Abstract
Extinction of drug-seeking behavior is a form of new and active learning. Facilitation of extinction learning is of clinical interest since cue exposure therapies for the treatment of addiction have largely been unsuccessful in preventing relapse, primarily due to the context specificity of extinction learning. Recently, several studies have shown that potentiation of glutamatergic transmission can facilitate extinction learning in rodent models of cocaine addiction. In this study we investigated the effects of the type 5 metabotropic glutamate receptor (mGluR5) positive allosteric modulator (PAM) 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) on the extinction and contextual reinstatement of methamphetamine-seeking behavior. Rats were trained and allowed to self-administer methamphetamine (0.1 mg/kg/infusion) in 2 hr daily sessions in Context A where self-administration chambers had distinct tactile, visual, auditory, and olfactory cues. Next, CDPPB (60 mg/kg) or vehicle was administered prior to subsequent extinction training sessions that were conducted in modified self-administration chambers (Context B) that were Context A. Following 16 days of extinction training in Context B, animals were placed back in Context A for assessment of contextual reinstatement of methamphetamine-seeking behavior. CDPPB failed to produce significant reductions in extinction responding or in the magnitude of contextual reinstatement of methamphetamine-seeking compared to vehicle treated controls. We postulate that numerous factors, including methamphetamine-induced changes in mGluR5 receptor expression or function, may have contributed to the observed lack of effects. Although these findings initially suggest that mGluR5 PAMs may be ineffective in facilitating extinction learning or preventing context-induced relapse in methamphetamine addiction, additional studies are warranted examining effects of other mGluR5 PAMs, particularly those with improved pharmacological properties and devoid of potential side effects at higher doses.
Keywords: Methamphetamine, mGluR5, Positive allosteric modulator, Extinction, Learning, Self-administration, Context, Renewal, Reinstatement
Introduction
Two of the most problematic obstacles to the successful treatment of drug addiction are the persistence of drug-seeking behavior during attempts at abstinence and the motivational salience of drug-associated cues and contexts. As a result of the associative over-learning that occurs during chronic drug use in the presence of specific environmental cues and contexts, drug-associated stimuli can trigger memories of prior drug use and drug craving, which increase the propensity for drug-seeking behavior and ultimately relapse[1,2]. Unfortunately, cue-exposure therapies based on extinction of the motivational salience of drug-associated cues has proven to be disappointingly ineffective in reducing relapse in drug addicts [3–5]. The ineffectiveness of these approaches has been ascribed to the inadequate consolidation and/or context-specificity of extinction learning [6–9].
Recently, various studies have demonstrated that pharmacological potentiation of glutamatergic transmission with ligands such as the N-methyl-D-aspartate (NMDA) partial agonist D-cycloserine (DCS), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor potentiators, or the cystine prodrug N-acetylcysteine reduce extinction responding following active drug self-administration, reduce re-acquisition of drug intake, and/or facilitate the extinction of a drug-induced conditioned place preference (CPP) [10–16]. These data suggest that potentiators of glutamatergic transmission may represent a novel class of adjunctive therapeutics that may facilitate extinction learning not only in the context of drug addiction, but other psychiatric disorders such as pathological anxiety [17–21].
Potentiation of the function of the type 5 metabotropic glutamate receptor (mGluR5) is an attractive target for enhancing the synaptic plasticity associated with glutamatergic neurotransmission that is thought to underlie learning and memory processes in both normal and pathological states. mGluR5 receptors are predominantly localized to the perisynaptic annulus of postsynaptic dendritic spines, where they are positively coupled to NMDA receptor function and mediate various forms of synaptic plasticity including learning and memory [22,23]. mGluR5 positive allosteric modulators (PAMs) enhance synaptic plasticity in the hippocampus [24], and improve spatial learning [24], recognition memory [25,26], and reverse deficits in various forms of learning induced by NMDA receptor antagonism [27–29]. In contrast, mGluR5 receptor antagonism (or genetic deletion) has been shown to have deleterious effects on learning and memory [30] including the extinction of conditioned fear [31]. These findings suggest an important role for mGluR5 receptors in various aspects of learning and memory, including extinction learning.
