Abstract
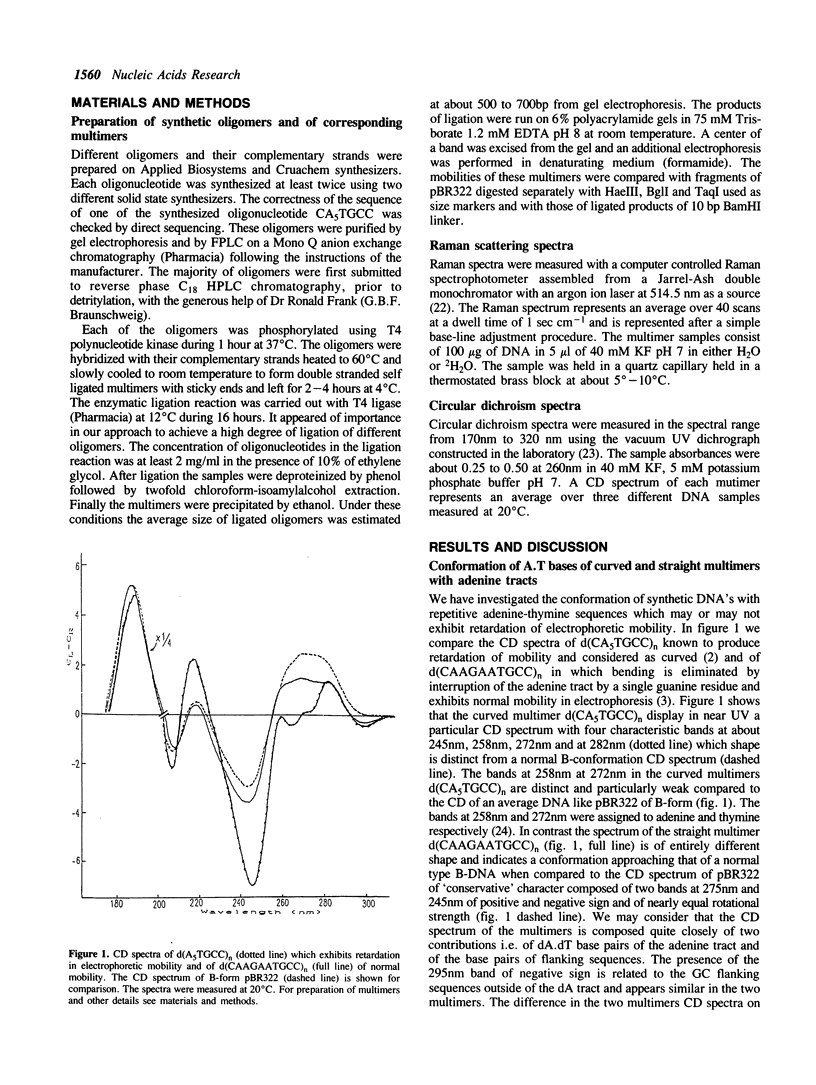
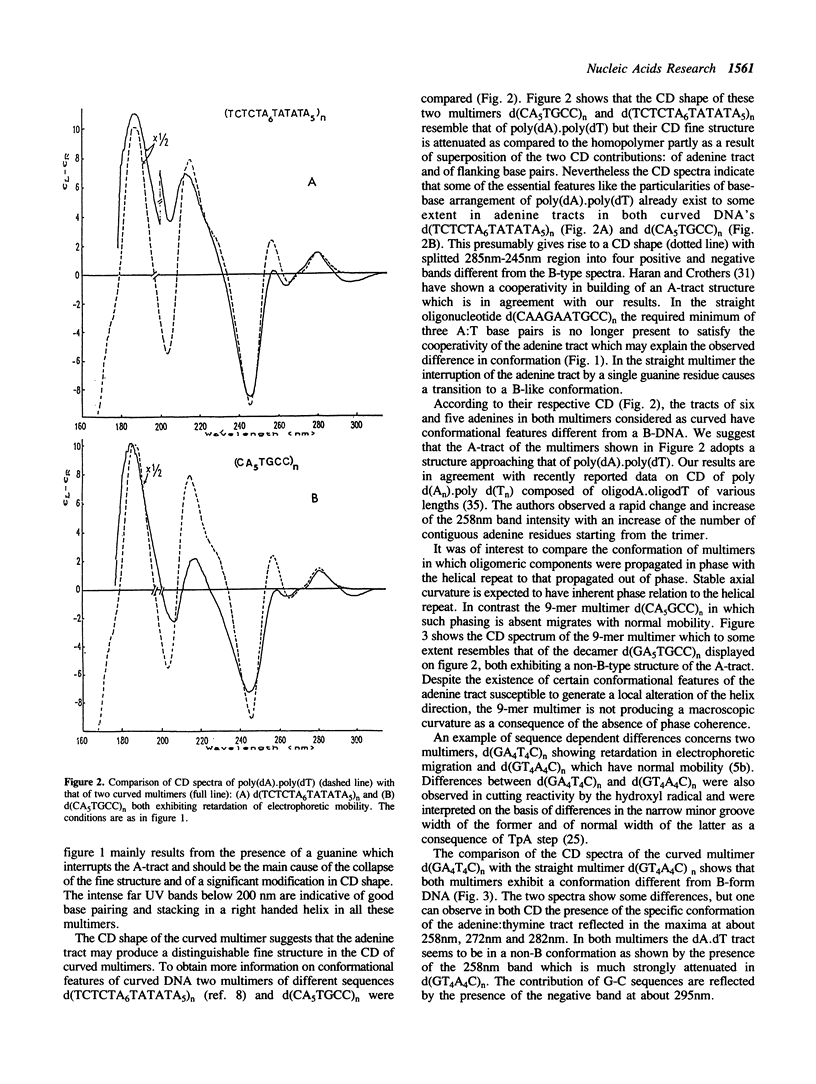
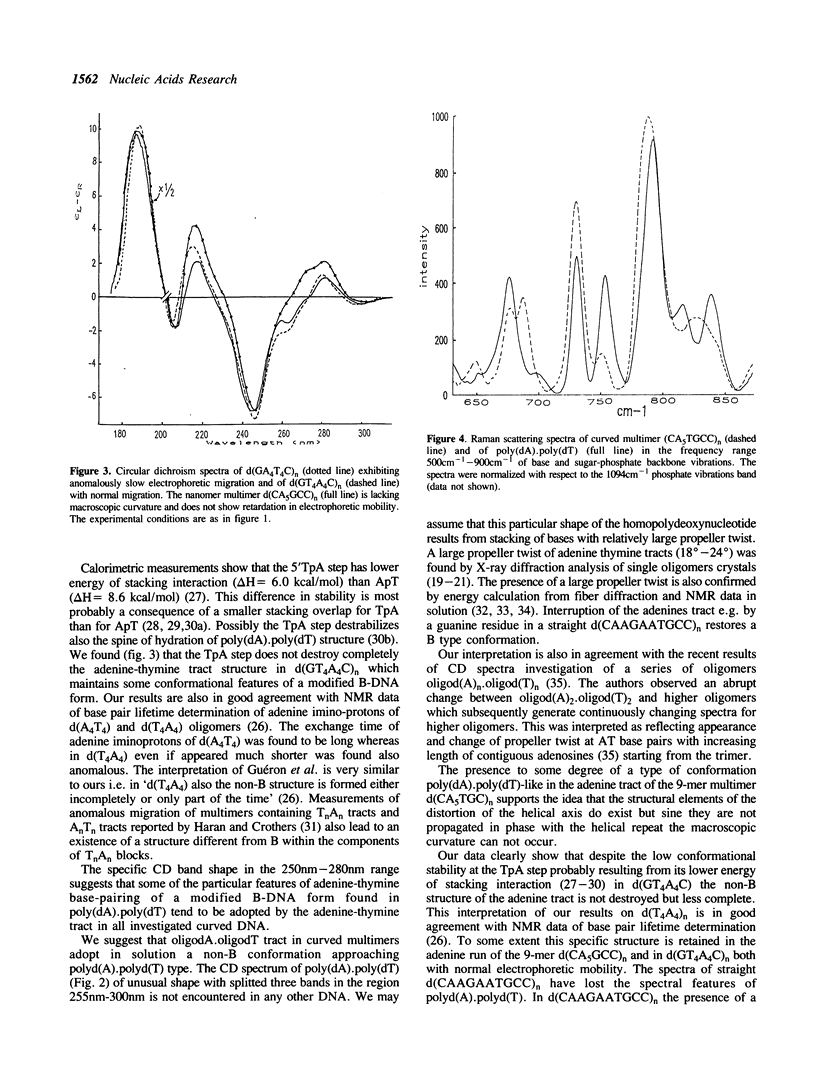
The conformation of DNA's with adenine-thymine tracts exhibiting retardation in electrophoretic migration and considered as curved were investigated in solution by CD and RAMAN spectroscopy. The following curved multimers with adenine tracts but of different flanking sequences d(CA5TGCC)n, d(TCTCTA6TATATA5)n, d(GA4T4C)n yield CD spectroscopic features indicating a non-B structure of the dA.dT tract with similarities to polyd(A).polyd(T). We suggest that adenine-thymine bases in these multimers contain some of the distinctive conformational features of poly(A).polyd(T) probably with large propeller twist found by NMR (Behling and Kearns, 1987) and by X-ray diffraction on oligonucleotides containing a tract of adenines (Nelson et al. 1987, Coll et al; 1987; DiGabriele et al. 1989). Some elements of distinctive CD features of the contiguous adenines run are also observed in the straight multi-9-mer d(CA5GCC)n which lacks in-phase relation to the helical repeat. Despite the presence of the TpA step in the straight multimer d(GT4A4)n, the altered dA.dT conformation is not completely destroyed. Interruption of adenine tract by a guanine in d(CAAGAATGCC)n leads to a B-like conformation and to a normal electrophoretic mobility. The Raman spectra reveal a rearrangement of the sugar-phosphate backbone of dA.dT tract in the multimer d(CA5TGCC)n with respect to that of polydA.polydT. This is reflected in the presence of an unique Raman band associated to C2'-endo sugar with a predominant contribution of C1'-exo puckering which is exhibited by the multimer whereas two distinct Raman bands characterize poly(dA).poly(dT) backbone conformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexeev D. G., Lipanov A. A., Skuratovskii IYa Poly(dA).poly(dT) is a B-type double helix with a distinctively narrow minor groove. 1987 Feb 26-Mar 4Nature. 325(6107):821–823. doi: 10.1038/325821a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Behling R. W., Kearns D. R. 1H two-dimensional nuclear Overhauser effect and relaxation studies of poly(dA).poly(dT) Biochemistry. 1986 Jun 3;25(11):3335–3346. doi: 10.1021/bi00359a037. [DOI] [PubMed] [Google Scholar]
