Abstract
Background
The fornix is the predominant outflow tract of the hippocampus, a brain region known to be affected early in the course of Alzheimer’s disease (AD). The aims of the present study were to: 1) examine the cross-sectional relationship between fornix DTI measurements (fractional anisotropy (FA), and mean (MD), axial (DA) and radial (DR) diffusivities), hippocampal volume, and memory performance, and 2) compare fornix DTI measures to hippocampal volumes as predictors of progression and transition from amnestic mild cognitive impairment (MCI) to AD dementia.
Methods
23 MCI participants with baseline hippocampal volumetry and diffusion tensor imaging received detailed evaluations at baseline, 3, 6, 12 months, and 2.5 years. Six participants converted to AD over the follow-up. Fornix and posterior cingulum DTI measurements and hippocampal volumes were ascertained using manual measures. Random effects models assessed each of the neuroimaging measures as predictors of decline on the MMSE, CDR-Sum of boxes and Memory z-scores; ROC analyses examined the predictive value for conversion to AD.
Results
There was a significant correlation between fornix FA and hippocampal volumes. However, only the fornix measurements (FA, MD, DR, DA) were cross-sectionally correlated with memory z-scores. Both fornix FA and hippocampal volumes were predictive of memory decline. Individually, fornix FA and MD and hippocampal volumes were very good predictors of progression with likelihood ratios>83, and better than 90% accuracy.
Conclusion
Fornix FA both cross-sectionally correlated with and longitudinally predicted memory decline and progression to AD. Manually-drawn fornix ROI shows comparable promise to hippocampal volume as a predictive biomarker of progression and warrants replication in a larger study.
Keywords: Fornix, Hippocampus, Mild Cognitive Impairment, Biomarker, Diffusion tensor imaging
1. Introduction
Individuals with amnestic mild cognitive impairment (aMCI) have an increased risk of progressing to dementia due to Alzheimer’s disease (AD-dementia) [1], and recent studies indicate that the majority of aMCI cases have AD pathology in their brains on autopsy [2, 3]. However, while a diagnosis of aMCI clearly increases the risk for progression to AD-dementia, many individuals with aMCI do not progress. Clinic-based longitudinal studies of aMCI cohorts suggest rates of instability (e.g. returning to normal cognition) for the aMCI diagnosis on the order of 10% but epidemiological studies show instability rates as high as 25–40% [4–6].. MCI cases that improve or do not decline (i.e. “non-progressors”) may not have Alzheimer’s pathology, or they have a milder form of the disease, and are, therefore, less likely to benefit from current and newly-developing AD-specific therapies. Thus, there is an inherent need to identify which aMCI cases will progress to AD-dementia.
Cerebrospinal fluid (CSF) measures of amyloid-beta(1-42) and phospho-tau, and amyloid plaque imaging (e.g. 11C-PIB PET, AV-45) may be good diagnostic and predictive markers of conversion [7–9]. However, their utility as biomarkers in the population is limited by their costs and invasiveness. More recently, less costly and invasive magnetic resonance (MR) imaging measures of hippocampal and global cortical atrophy have been found to be as good as, or superior to, CSF measures in predicting aMCI progression [10, 11]. Hippocampal atrophy also correlates with memory performance [12–15], although the strength of the association can vary based on the diagnostic group(s) examined (e.g. normal control, MCI, AD-dementia) and on the type of memory tests incorporated [12, 13, 16]. However, hippocampal atrophy has not been clinically utilized as a single AD diagnostic marker due to the large variability within diagnostic groups and because atrophy can be found in other dementias as well as normal aging.
The fornix is the predominant outflow tract of the hippocampus, connecting the hippocampus with the septal nuclei and with the mammillary bodies in the hypothalamus. An initial study examining the fornix in PS1 and PS2 mutation carriers reported that, compared to non-carriers, presymptomatic mutation carriers had significantly reduced fornix fractional anisotropy (FA), measured by diffusion tensor imaging (DTI) [17]. While the cingulum bundle FA was also reduced in the presymptomatic carriers, this finding was not significant, suggesting the fornix may be a more sensitive, early pathological marker. Similarly, we have previously shown that among a group of cognitively normal controls, aMCI and early AD-dementia cases, that fornix FA is reduced with increasing severity of the disease [18]. In light of these findings and the need to identify a biomarker to predict progression from aMCI to AD, the aims of the present study were to: 1) examine the cross-sectional correlation between the fornix and posterior cingulate white matter integrity using DTI, hippocampal volumes measured with structural MR methods, and memory performance; 2) compare the imaging parameters as indicators of cognitive and functional decline; and 3) compare neuroimaging and other AD-related variables in predicting progression from aMCI to AD-dementia.
2. Methods
2.1. Participants
Participants were community-dwelling volunteers who enrolled in a longitudinal study examining the utility of neuroimaging measures as biomarkers of AD progression. Recruiting methods have previously been described [18]. Briefly, participants were recruited from the Clinical Core of the Johns Hopkins Alzheimer’s Disease Research Center (ADRC) and from memory clinics associated with Johns Hopkins Medicine. Participants were diagnosed with Amnestic Mild Cognitive Impairment (MCI) if they were non-demented participants with mild memory problems, Clinical Dementia Rating (CDR)=0.5, and met Mayo criteria for amnestic MCI, single or multiple domains [19]. All participants were required to be older than 55 years, have no history of a neurological disease or of a major psychiatric illness, and have an informant who could provide information about their daily function. Twenty-five MCI participants completed the baseline examination, 23 of whom had available both hippocampal volumes and DTI scans and comprised the sample for the present analyses. Informed consent was obtained prior to the initiation of the study in accordance with the requirements of the Johns Hopkins Institutional Review Board. Consent procedures followed the guidelines endorsed by the Alzheimer’s Association for participation of cognitively impaired individuals [20].
