T., Tabrizi A., Irvine B. D., Bell G. I., Hallewell R. A. Sequence of a cDNA coding for human glutathione peroxidase confirms TGA encodes active site selenocysteine. Nucleic Acids Res. 1987 Jul 10;15(13):5484–5484. doi: 10.1093/nar/15.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. P., Hsu B. L., Reddy P. S., Li N. Q., Thyagaraju K., Reddy C. C., Tam M. F., Tu C. P. Expression of glutathione peroxidase I gene in selenium-deficient rats. Nucleic Acids Res. 1988 Jun 24;16(12):5557–5568. doi: 10.1093/nar/16.12.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R., Wendel A. Selenium and drug metabolism--II. Independence of glutathione peroxidase and reversibility of hepatic enzyme modulations in deficient mice. Biochem Pharmacol. 1984 Jun 15;33(12):1923–1928. doi: 10.1016/0006-2952(84)90548-3. [DOI] [PubMed] [Google Scholar]
- Saedi M. S., Smith C. G., Frampton J., Chambers I., Harrison P. R., Sunde R. A. Effect of selenium status on mRNA levels for glutathione peroxidase in rat liver. Biochem Biophys Res Commun. 1988 Jun 16;153(2):855–861. doi: 10.1016/s0006-291x(88)81174-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukenaga Y., Ishida K., Takeda T., Takagi K. cDNA sequence coding for human glutathione peroxidase. Nucleic Acids Res. 1987 Sep 11;15(17):7178–7178. doi: 10.1093/nar/15.17.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde R. A., Evenson J. K. Serine incorporation into the selenocysteine moiety of glutathione peroxidase. J Biol Chem. 1987 Jan 15;262(2):933–937. [PubMed] [Google Scholar]
- Takahashi K., Newburger P. E., Cohen H. J. Glutathione peroxidase protein. Absence in selenium deficiency states and correlation with enzymatic activity. J Clin Invest. 1986 Apr;77(4):1402–1404. doi: 10.1172/JCI112449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C. D., Rea H. M., Doesburg V. M., Robinson M. F. Selenium concentrations and glutathione peroxidase activities in whole blood of New Zealand residents. Br J Nutr. 1977 May;37(3):457–460. doi: 10.1079/bjn19770049. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., von Bahr C., Mannervik B. Glutathione transferases from human liver. Methods Enzymol. 1985;113:499–504. doi: 10.1016/s0076-6879(85)13065-x. [DOI] [PubMed] [Google Scholar]
- Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- Whanger P. D., Beilstein M. A., Thomson C. D., Robinson M. F., Howe M. Blood selenium and glutathione peroxidase activity of populations in New Zealand, Oregon, and South Dakota. FASEB J. 1988 Nov;2(14):2996–3002. doi: 10.1096/fasebj.2.14.3181654. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Takekoshi S., Watanabe K., Fujii-Kuriyama Y. Determination of nucleotide sequence of cDNA coding rat glutathione peroxidase and diminished expression of the mRNA in selenium deficient rat liver. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1024–1028. doi: 10.1016/0006-291x(88)90242-2. [DOI] [PubMed] [Google Scholar]