- Benevides J. M., Wang A. H., van der Marel G. A., van Boom J. H., Thomas G. J., Jr Crystal and solution structures of the B-DNA dodecamer d(CGCAAATTTGCG) probed by Raman spectroscopy: heterogeneity in the crystal structure does not persist in the solution structure. Biochemistry. 1988 Feb 9;27(3):931–938. doi: 10.1021/bi00403a014. [DOI] [PubMed] [Google Scholar]
- Brahms S., Brahms J. Determination of protein secondary structure in solution by vacuum ultraviolet circular dichroism. J Mol Biol. 1980 Apr;138(2):149–178. doi: 10.1016/0022-2836(80)90282-x. [DOI] [PubMed] [Google Scholar]
- Brahms S., Nakasu S., Kikuchi A., Brahms J. G. Structural changes in positively and negatively supercoiled DNA. Eur J Biochem. 1989 Sep 15;184(2):297–303. doi: 10.1111/j.1432-1033.1989.tb15019.x. [DOI] [PubMed] [Google Scholar]
- Brahms S., Vergne J., Brahms J. G., Di Capua E., Bucher P., Koller T. Natural DNA sequences can form left-handed helices in low salt solution under conditions of topological constraint. J Mol Biol. 1982 Dec 5;162(2):473–493. doi: 10.1016/0022-2836(82)90539-3. [DOI] [PubMed] [Google Scholar]
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. Structural details of an adenine tract that does not cause DNA to bend. Nature. 1988 Feb 4;331(6155):455–457. doi: 10.1038/331455a0. [DOI] [PubMed] [Google Scholar]
- Calladine C. R., Drew H. R., McCall M. J. The intrinsic curvature of DNA in solution. J Mol Biol. 1988 May 5;201(1):127–137. doi: 10.1016/0022-2836(88)90444-5. [DOI] [PubMed] [Google Scholar]
- Calladine C. R., Drew H. R. Principles of sequence-dependent flexure of DNA. J Mol Biol. 1986 Dec 20;192(4):907–918. doi: 10.1016/0022-2836(86)90036-7. [DOI] [PubMed] [Google Scholar]
- Calladine C. R. Mechanics of sequence-dependent stacking of bases in B-DNA. J Mol Biol. 1982 Oct 25;161(2):343–352. doi: 10.1016/0022-2836(82)90157-7. [DOI] [PubMed] [Google Scholar]