We have recently demonstrated that the mGluR5 PAM 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide (CDPPB) facilitates the extinction of a cocaine-induced CPP [32] as well as cocaine-seeking following intravenous self-administration [33]. To determine if these findings generalize to extinction following self-administration of other psychostimulants, the present study sought to determine if enhancement of mGluR5 receptor function would enhance extinction learning following intravenous methamphetamine self-administration. Since mGluR5 PAMs have been shown to increase synaptic plasticity in the hippocampus and improve performance in hippocampus-dependent learning tasks [24], we also sought to determine if CDPPB would reduce or eliminate contextual reinstatement of methamphetamine-seeking behavior, which is known to be mediated by the hippocampus [34,35].
Methods
Subjects
All experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Thirty male Sprague-Dawley rats (Harlan, Indianapolis, IN), approximately 60 days old (250–275 g), were received at MUSC in three cohorts, each spaced approximately two months apart. Prior to shipment, rats were surgically implanted with silastic rounded-tip jugular vein catheters by Harlan Laboratories Surgical Services, and catheters were filled with HepLock solution (50% v/v glycerol and 50% 70 U/ml heparinized saline) to maintain patency during shipment. Upon arrival, rats were individually housed in standard Plexiglas cages with recycled paper bedding in an environmentally-controlled room (230C, ~65% humidity) on a 12-hour reversed light-dark cycle (lights off at 0800). All rats were allowed a one-week acclimation period prior to vascular access port implantation, and a one week recovery period following surgery during which all rats received free access to food and water. Following recovery from surgery, rats were maintained under mild food restriction conditions in which each rat received 20 g of food/day and this schedule was maintained throughout the remainder of the experiment. Animals were weighed daily to ensure adequate body weight. All behavioral testing (except for the overnight lever press training session) took place Monday – Friday during the rat’s dark cycle between 1000 and 1200 h.
Apparatus
Behavioral testing was conducted in 12 self-administration chambers (ENV-008; Med Associates Inc., St. Albans, VT) that were interfaced to a PC computer and located in sound attenuating melamine enclosures equipped with ventilation fans. The chambers (28 × 27 × 22 cm) consisted of two aluminum walls and two clear Plexiglas walls. The ceiling was also constructed of Plexiglas with a 3-cm diameter hole cut in the center to allow a drug delivery tether to pass through. The floor consisted of parallel stainless steel rods (0.48 cm diameter) placed 1.6 cm apart. Each chamber contained a 45mg pellet dispenser, a house light located 1.25 cm from the ceiling, a Sonalert speaker that provided an auditory stimulus (~ 65 dB, 2900 Hz) during drug infusion, one retractable response lever, one stationary response lever, and two 2.5 cm stimulus cue lights located above each response lever. Response levers were located 7 cm above the floor of the chamber. Centered between the levers was a 5x5 cm food pellet receptacle. Each chamber was outfitted with a single-speed automated drug infusion pump (PHM-100; Med Associates). Tygon microbore tubing (0.5 mm ID) was used to connect the syringe containing the drug solution to a single-channel liquid swivel that was mounted to the top of the chamber enclosure. The swivel was then connected to the vascular access port using Tygon microbore tubing that was protected by a stainless steel tether (Plastics One, Roanoke, VA). All experimental parameters were controlled using Med PC IV software (MedAssociates). In order to assess contextual reinstatement using the ABA renewal paradigm [36], self-administration chambers were temporarily modified to create two distinct contexts (Contexts A and B) that differed in visual, auditory, olfactory, and tactile cues. For Context A (self-administration and contextual reinstatement), the self-administration chambers were used as described above. In addition, a vanilla scented foam air freshener was used as an olfactory stimulus, and the chamber fan was activated to provide an auditory stimulus. For Context B (extinction), a smooth acrylic floor was inserted on top of the stainless steel bar floor to create a different tactile environment, and acrylic panels alternating in 2.5 cm vertical black and white stripes were added to the front and rear clear Plexiglas walls to create different visual properties of the chamber. In addition, the ventilation fan was not activated during testing in Context B to provide a different auditory environment, and a pine scented air freshener was used as a different olfactory cue. All other aspects of the chambers remained the same during testing in Context B.