2.2. Assessments
Each participant received a detailed medical evaluation at baseline, three months, six months and one-year later, including: (1) a medical, psychiatric and neurologic history; (2) a neuropsychological battery; (3) a physical, psychiatric and neurological examination; (4) an assessment of clinical severity using the CDR scale [21]; (5) MRI scan; and (6) a blood draw. An additional in-person clinical examination was conducted approximately 2.5 years after baseline for the purpose of determining clinical progression from MCI to AD dementia, based on a consensus diagnosis by ADRC investigators.
The neuropsychological battery included the Mini-Mental State Exam (MMSE) [22]; Alzheimer’s Disease Assessment Scale – cognitive portion (ADAS-Cog) [23]; California Verbal Learning Test (CVLT) [24]; the Logical Memory Story A from the Wechsler Memory Scale (WMS) [25]; Trail Making Test (TMT) Parts A (TMT-A) and B (TMT-B) [26]; and the total score on the Controlled Oral Word Association test for category and letter fluency [27]. A composite memory score (“Memory z-scores”) was computed using the baseline means and standard deviations for the cognitively healthy subjects enrolled in this study. The memory z-scores included the following tests: CVLT-total correct trials 1 to 5, CVLT-short delayed free recall, CVLT-long delayed free recall, WMS-immediate memory, and WMS-delayed memory.
The CDR [21] examines functioning in six domains: memory, orientation, judgment/problem solving, community affairs, home/hobbies, and personal care. Scores include a composite score (CDR-composite) and Sum of Boxes (CDR-Sum), which is the sum of ratings in each of six domains with a range of 0 (no impairment) to 30 (maximum impairment in all domains). CDR-SB was chosen herein, instead of the composite, because of its greater range and demonstrated sensitivity to change in MCI and AD demented (e.g. [28]).
2.3. MRI acquisition
MRI images were acquired on a 3.0 Tesla (3T) scanner (Philips Medical Systems, Best, The Netherlands) at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute. A Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE) scan and a DTI scan was acquired. The MPRAGE scan was conducted according to the protocol of the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
DTI images were acquired using a SENSE head coil on the 3T scanner, equipped with Dual Quasar gradients (up to 80 mT/m). For acquisition, an eight-element arrayed RF coil, converted to six-channel to be compatible with the six-channel receiver, was used. For DTI acquisitions, a single-shot spin echo - echo planar sequence (SE-EPI) was used, with diffusion gradients applied in 32 non-collinear directions and b = 700 s/mm2. Five additional reference images with least diffusion weighting (b = 33 s/mm2) were also acquired. Fifty to sixty axial slices were acquired to cover the entire cerebral hemispheres and the cerebellum, parallel to the AC-PC line. The field of view, the size of the acquisition matrix, and the slice thickness were 212 × 212 mm/96×96/2.2 mm. Other imaging parameters were: TR > 7,000 ms and TE = 80 ms; and SENSE reduction factor = 2.5. To improve the signal-to-noise ratio, two datasets were acquired, leading to a total acquisition time of 7 minutes.
2.3.1. Hippocampal Volume Measurement
Right and left hippocampal volumes were calculated by voxel counting on binary hippocampal images segmented on T1-MRI data as previously described [29]. A template-based segmentation algorithm was used to segment the binary hippocampal images for the baseline scans. Briefly, a representative elderly normal subject was selected as the template and left and right hippocampi were segmented manually. A total of 38 landmarks were placed on the hippocampus similar to the procedure described in Csernansky et al [30]. First, the head and the tail landmarks were placed to identify the principal axis of the structure. Nine slices at equal distance from each other and perpendicular to this principal axis were then identified and a total of four landmarks were placed at the anterior, inferior, posterior, and superior midpoints in each slice. Using these landmarks, the subvolume images were then created around the hippocampi and the landmarks were used to calculate a rigid transformation [31] between the template and individual subject subvolumes. The large deformation diffeomorphic metric mapping (LDDMM) landmark matching algorithm [32] was used to register the subvolumes further. These transformations were subsequently used to move the template hippocampi segmentation onto each subject’s MR scan and to create an initial hippocampus segmentation for the subject. The alternating kernel mixture (AKM) [33] method was used to segment the subject subvolume image into Grey Matter (GM)/white matter (WM)/CSF regions. Using this AKM segmentation, any mislabeled WM and CSF voxels were removed from the initial hippocampus segmentation. The right and left hippocampi were initially examined separately. However, as there were no differences in the correlations or predictive performances for the right vs. left hippocampus, the two volumes were averaged. In order to control for head size, the outcome measure was the ratio of the total hippocampal volume to the intracranial volume (ICV).