- Chuprina V. P. Anomalous structure and properties of poly (dA).poly(dT). Computer simulation of the polynucleotide structure with the spine of hydration in the minor groove. Nucleic Acids Res. 1987 Jan 12;15(1):293–311. doi: 10.1093/nar/15.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGabriele A. D., Sanderson M. R., Steitz T. A. Crystal lattice packing is important in determining the bend of a DNA dodecamer containing an adenine tract. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1816–1820. doi: 10.1073/pnas.86.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Diekmann S. Temperature and salt dependence of the gel migration anomaly of curved DNA fragments. Nucleic Acids Res. 1987 Jan 12;15(1):247–265. doi: 10.1093/nar/15.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann S., Wang J. C. On the sequence determinants and flexibility of the kinetoplast DNA fragment with abnormal gel electrophoretic mobilities. J Mol Biol. 1985 Nov 5;186(1):1–11. doi: 10.1016/0022-2836(85)90251-7. [DOI] [PubMed] [Google Scholar]
- Edmondson S. P., Johnson W. C., Jr Base tilt of poly[d(A)]-poly[d(T)] and poly[d(AT)]-poly[d(AT)] in solution determined by linear dichroism. Biopolymers. 1985 May;24(5):825–841. doi: 10.1002/bip.360240508. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Bond P. J., Peticolas W. L. Characterization of the A in equilibrium B transition of DNA in fibers and gels by laser Raman spectroscopy. Biopolymers. 1975 Jun;14(6):1245–1257. doi: 10.1002/bip.1975.360140613. [DOI] [PubMed] [Google Scholar]
- Fratini A. V., Kopka M. L., Drew H. R., Dickerson R. E. Reversible bending and helix geometry in a B-DNA dodecamer: CGCGAATTBrCGCG. J Biol Chem. 1982 Dec 25;257(24):14686–14707. [PubMed] [Google Scholar]
- Goodwin D. C., Brahms J. Form of DNA and the nature of interactions with proteins in chromatin. Nucleic Acids Res. 1978 Mar;5(3):835–850. doi: 10.1093/nar/5.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J., Bleyman M., Rauch C. A., Kitchin P. A., Englund P. T. Visualization of the bent helix in kinetoplast DNA by electron microscopy. Cell. 1986 Aug 29;46(5):717–724. doi: 10.1016/0092-8674(86)90347-8. [DOI] [PubMed] [Google Scholar]