Vascular access port implantation
Rats with pre-implanted jugular vein catheters were anesthetized with isoflurane (2%) vaporized in medical grade breathing air at a flow rate of 0.4 L/min. Following induction of anesthesia, carprofen (2.5 mg/kg s.c) was administered to minimize post-surgical discomfort. The skin area between the scapulae where the catheter exited the dorsum was shaved and scrubbed with betadine and 0.1% v/v H2O2. A 2 cm incision was made to connect the catheter to a backmount threaded vascular access port (Plastics One). The access port was stabilized to the surrounding tissue using a polyethylene mesh collar and the wound was sutured closed with 3-0 Vicryl sutures and treated with 2% bacitracin/polymixin B/neomycin and 5% xylocaine (Henry Schein Veterinary Supply, Melville, NY). The HepLock solution was then evacuated from the catheter and the catheter was flushed with 0.2 ml heparin (70 U/ml). The access port was then sealed with a piece of Tygon tubing closed at one end and a protective cap was screwed onto the access port. Rats were then allowed 7 days of recovery from surgery, during which they received daily intravenous infusions of 0.1 ml antibiotic solution (100 mg/ml cefazolin) and 0.2 ml heparin (70 U/ml) to minimize postsurgical infection and maintain catheter patency. Rats were also given daily injections of carprofen (2.5 mg/kg s.c.) to minimize postsurgical discomfort.
Self-administration procedures
Training
Following recovery from surgery, all rats underwent food-reinforced lever press training that consisted of a single, 16 hr overnight session in which presses on the active lever were reinforced with a single 45 mg food pellet (Bio-Serv, Frenchtown, NJ) on an FR1 schedule of reinforcement. Presses on the other (inactive) lever had no programmed consequences. Most rats acquired operant responding and lever discrimination during the single overnight training session; however, several rats required a second overnight training session conducted during the subsequent night in order to learn the operant task.
Methamphetamine self-administration (context A)
Self-administration of methamphetamine procedures began on the day following lever press training and were conducted in Context A. Prior to each session, rats were weighed, injected with 0.1 mL of heparin to ensure catheter patency, placed in the test chambers, and infusion tethers were connected to the vascular access port. Upon initiation of the session, the active lever was inserted into the chamber, the houselight was illuminated, and the ventilation fan was activated. Following each press on the active lever (FR1 schedule of reinforcement), the syringe pump was activated for 2 sec to deliver methamphetamine (0.1 mg/kg) in a volume of 0.06 ml. During the 2 sec drug infusion, the stimulus light above the lever was illuminated and the tone was presented. Following each methamphetamine infusion, presentation of the stimulus light and tone was terminated and a 20 sec timeout commenced during which additional active lever presses were recorded but had no programmed consequences. Responses on the inactive lever were also recorded but had no programmed consequence at any time during the experiment. Methamphetamine self-administration sessions were 2 hr in duration and were conducted daily (M-F) from 1000–1200 for a total of 12 sessions. Following the last session of self-administration training, a matching procedure based on the number of active lever presses was used to assign the rats to either the CDPPB (n=16) or vehicle control group (n=14) with equal levels of responding.
Extinction (context B)
Extinction of lever pressing was conducted in Context B and rats were assigned to different chambers than those during drug self-administration to create a spatially unique environment. Twenty min prior to each extinction session, rats were weighed and given a subcutaneous injection of CDPPB (60 mg/kg) or vehicle (10% v/v Tween 80) in a volume of 1 ml/kg. This dose was chosen based on our previous findings of effectiveness in reducing extinction responding following cocaine self-administration [33], as well as an observed lack of efficacy of a lower (30 mg/kg) dose (n=6, data not shown). During the extinction sessions, rats were not connected to the infusion tether and did not receive any drug infusions (to further distinguish the extinction context from Context A). All other contingencies and drug-related cues present during self-administration of methamphetamine (i.e., activation of the infusion pump, presentation of the stimulus light and tone, and a 20-sec timeout period) were present during extinction. Extinction sessions were 2 hr in duration and were conducted M-F between 1000–1200 for a total 16 sessions.
Contextual reinstatement (context A)
On the day immediately following the last day of extinction training, all rats underwent a single session of testing contextual reinstatement of methamphetamine-seeking behavior (i.e., renewal testing) by placing them in the chambers configured as in Context A. Upon placement in the chambers, rats were attached to the infusion pump tether and all of the contingencies and drug-related stimuli present during drug self-administration were present. However, no drug was infused during reinstatement testing. The single reinstatement test session was 2 hr in duration and conducted at the same time of day as self-administration and extinction sessions.
Drugs
Methamphetamine hydrochloride was obtained from Sigma-Aldrich Inc. (St. Louis, MO) and dissolved in sterile saline (0.9% w/v sodium chloride) for intravenous infusion. CDPPB was custom synthesized by Azopharma Product Development Group (Hollywood, FL) according to previously published methods [37] and suspended in 10% v/v Tween 80 (Sigma-Aldrich).