2.4 DTI Data Processing
The DTI datasets were transferred to a personal computer running a Windows platform and were processed using DtiStudio (mri.kennedykrieger.org or www.DtiStudio.org) [34]. Images were first realigned by affine transformation using Automatic Image Registration [35], in order to remove any potential small bulk motion and Eddy-current distortion. The six elements of the diffusion tensor were calculated for each pixel using multivariant linear fitting. After diagonalization, three eigenvalues and eigenvectors were obtained. For the anisotropy map, fractional anisotropy (FA) was used [36]. The eigenvector (v1) associated with the largest eigenvalue was used as an indicator for fiber orientation. A 24-bit color-coded orientation map was created by assigning red, green, and blue channels to the x (right-left), y (anterior-posterior), and z (superior-inferior) components of the v1 and its intensity was modulated by FA. The mean diffusivity (MD) was calculated as an average of the three eigenvalues. The parallel, or axial, diffusion (DA) was identical to the first eigenvalue, and the radial diffusion (DR) was defined as an average of the second and third eigenvalues.
2.5 DTI regions of interest
Protocols were developed to identify specific fiber tracts and to manually delineate regions of interest (ROI) within the fiber tracts using the in-house software MriStudio/RoiEditor (www.MriStudio.org or mri.kennedykrieger.org), as previously described [18]. Eight ROIs were originally identified and manually drawn with high reliability (mean interclass correlation = 0.87; range = 0.82–0.95) using standardized guidelines based on location, color, and size. For the purpose of the present study, we focused on efferent tracks of the hippocampus, including the fornix and posterior cingulum. These ROIs were identified as follows (Fig. 1): 1) fornix: the body of the fornix drawn in two adjacent axial slices using the ventral midbrain and splenium of the corpus callosum as anatomic landmarks; 2) cingulum bundle: drawn on the posterior portion of the cingulum bundle on the same axial slices as the body of the fornix. As there was little difference between consecutive slices of the fornix or posterior cingulum in relation to outcomes, we took the average of the two slices. Left and right sides of the posterior cingulum were initially examined separately. As there was little difference in the results, the DTI measurements (FA, MD, DA, DR) of the two sides was averaged for the analyses presented here. Thus, we had single measures of FA, MD, DA, and DR for both the fornix and the posterior cingulum.
Figure 1.
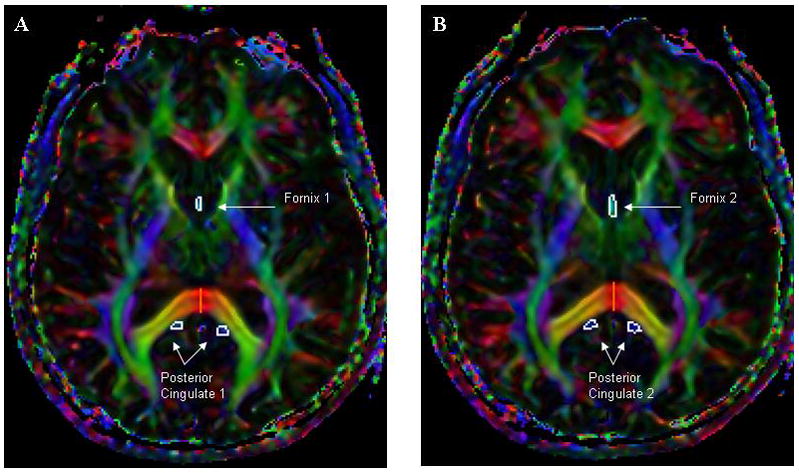
Regions of interest on two consecutive slices (A and B) for the fornix and posterior cingulate.
2.6 Statistical analysis
The demographic and health-related characteristics were examined by MCI converter status (stable vs. converter) using Fischer’s Exact Test for dichotomous variables and students t-tests for continuous variables. Individuals who progressed to a clinical consensus-conferenced diagnosis of AD-dementia over the follow-up were considered converters; those who remained with a diagnosis of MCI were considered to be stable. None of the participants reverted from a diagnosis of MCI to a cognitive normal control. MD, DA, DR and the hippocampal volume/ICV ratio were transformed by multiplying by 10^2 in order to have comparable units to the FA. Partial correlation coefficients were used to examine the cross-sectional baseline correlations between the fornix and posterior cingulum FA, MD, DA and DR values and hippocampal volume, adjusting for baseline age; and between the fornix and posterior cingulate FA, MD, DA, and DR values, hippocampal volume, and memory z-scores adjusting for age and years of education. Random effects models were used to examine the baseline neuroimaging measures and decline on the MMSE, CDR-sum of boxes (CDR-Sum), and memory z-score. This approach allows us to assess the effects of key fixed factors, such as age, on the average rate of change in each outcome while accounting for the dependence between within-subject repeated measures. Multivariate models controlled for age and education. We initially examined time as a random effect, but this variable did not contribute to the model in terms of log-likelihood values so only a random intercept was incorporated in the final models.
The predictive values of the fornix and posterior cingulate FA, MD, DA, and DR and hippocampal/ICV ratio were calculated using receiver operating characteristics (ROC) analyses. The area under the ROC curve (AUC) and 95% confidence intervals were estimated and sensitivity, specificity, and accuracy (percent correctly classified) were computed. The a priori p-value was set at p<0.05. All analyses were conducted using STATA Version 10.0 (StataCorp, College Station, TX).
3. Results
All 23 MCI participants were followed for at least one year; 20 were followed up to 2.5 years. There were no demographic or clinical differences between the three participants without a follow-up at 2.5 years and the 20 participants with a follow-up. Six of the 23 MCI participants (26.1%) progressed to a diagnosis of AD over the 2.5 years. Those who progressed had lower MMSE and memory z-scores at baseline compared to those who did not progress (Table 1). There were no differences in demographics such as age, education or APOE E4 allele status by progression status, nor was there a significant difference for CDR-Sum.