- Gudibande S. R., Jayasena S. D., Behe M. J. CD studies of double-stranded polydeoxynucleotides composed of repeating units of contiguous homopurine residues. Biopolymers. 1988 Dec;27(12):1905–1915. doi: 10.1002/bip.360271205. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Evidence for the existence of stable curvature of DNA in solution. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4632–4636. doi: 10.1073/pnas.81.15.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence-directed curvature of DNA. Nature. 1986 May 22;321(6068):449–450. doi: 10.1038/321449a0. [DOI] [PubMed] [Google Scholar]
- Haran T. E., Crothers D. M. Cooperativity in A-tract structure and bending properties of composite TnAn blocks. Biochemistry. 1989 Apr 4;28(7):2763–2767. doi: 10.1021/bi00433a003. [DOI] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Crothers D. M. Calibration of DNA curvature and a unified description of sequence-directed bending. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1763–1767. doi: 10.1073/pnas.85.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H. S., Crothers D. M. Chemical determinants of DNA bending at adenine-thymine tracts. Biochemistry. 1987 Jun 16;26(12):3745–3748. doi: 10.1021/bi00386a070. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Martinson H. G. Nucleosomes will not form on double-stranded RNa or over poly(dA).poly(dT) tracts in recombinant DNA. Nucleic Acids Res. 1981 Dec 21;9(24):6869–6888. doi: 10.1093/nar/9.24.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J. L., Charretier E., Kochoyan M., Guéron M. Evidence from base-pair kinetics for two types of adenine tract structures in solution: their relation to DNA curvature. Biochemistry. 1988 Dec 13;27(25):8894–8898. doi: 10.1021/bi00425a004. [DOI] [PubMed] [Google Scholar]
- Lipanov A. A., Chuprina V. P. The structure of poly(dA):poly(dT) in a condensed state and in solution. Nucleic Acids Res. 1987 Jul 24;15(14):5833–5844. doi: 10.1093/nar/15.14.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Prunell A., Goulet I., Jacob Y., Goutorbe F. The smaller helical repeat of poly(dA) . poly(dT) relative to DNA may reflect the wedge property of the dA . dT base pair. Eur J Biochem. 1984 Jan 16;138(2):253–257. doi: 10.1111/j.1432-1033.1984.tb07909.x. [DOI] [PubMed] [Google Scholar]