Statistical analyses
The primary dependent measures were the number of active and inactive lever presses emitted during each 2 hr self-administration, extinction, or reinstatement session. The number of methamphetamine infusions per session, duration of active and inactive lever presses (as a measure of gross motor changes to drug- and non-drug-directed behavior), and the number of time-out responses were also assessed. All data were analyzed using either a repeated measures ANOVA with treatment (vehicle or CDPPB) as the between-groups factor and session as the repeated measures factor, or as a one-way ANOVA with treatment as the between groups factor. Because a matching procedure based on the number of active lever presses emitted during drug self-administration was used to create treatment groups with equivalent levels of responding, only the statistical analyses from the extinction and reinstatement conditions are reported. Significant interactions, where applicable, were further investigated using Bonferroni post-hoc tests. The level for statistical significance was set at p < 0.05.
Results
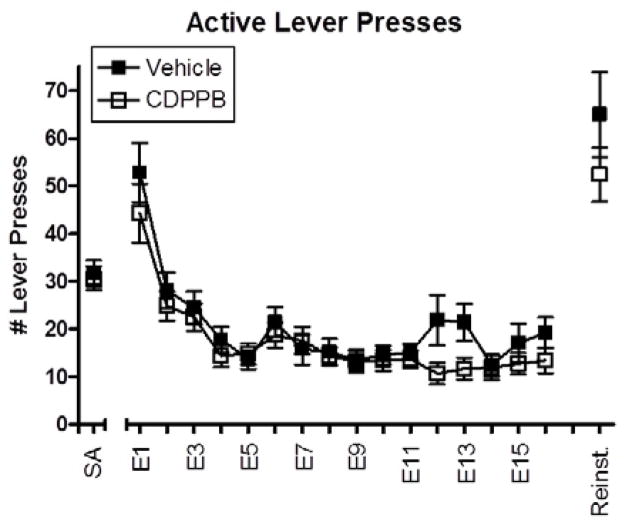
Figure 1 shows the number of active lever presses emitted during the methamphetamine self-administration (SA), extinction, and reinstatement conditions. A significant contextual reinstatement of methamphetamine-seeking was evidenced by a significant increase in the number of active lever presses during the reinstatement test session as compared to the last day of extinction training (F(1,28) = 71.220, p < 0.001). During extinction training, CDPPB produced a slight trend towards a reduction in the overall number of active lever presses (mean 20.24 vs. 16.98 lever presses per session for vehicle and CDPPB-treated animals, respectively), but this difference was not statistically significant (F(1,28) = 1.636, p = 0.211). Similarly, no interaction between treatment group and session was observed (F(15,420) = 1.071, p = 0.382), suggesting that the pattern of active lever responding throughout extinction was similar for both groups. The number of active lever presses during reinstatement testing was not significantly different between CDPPB and vehicle-treated rats (F(1,29) = 1.499, p = 0.231). Lastly, when active lever presses during contextual reinstatement were separated into those resulting in cue presentation and those during the 20 sec time-out period (time-out responses), no significant differences were observed between treatment groups (F(1,29) = .295, p = 0.592 and F(1,29) = 3.184, p = 0.085 for initial and time-out responding, respectively).
Figure 1.
Number of active lever presses emitted per 2 hr session during the last 5 days of methamphetamine self-administration (SA), 16 days of extinction training (E1–E16) and contextual reinstatement (Reinst). Data (mean ± SEM) from animals treated with vehicle during extinction (n=14) are shown in filled symbols, and from animals treated with CDPPB during extinction (n=16) are shown in open symbols.
Figure 2 plots the number of inactive lever presses during the SA, extinction, and reinstatement conditions. There was no difference in overall number of inactive lever presses during extinction (F(1,28) = 1.389, p = 0.249) or during contextual reinstatement (F(1,29) = 0.354, p = 0.557). However, an analysis of the duration of each inactive lever press revealed CDPPB-treated rats exhibited significantly longer lever presses than vehicle-treated rats during contextual reinstatement testing (mean duration 1.61 and 0.67 s in CDPPB- and vehicle-treated rats, respectively; F(1,28) = 6.224, p = 0.019). No group differences in active lever press duration were observed during the SA, extinction, and reinstatement conditions.
Figure 2.