Table 1.
Demographic and Cognitive Characteristics of the MCI participants and by Converter Status
| Variable | All (n=23) mean(SD)/N(%) |
Stable MCI (n=17) | Converted to AD (n=6) | p-value |
|---|---|---|---|---|
|
| ||||
| mean(SD)/N(%) | mean(SD)/N(%) | |||
| Age (yrs) | 75.6 (5.5) | 74.5 (5.8) | 78.7 (2.9) | 0.112 |
| Female | 7 (30.4%) | 5 (29.4%) | 2 (33.3%) | 0.618 |
| Education (yrs) | 15.6 (3.1) | 15.3 (3.1) | 16.3 (3.4) | 0.497 |
| APOE E4 allele | 10/22 (45.5%) | 9/16 (56.3%) | 1/6 (16.7%) | 0.162 |
| Dementia Med | 4/22 (18.2%) | 2/16 (12.5%) | 2/6 (33.3%) | 0.292 |
| CDR-Sum | 1.3 (0.8) | 1.2 (0.8) | 1.7 (0.7) | 0.229 |
| MMSE | 26.7 (2.1) | 27.2 (2.0) | 25.0 (1.3) | 0.018 |
| Memory Z-score | −2.0 (1.2) | −1.5 (1.0) | −3.2 (0.3) | 0.001 |
| CVLT-total | 34.2 (12.9) | 38.6 (12.1) | 22.5 (5.5) | 0.006 |
| CVLT-sdfr | 5.4 (3.7) | 7.1 (2.6) | 0.7 (0.8) | <0.001 |
| CVLT-ldfr | 5.5 (3.9) | 6.8 (3.5) | 1.8 (2.3) | 0.004 |
| Wechsler-Imm | 8.2 (3.1) | 8.8 (3.3) | 6.5 (1.8) | 0.128 |
| Wechsler-Del | 6.0 (4.2) | 7.1 (4.1) | 2.8 (2.7) | 0.031 |
| GDS | 1.5 (1.2) | 1.5 (1.1) | 1.2 (1.6) | 0.609 |
Abbreviations: MCI=amnestic mild cognitive impairment; AD=Alzheimer’s disease; CDR-Sum = Clinical Dementia Rating, Sum of Boxscores; MMSE=Mini-mental State Examination; CVLT=California Verbal Learning Test; GDS=15-item Geriatric Depression Scale
Fischer’s Exact test for categorical variables; Students t-test for continuous variables
Examining the baseline partial correlation coefficients among the DTI variables and hippocampal/ICV ratio, controlling for age, only a higher fornix FA strongly and significantly correlated with a larger hippocampal/ICV ratio (r = 0.438, p = 0.041). Higher fornix MD, DA, and DR also correlated with smaller hippocampal/ICV ratios, but these results did not reach significance at the p < 0.05 level (Table 2). The posterior cingulate DTI measures did not significantly correlate with the total hippocampal volume/ICV ratio (p>0.05). Examining the partial correlations between all neuroimaging measures and memory z-scores, controlling for age and education, only fornix FA (r = 0.441, p = 0.051), MD (r = −0.464, p = 0.039), DA (r = −0.448, p = 0.048), and DR (r = −0.458, p = 0.043) correlated with memory z-scores (Table 2). Neither the hippocampal/ICV ratio nor the posterior cingulate DTI measures trended towards an association with memory z-scores.
Table 2.
Cross-sectional Correlations Between the Fornix, Posterior Cingulate, Hippocampal/ICV Ratio, and Memory Z-score
| Total Hippocampal Volume/ICV* | Memory Z-score* | |||
|---|---|---|---|---|
| corr | p-value | corr | p-value | |
| Fornix | ||||
| Fractional Anisotropy (FA) | 0.438 | 0.041 | 0.441 | 0.051 |
| Mean Diffusivity (MD) | −0.281 | 0.205 | −0.464 | 0.039 |
| Axial Diffusivity (DA) | −0.209 | 0.350 | −0.448 | 0.048 |
| Radial Diffusivity (DR) | −0.212 | 0.344 | −0.458 | 0.043 |
| Posterior Cingulate | ||||
| Fractional Anisotropy (FA) | 0.360 | 0.100 | −0.120 | 0.615 |
| Mean Diffusivity (MD) | −0.088 | 0.697 | 0.009 | 0.969 |
| Axial Diffusivity (DA) | 0.200 | 0.371 | −0.267 | 0.255 |
| Radial Diffusivity (DR) | −0.324 | 0.141 | 0.105 | 0.659 |
| Hippocampal Volume/ICV | 0.182 | 0.444 | ||
ICV = intracranial volume
Controlling for age (imaging) and age and education (memory Z-score)
The baseline neuroimaging measures were next examined as predictors of progression on the MMSE, CDR-sum and memory z-scores using random effects models. In multivariable analyses, controlling for age and education, only fornix FA (b = 7.62, 95% CI: 0.91–14.35), DA (b=−18.52, 95% CI: −34.31, −2.74) and DR (b=−20.56, 95% CI: −37.37, −3.76) were associated with better memory z-scores at baseline. Fornix DA and DR were also associated with CDR-Sum at baseline (Table 3). In longitudinal analyses, both the low fornix FA and low hippocampal/ICV ratio were predictive of decline on the MMSE, CDR-sum, and memory z-scores over 2.5 years of follow-up (Table 3). Fornix MD was predictive of decline on the MMSE and CDR-sum, but not on memory z-scores and the fornix DA was only predictive of MMSE decline. Interestingly, the posterior cingulate MD (b = −0.04, 95% CI: −0.06 – −0.01) and DR (b = − 0.06, 95% CI: −0.12, −0.01), but not FA or DA, was also predictive of memory decline but not decline on the MMSE or CDR-sum.