- Prunell A. Nucleosome reconstitution on plasmid-inserted poly(dA) . poly(dT). EMBO J. 1982;1(2):173–179. doi: 10.1002/j.1460-2075.1982.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov E. N., Sussman J. L. The pitch of chromatin DNA is reflected in its nucleotide sequence. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3816–3820. doi: 10.1073/pnas.77.7.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky L. E., Trifonov E. N. Estimation of wedge components in curved DNA. Nature. 1987 Apr 16;326(6114):720–722. doi: 10.1038/326720a0. [DOI] [PubMed] [Google Scholar]
- Ulanovsky L., Bodner M., Trifonov E. N., Choder M. Curved DNA: design, synthesis, and circularization. Proc Natl Acad Sci U S A. 1986 Feb;83(4):862–866. doi: 10.1073/pnas.83.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswamitra M. A., Kennard O., Jones P. G., Sheldrick G. M., Salisbury S., Favello L., Shakked Z. DNA double helical fragment at atomic resolution. Nature. 1978 Jun 22;273(5664):687–688. doi: 10.1038/273687a0. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Harrell J. T. Characteristics and variations of B-type DNA conformations in solution: a quantitative analysis of Raman band intensities of eight DNAs. Biochemistry. 1986 May 6;25(9):2664–2671. doi: 10.1021/bi00357a056. [DOI] [PubMed] [Google Scholar]
- Westhof E. Re-refinement of the B-dodecamer d(CGCGAATTCGCG) with a comparative analysis of the solvent in it and in the Z-hexamer d(5BrCG5BrCG5BrCG). J Biomol Struct Dyn. 1987 Dec;5(3):581–600. doi: 10.1080/07391102.1987.10506414. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yoon C., Privé G. G., Goodsell D. S., Dickerson R. E. Structure of an alternating-B DNA helix and its relationship to A-tract DNA. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6332–6336. doi: 10.1073/pnas.85.17.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kitzing E., Diekmann S. Molecular mechanics calculations of dA12.dT12 and of the curved molecule d(GCTCGAAAAA)4.d(TTTTTCGAGC)4. Eur Biophys J. 1987;15(1):13–26. doi: 10.1007/BF00255031. [DOI] [PubMed] [Google Scholar]


