Abstract
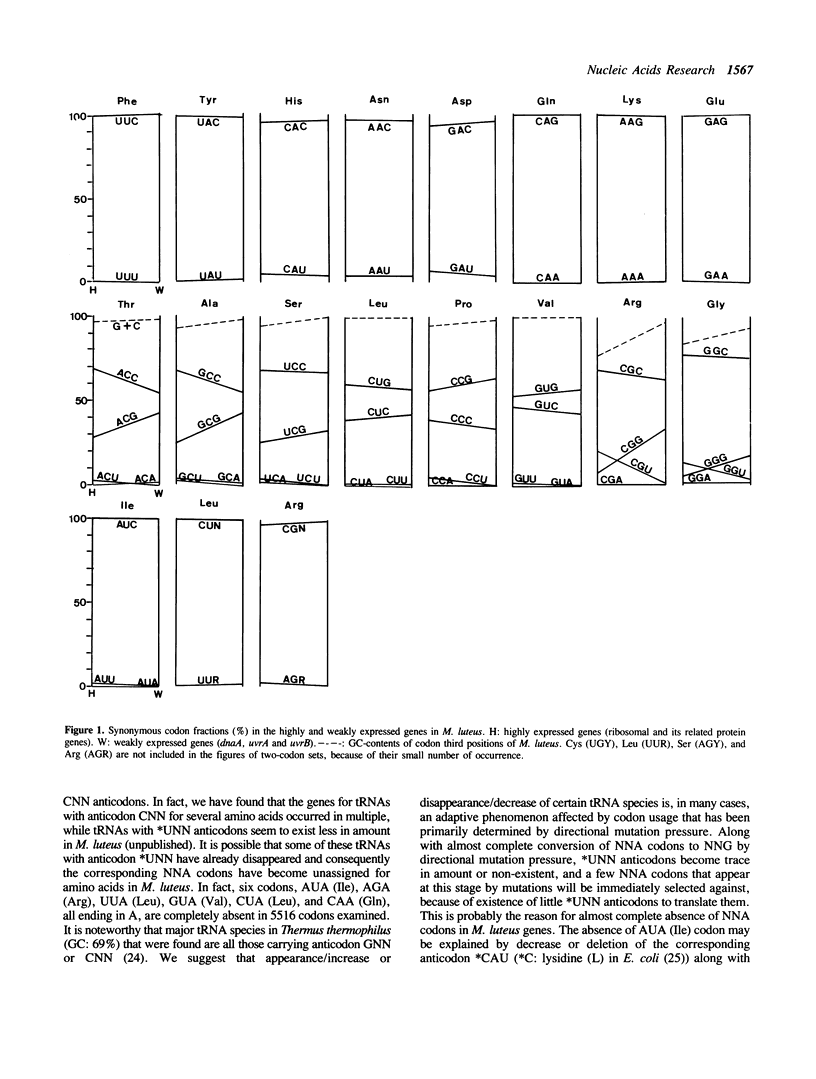
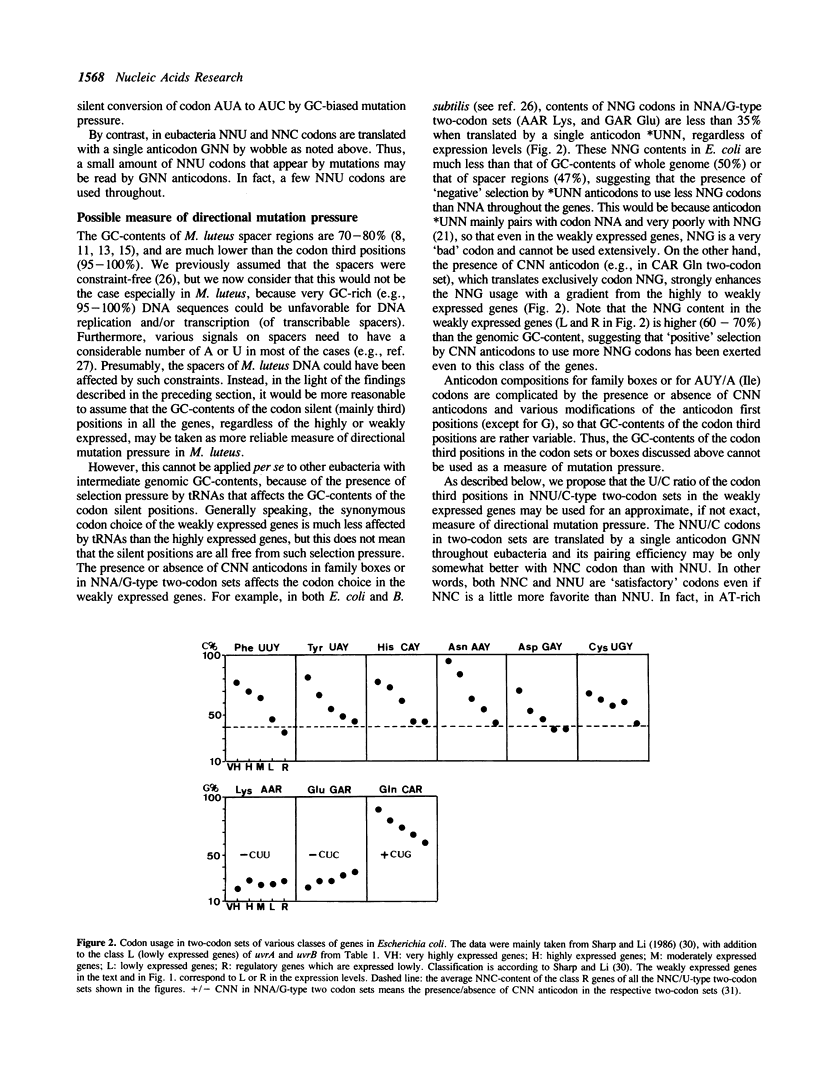
The GC (G + C, or G or C)-contents of codon silent positions in all two-codon sets and three codons AUY/A (IIe), and in most of the family boxes of Micrococcus luteus (genomic GC-content: 74%) are 95% to 100% in both the highly and weakly expressed genes. In some family boxes, there is a decrease in NNC codons and an increase in NNG codons from the highly expressed to weakly expressed genes without apparent involvement of NNU and NNA codons. From these observations, we conclude that the selective use of synonymous codons in M. luteus may be largely determined by GC-biased mutation pressure and that in the highly expressed genes tRNAs would act as a weak selection pressure in some family boxes. Available data suggest that the effect of selection pressure by tRNAs on the synonymous codon choice becomes more apparent in the highly expressed genes in eubacteria with intermediate GC-contents such as Escherichia coli and Bacillus subtilis, and that the U/C ratio of the codon third positions in NNU/C-type two-codon sets in the weakly expressed genes would represent the approximate magnitude of directional mutation pressure throughout eubacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Arikan E., Kulkarni M. S., Thomas D. C., Sancar A. Sequences of the E. coli uvrB gene and protein. Nucleic Acids Res. 1986 Mar 25;14(6):2637–2650. doi: 10.1093/nar/14.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1982;1(9):1043–1048. doi: 10.1002/j.1460-2075.1982.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Sancar A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J Biol Chem. 1986 Apr 15;261(11):4895–4901. [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Jukes T. H., Bhushan V. Silent nucleotide substitutions and G + C content of some mitochondrial and bacterial genes. J Mol Evol. 1986;24(1-2):39–44. doi: 10.1007/BF02099949. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Yokoyama S., Horie N., Matsuda A., Ueda T., Yamaizumi Z., Kuchino Y., Nishimura S., Miyazawa T. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J Biol Chem. 1988 Jul 5;263(19):9261–9267. doi: 10.1351/pac198961030573. [DOI] [PubMed] [Google Scholar]
- Muto A., Osawa S. The guanine and cytosine content of genomic DNA and bacterial evolution. Proc Natl Acad Sci U S A. 1987 Jan;84(1):166–169. doi: 10.1073/pnas.84.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Fujita N., Ohama T., Osawa S., Ishihama A. Micrococcus luteus, a bacterium with a high genomic G + C content, contains Escherichia coli-type promoters. Mol Gen Genet. 1989 Sep;218(3):384–389. doi: 10.1007/BF00332399. [DOI] [PubMed] [Google Scholar]