Table 3.
DTI measures and hippocampal volumes predict decline in MCI over 2.5 years
| Univariate | Multivariate* | |||||||
|---|---|---|---|---|---|---|---|---|
| b (95% CI) | p | b* time (95% CI) | p | b (95% CI) | p | b* time (95% CI) | p | |
| Fornix FA | ||||||||
| mmse | 14.45 (3.48, 25.41) | 0.010 | 0.03 (0.01, 0.04) | <0.001 | 7.05 (−3.39, 17.50) | 0.186 | 0.03 (0.01, 0.04) | <0.001 |
| cdrsum | −3.36 (−7.84, 1.12) | 0.141 | −0.01 (−0.02, −0.01) | <0.001 | −3.44 (−8.49, 1.61) | 0.182 | −0.01 (−0.02, −0.01) | <0.001 |
| memory Z | 10.06 (3.93, 16.19) | 0.001 | 0.01 (0.001, 0.01) | 0.020 | 7.62 (0.91, 14.35) | 0.026 | 0.01 (0.001, 0.01) | 0.020 |
| Fornix MD (x10-2) | ||||||||
| mmse | −14.07 (−24.54, −3.60) | 0.008 | −0.02 (−0.04, −0.01) | 0.001 | −7.06 (−16.85, 2.74) | 0.158 | −0.02 (−0.03, −0.01) | 0.001 |
| cdrsum | 3.64 (−0.43, 7.73) | 0.079 | 0.01 (0.005, 0.02) | 0.001 | 3.72 (−0.81, 8.25) | 0.108 | 0.01 (0.005, 0.02) | 0.001 |
| memory Z | −8.74 (−14.81, −2.67) | 0.005 | −0.004 (−0.01, 0.001) | 0.151 | −6.09 (−12.56, 0.38) | 0.065 | −0.004 (−0.009, 0.001) | 0.158 |
| Fornix DA (x10-2) | ||||||||
| mmse | −37.54 (−62.16, −12.93) | 0.003 | −0.04 (0.07, −0.01) | 0.022 | −19.18 (−44.08, 5.73) | 0.131 | −0.04 (−0.07, −0.005) | 0.023 |
| cdrsum | 10.36 (0.43, 20.28) | 0.041 | 0.01 (−0.001, 0.03) | 0.060 | 11.65 (0.27, 23.03) | 0.045 | 0.01 (−0.001, 0.03) | 0.057 |
| memory Z | −23.85 (−37.80, −9.90) | 0.001 | −0.0004 (−0.01−0.01) | 0.945 | −18.52 (−34.31, −2.74) | 0.021 | −0.001 (−0.01, 0.01) | 0.947 |
| Fornix DR (x10-2) | ||||||||
| mmse | −40.54 (−69.44, −11.64) | 0.006 | −0.02 (−0.06, 0.01) | 0.160 | −19.53 (−47.07, 8.01) | 0.165 | −0.02 (−0.06, 0.01) | 0.156 |
| cdrsum | 14.04 (3.70, 24.38) | 0.008 | 0.01 (−0.002, 0.03) | 0.092 | 15.40 (3.95, 26.85) | 0.008 | 0.01 (−0.002, 0.03) | 0.089 |
| memory Z | −26.52 (−42.07, −10.98) | 0.001 | 0.002 (−0.01, 0.01) | 0.721 | −20.56 (−37.37, −3.76) | 0.016 | 0.002 (−0.01, 0.01) | 0.726 |
| Posterior Cingulate FA | ||||||||
| mmse | −8.36 (−31.77, 15.05) | 0.484 | 0.01 (−0.02, 0.03) | 0.709 | −5.26 (−23.29, 12.78) | 0.568 | 0.004 (−0.02, 0.03) | 0.713 |
| cdrsum | 0.68 (−7.92, 9.28) | 0.876 | −0.002 (−0.01, 0.01) | 0.753 | 0.55 (−7.92, 9.04) | 0.898 | −0.002 (−0.02, 0.01) | 0.744 |
| memory Z | −3.69 (−16.35, 8.96) | 0.567 | 0.004 (−0.004, 0.01) | 0.372 | −2.86 (−14.07, 8.35) | 0.617 | 0.04 (−0.004, 0.01) | 0.375 |
| Posterior Cingulate MD (x10-2) | ||||||||
| mmse | −2.39 (−52.93, 48.16) | 0.926 | −0.02 (−0.06, 0.03) | 0.428 | −9.48 (−48.21, 29.26) | 0.632 | −0.02 (−0.06, 0.03) | 0.444 |
| cdrsum | 2.61 (−15.54, 20.77) | 0.778 | 0.02 (−0.005, 0.04) | 0.129 | 3.26 (−14.80, 21.31) | 0.724 | 0.02 (−0.005, 0.04) | 0.129 |
| memory Z | 10.26 (−17.49, 38.01) | 0.469 | −0.04 (−0.06, −0.01) | 0.006 | 8.98 (−16.00, 33.96) | 0.481 | −0.04 (−0.06, −0.01) | 0.006 |
| Posterior Cingulate DA (x10-2) | ||||||||
| mmse | −170.16 (−335.79, −4.54) | 0.044 | 0.01 (−0.17, 0.19) | 0.903 | −114.15 (−245.63, 17.33) | 0.089 | 0.01 (−0.17, 0.19) | 0.919 |
| cdrsum | 53.06 (−8.67, 14.79) | 0.092 | −0.01 (−0.09, 0.08) | 0.868 | 49.70 (−12.40, 111.79) | 0.117 | −0.01 (−0.09, 0.08) | 0.866 |
| memory Z | −67.43 (−159.22, 24.36) | 0.150 | −0.01 (−0.08, 0.05) | 0.691 | −47.52 (−131.28, 36.24) | 0.266 | −0.01 (−0.08, 0.05) | 0.679 |
| Posterior Cingulate DR (x10-2) | ||||||||
| mmse | 7.05 (−173.83, 188.13) | 0.938 | −0.16 (−0.33, 0.01) | 0.071 | 23.07 (−115.20, 161.33) | 0.744 | −0.15 (−0.32, 0.01) | 0.073 |
| cdrsum | −12.40 (−78.51, 53.70) | 0.713 | 0.03 (−0.05, 0.11) | 0.446 | −17.52 (−82.33, 47.28) | 0.596 | 0.03 (−0.05, 0.11) | 0.442 |
| memory Z | 13.23 (−84.41, 110.88) | 0.790 | −0.06 (−0.12, −0.01) | 0.027 | 23.31 (−62.56, 109.18) | 0.595 | −0.06 (−0.12, −0.01) | 0.027 |
| Hippocampal/ICV Ratio (x10-2) | ||||||||
| mmse | 13.31 (−14.51, 41.14) | 0.348 | 0.11 (0.07, 0.15) | <0.001 | −4.41 (−30.32, 21.52) | 0.739 | 0.11 (0.07, 0.15) | <0.001 |
| cdrsum | −2.44 (−12.98, 8.11) | 0.650 | −0.05 (−0.06, −0.03) | <0.001 | −2.89 (−14.98, 9.21) | 0.640 | −0.05 (−0.06, −0.03) | <0.001 |
| memory Z | 11.90 (−3.99, 27.78) | 0.142 | 0.03 (0.01, 0.04) | <0.001 | 4.37 (−12.95, 21.70) | 0.621 | 0.03 (0.01, 0.04) | <0.001 |
Controlling for age and education
Lastly, we examined the classification accuracy of the neuroimaging measures and other factors with respect to progression from MCI to AD using ROC analyses. Both the fornix FA and MD and hippocampal/ICV ratio were very good predictors of progression with AUC >0.90, likelihood ratios (LR)>83, and better than 90% accuracy (Table 4). Comparison of the fornix (FA, MD, DA or DR) and hippocampal AUC scores did not show statistically significant differences. Similarly, because the AUC and LRs were so high, the combination of the fornix FA or MD and hippocampus did not improve on the predictive value of each measure in isolation. It is important to point out, however, that the predictive value of these neuroimaging measures using ROC analyses only trended towards significance (p~0.1) to be better than the predictive value of the MMSE and CDR-sum. This is likely due to the small sample size and large confidence intervals because the comparison of likelihood ratios clearly indicate that the fornix FA and MD and hippocampal/ICV ratio are superior to the clinical measures.
Table 4.
Receive Operating Characteristic (ROC) Analyses for Predictors of Conversion from MCI to AD
| Predictor | AUC [95% CI] | Optimal Cut-off | Sensitivity | Specificity | Accuracy | Likelihood Ratio |
|---|---|---|---|---|---|---|
| Age | ||||||
| Higher Age (years) | 0.71 (0.48, 0.93) | >80 | 66.70% | 70.60% | 69.60% | 2.27 |
| CDR-Sum | ||||||
| Higher CDR-Sum | 0.73 (0.46, 0.99) | >2 | 50.00% | 88.30% | 78.30% | 4.27 |
| MMSE | ||||||
| Lower MMSE | 0.82 (0.65–0.99) | <24 | 50.00% | 88.24% | 78.26% | 4.25 |
| Posterior Cingulate | ||||||
| Low FA | 0.62 (0.32, 0.92) | <0.43 | 50.00% | 82.35% | 73.90% | 2.83 |
| High MD (x10−2) | 0.73 (0.46, 0.99) | >0.248 | 83.33% | 76.47% | 78.26% | 3.54 |
| High DA (x10−2) | 0.58 (0.34, 0.82) | >0.134 | 16.67% | 82.35% | 65.22% | 0.94 |
| High DR (x10−2) | 0.73 (0.43, 1.00) | >0.61 | 83.33% | 82.35% | 82.61% | 4.72 |
| Fornix | ||||||
| Low FA | 0.95 (0.85, 1.00) | <0.435 | 83.30% | 100.00% | 95.70% | >83.00* |
| High MD (x10−2) | 0.92 (0.79, 1.00) | >0.41 | 83.30% | 94.12% | 91.30% | 14.17 |
| High DA (x10−2) | 0.79 (0.60, 0.98) | >0.22 | 66.67% | 82.35% | 78.26% | 3.78 |
| High DR (x10−2) | 0.86 (0.71, 1.00) | >0.098 | 100.00% | 82.35% | 86.96% | 5.67 |
| Hippocampal Volume | ||||||
| Low Volume/ICV ratio (x10−2) | 0.98 (0.93, 1.00) | <0.13 | 83.30% | 100.00% | 95.70% | >83.00* |
The likelihood ratio (Sensitivity/1-Specificity) could not be calculated since the demoninator was 0. For purposes of estimation, the denominator was replaced with 1.