- Ohama T., Muto A., Osawa S. Spectinomycin operon of Micrococcus luteus: evolutionary implications of organization and novel codon usage. J Mol Evol. 1989 Nov;29(5):381–395. doi: 10.1007/BF02602908. [DOI] [PubMed] [Google Scholar]
- Ohama T., Yamao F., Muto A., Osawa S. Organization and codon usage of the streptomycin operon in Micrococcus luteus, a bacterium with a high genomic G + C content. J Bacteriol. 1987 Oct;169(10):4770–4777. doi: 10.1128/jb.169.10.4770-4777.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo S., Muto A., Kawauchi Y., Yamao F., Osawa S. The ribosomal protein gene cluster of Mycoplasma capricolum. Mol Gen Genet. 1987 Dec;210(2):314–322. doi: 10.1007/BF00325700. [DOI] [PubMed] [Google Scholar]
- Osawa S., Jukes T. H. Evolution of the genetic code as affected by anticodon content. Trends Genet. 1988 Jul;4(7):191–198. doi: 10.1016/0168-9525(88)90075-3. [DOI] [PubMed] [Google Scholar]
- Osawa S., Ohama T., Yamao F., Muto A., Jukes T. H., Ozeki H., Umesono K. Directional mutation pressure and transfer RNA in choice of the third nucleotide of synonymous two-codon sets. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1124–1128. doi: 10.1073/pnas.85.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- SUEOKA N. On the genetic basis of variation and heterogeneity of DNA base composition. Proc Natl Acad Sci U S A. 1962 Apr 15;48:582–592. doi: 10.1073/pnas.48.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Clarke N. D., Griswold J., Kennedy W. J., Rupp W. D. Identification of the uvrB gene product. J Mol Biol. 1981 May 5;148(1):63–76. doi: 10.1016/0022-2836(81)90235-7. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24(1-2):28–38. doi: 10.1007/BF02099948. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D. C., Sharp P. M. Synonymous codon usage in Bacillus subtilis reflects both translational selection and mutational biases. Nucleic Acids Res. 1987 Oct 12;15(19):8023–8040. doi: 10.1093/nar/15.19.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota S., Nakayama H. Evidence for a Micrococcus luteus gene homologous to uvrB of Escherichia coli. Mol Gen Genet. 1988 Jul;213(1):21–29. doi: 10.1007/BF00333393. [DOI] [PubMed] [Google Scholar]
- Shiota S., Nakayama H. Micrococcus luteus homolog of the Escherichia coli uvrA gene: identification of a mutation in the UV-sensitive mutant DB7. Mol Gen Genet. 1989 Jun;217(2-3):332–340. doi: 10.1007/BF02464901. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. M., Archer R. H., Lindahl L. The nucleotide sequence of the Escherichia coli fus gene, coding for elongation factor G. Nucleic Acids Res. 1984 Feb 24;12(4):2181–2192. doi: 10.1093/nar/12.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Zurawski S. M. Structure of the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1985 Jun 25;13(12):4521–4526. doi: 10.1093/nar/13.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]