FA = fractional anisotropy; MD = mean diffusivity; DA = axial diffusivity; DR = radial diffusivity
4. Discussion
This study examined the relationship between MRI measures including hippocampal volume and fornix and posterior cingulum (PC) white matter integrity, their relation to memory, and their predictive value for cognitive decline and progression from aMCI to AD dementia. Cross-sectionally, fornix white matter integrity (FA, MD, DA, and DR) correlated more strongly with memory performance than the hippocampal/ICV ratio or PC white matter integrity. Longitudinally, both fornix FA and hippocampal/ICV ratio were predictive of decline in memory performance and progression from MCI to AD dementia over 2.5 yrs. Fornix white matter integrity (FA, MD, DA, and DR) was as good as the hippocampal volume in predicting progression from MCI to dementia. Since the fornix (FA, DA and DR) also cross-sectionally correlated with memory performance, the fornix may be a better correlate of early disease progression, and a clinically useful biomarker. Additional studies with larger sample sizes are warranted to confirm these results.
The disruption of white matter, including axonal and dendritic integrity, occurs early in the AD process, and has been demonstrated in both animal and in vivo human studies [37–39]. One evolving hypothesis is that disruption of white matter tracts reflects the susceptibility of late-myelinating regions to the effects of aging and AD. These white matter changes make individuals particularly vulnerable to the clinical manifestations of AD [39] and occur before neuronal degradation and atrophy are detectable. DTI was developed to measure the integrity of white matter fiber bundles in the nervous system [40, 41]. It is thought to be especially sensitive to microstructural alterations in nerve fibers [42], and a sensitive early indicator of Alzheimer pathology [43]. There are multiple indices that can be measured with DTI. FA is a measure of anisotropic water diffusion and reflects the degree of directionality of cellular structures within the fiber tracts and, therefore, the structural integrity of tracts [44]. MD, a measure of randomized mean water diffusion, represents a loss of barriers restricting the water motion, partial breakdown of tissue cytoarchitecture [44] or demyelinating processes [39]. However, FA and MD alterations could be due to changes of diffusion either parallel or perpendicular to the principal direction of the tensor. Thus, in the present study we also examined DA and DR. Axial diffusivity (DA) is a measure of parallel diffusivity and is thought to be an indicator of axonal damage while radial diffusivity (DR) is a measure of perpendicular diffusivity and thought to be an indicator of myelin breakdown [45].
Previous DTI studies using the ROI approach have focused on hippocampal or posterior cingulum FA, MD, or apparent diffusion coefficient (ADC) (e.g. [46–48]. These studies suggest that hippocampal MD and ADC is higher, and FA lower, in MCI cases compared to cognitively normal controls. One study that compared hippocampal volumetry and DTI measures reported poor correlation between the volumetry and DTI measures [48], and that left hippocampal MD was the strongest independent correlate of poor verbal memory performance and also best separated MCI cases from normal controls. Another study reported higher hippocampal ADC values in MCI cases who converted to AD dementia compared to those who did not convert over two years [49].
In contrast to these studies, we focused directly on the fornix, the predominant outflow tract of the hippocampus that connects it with the septal nuclei and mamillary bodies in the hypothalamus. The posterior fornix fibers further connect to anterior thalamic nuclei and to cingulate cortex. Prior studies have demonstrated that limbic projections and pathways (e.g. fornix) connecting to the frontal lobes are preferentially affected early in the course of AD [39, 50]. An initial study examining the fornix in PS1 and PS2 mutation carriers reported that, compared to non-carriers, presymptomatic mutation carriers had significantly reduced fornix FA [17]. While the cingulum bundle FA was also reduced in the presymptomatic carriers, this finding was not significant, suggesting the fornix may be a more sensitive, early pathological marker. Our results, among MCI cases, are congruent with this finding such that the fornix, but not the PC, correlated cross-sectionally with memory performance, and predicted longitudinal decline in memory and progression to AD. While the PC MD and DR were predictive of memory decline, the fornix was a much better predictor of clinical conversion to AD dementia and is likely a more useful correlate of disease progression.
As mentioned, the majority of DTI studies to date have primarily reported on FA and MD. Of those using a ROI approach, FA has been the most sensitive [18, 51] although another study reported that MD was more sensitive [46]. It has been suggested that ROI-based approaches may inflate the significance of FA over MD [52]. Indeed, a recent cross-sectional voxel-wise analysis of 25 AD patients and 13 elderly controls using tract-based spatial statistics reported alterations in diffusivity (MD, DA and DR) were more significant and more sensitive than FA in temporo-parietal white matter, posterior cingulum, splenium and fornix [52]. However, another recent cross-sectional study, using an atlas-based tractography approach, of 25 AD patients, 19 MCI, and 15 cognitively normal controls reported group differences that varied by DTI measurement and tract [53]. FA was significantly different among groups only in the fornix; DA and MD in all white matter tracts except the fornix; and DR was altered in the fornix and interior longitudinal fasciculus. These data suggest that the sensitivity of the DTI measurements may vary by region and tract of interest.
In the present ROI-based study of participants with MCI, we also examined DA and DR in addition to FA and MD. While DA and DR were more strongly associated with cognitive performance cross-sectionally (Table 2), fornix FA and MD were more strongly predictive of longitudinal decline. Thus, it is also possible that the sensitivity of each DTI measures varies by diagnostic group and over time. Ultimately, a longitudinal study of normal controls, MCI and AD participants using various approaches (ROI-based, voxel-based and tract-based) are needed to better understand these conflicting results and to determine the relationship of the methodology to any differences in findings between FA, MD, DA and DR. This is particularly important in further understanding the pathology of AD in terms of whether damage to white matter is due to secondary degeneration or primary myelin damage [45].
Limitations of this study warrant consideration. First, while the expected percentage (10–15%/year) of individuals with MCI converted to AD, the overall sample size was small. Second, a possible confounder in this study is that atrophy of the fornix could also contribute to decreased FA. The smaller the fornix, the more CSF could be included in a given voxel size. Since CSF has FA near 0, the partial volume effect of increased CSF in a voxel would cause the mean FA within that voxel to be decreased. While we cannot completely eliminate the partial volume effect, we did use high-resolution MPRAGE and Large Deformation Diffeomorphic Metric Mapping (LDDMM) analysis, in an attempt to reduce the likelihood of this problem.
A recently-published phase I clinical trial evaluating the effects of fornix deep brain stimulation (F-DBS) in AD patients supports the relevance of the fornix to the pathophysiology of AD [54]. Thus, this biomarker could be a useful measure to enrich a clinical trial with those most likely to progress.
Acknowledgments
The authors would like to thank Dr. Gwenn Smith for comments on the manuscript. This research was funded in part by grants from GlaxoSmithKline, the National Institute on Aging (P50-AG005146, P50-AG021334, R21AG033774, and R21NS060271-01) and the National Institute of Research Resources (NCRR, P41-RR15241). NCRR is a component of the National Institutes of Health (NIH). The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
The corresponding author had full access to all the data in the study and had final responsibility of the decision to submit for publication
All other authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–3. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 3.Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–81. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 4.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–9. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie K, Touchon J. Mild cognitive impairment: conceptual basis and current nosological status. Lancet. 2000;355:225–8. doi: 10.1016/S0140-6736(99)06155-3. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 8.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teipel SJ, Meindl T, Grinberg L, Heinsen H, Hampel H. Novel MRI techniques in the assessment of dementia. Eur J Nucl Med Mol Imaging. 2008;35:S58–69. doi: 10.1007/s00259-007-0703-z. [DOI] [PubMed] [Google Scholar]
- 12.Apostolova LG, Morra JH, Green AE, Hwang KS, Avedissian C, Woo E, et al. Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. Neuroimage. 2010;5:488–99. doi: 10.1016/j.neuroimage.2009.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.09.005. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol Aging. 2010;31:1089–98. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Ringman JM, O’Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer’s disease mutations. Brain. 2007;130:1767–76. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- 18.Mielke MM, Kozauer NA, Chan KC, Cheorge M, Toroney J, Zerrate M, et al. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2009;46:47–55. doi: 10.1016/j.neuroimage.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Alzheimer’s Association: Research Consent for Cognitively Impaired Adults. Recommendations for Institutional Review Boards and Investigators. Alzheimer Dis Assoc Disord. 2004;18:171–175. doi: 10.1097/01.wad.0000137520.23370.56. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S13–21. [PubMed] [Google Scholar]
- 24.Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–30. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. The Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1988. [Google Scholar]
- 26.Reitan R. Trail Making Test: Manual for administration, scoring and interpretation. Indianapolis: Department of Neurology, Indiana University Medical Center; 1958. [Google Scholar]
- 27.Benton A, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1976. [Google Scholar]
- 28.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–80. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 29.Mielke MM, Haughey NJ, Ratnam Bandaru VV, Schech S, Carrick R, Carlson MC, et al. Plasma ceramides are altered in mild cognitive impairment and predict cognitive decline and hippocampal volume loss. Alzheimers Dement. 2010;6:378–85. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95:11406–11. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umeyama S. Least-squares estimation of transformation parameters between two point patterns. IEEE Trans Pattern Analysis and Machine Intelligence. 1991;13:376–380. [Google Scholar]
- 32.Joshi S, Miller MI. Landmark matching via large deformation diffeomorphisms. Image Processing, IEEE Trans Image Process. 2000;9:1357–1370. doi: 10.1109/83.855431. [DOI] [PubMed] [Google Scholar]
- 33.Priebe C, Marchette DJ. Alternating kernel and mixture density estimates. Comput Stat Data Anal. 2000;35:43–65. [Google Scholar]
- 34.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 36.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 37.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–62. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 38.Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer’s type. Biochemical and neuropathological correlates. Brain. 1988;111:1425–39. doi: 10.1093/brain/111.6.1425. [DOI] [PubMed] [Google Scholar]
- 39.Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25:843–51. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–67. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 41.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–55. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- 43.Kowall NW, Kosik KS. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer’s disease. Ann Neurol. 1987;22:639–43. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- 44.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–34. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 45.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–85. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 46.Fellgiebel A, Wille P, Muller MJ, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord. 2004;18:101–8. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- 47.Kantarci K, Petersen RC, Boeve BF, et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005;64:902–4. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller MJ, Greverus D, Dellani PR, et al. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28(4):1033–42. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 49.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72(17):1519–25. doi: 10.1212/WNL.0b013e3181a2e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–9. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133:529–39. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- 53.Pievani M, Agosta F, Pagani E, et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31:1862–75. doi: 10.1002/hbm.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laxton AW, Tang-Wai DF, McAndrews MP, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68:521–34. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]