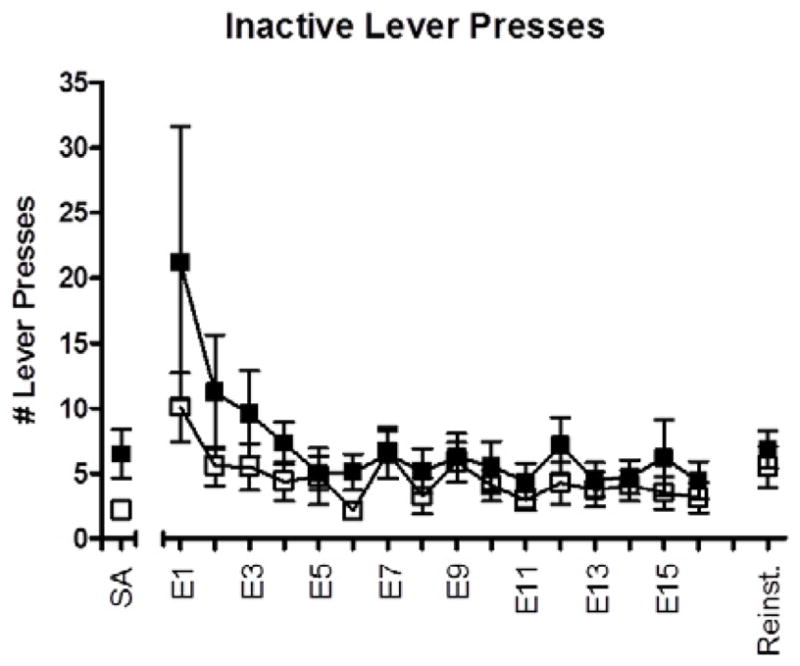
Number of inactive lever presses emitted per 2 hr session during the last 5 days of methamphetamine self-administration (SA), 16 days of extinction training (E1–E16) and contextual reinstatement (Reinst). Data (mean ± SEM) from animals treated with vehicle during extinction (n=14) are shown in filled symbols, and from animals treated with CDPPB during extinction (n=16) are shown in open symbols.
Discussion
Results from the present study show that the mGluR5 PAM CDPPB did not facilitate the acquisition of extinction learning following methamphetamine self-administration, nor did treatment with this compound alter context-induced reinstatement of methamphetamine-seeking behavior. Based on these observations, it could be speculated that mGluR5 PAMs may be of limited therapeutic benefit in cue exposure-based therapies for the treatment of addiction to methamphetamine, which is characterized by high rates of relapse and a lack of approved medications for the treatment of this disorder [38–40]. However, such a conclusion may currently be premature, as this is the first study examining the effects of an mGluR5 PAM on extinction and reinstatement of methamphetamine-seeking behavior. Clearly, additional studies examining the effects of other mGluR5 PAMs with greater brain penetrance, improved solubility in aqueous solutions, and fewer side effects at higher doses are warranted to further explore the potential efficacy of mGluR5 PAMs in the treatment of methamphetamine addiction.
The observed lack of effects of CDPPB in the present study were somewhat surprising, since we have previously demonstrated that similar doses of CDPPB facilitate the extinction of cocaine CPP [32] as well as cocaine-seeking behavior in rats with a history of intravenous cocaine self-administration [33]. The reasons for these discordant results are currently unknown. While administration of CDPPB prior to each extinction session had no effect on the acquisition of extinction learning in the present study, it is possible that administration of CDPPB following each extinction session might have some effects on the consolidation of extinction learning, as we have previously demonstrated [33]. In addition, levels of extinction responding prior to reinstatement testing were slightly higher than those that we and others have observed following cocaine self-administration. As a consequence, higher levels of extinction responding may have influenced the effects of CDPPB when administered prior to extinction training sessions, as well as subsequent context-induced reinstatement.
Moreover, cocaine and methamphetamine have different pharmacological effects on monoaminergic neurotransmission which may have contributed to the observed lack of effects of CDPPB. Cocaine blocks the reuptake of monoamines by inhibiting plasma membrane monoamine transporters, while methamphetamine acts as a pseudo substrate for vesicular monoamine transporters, displacing monoamines from synaptic vesicles and causing an accumulation of these neurotransmitters in the cytoplasm of the presynaptic terminal, which are then released into the synaptic cleft due the ability of methamphetamine to reverse plasma membrane monoamine transporters. Precisely how the different mechanisms of action of these two psychostimulants affect glutamatergic transmission at mGluR5 receptors, and thus possible differences in the efficacy of mGluR5 PAMs, requires further study.
It is also possible that higher doses of CDPPB may be necessary to produce effects on extinction and/or contextual reinstatement in rats with a history of methamphetamine self-administration. However, we do not believe that doses of CDPPB higher than 60 mg/kg would produce observable effects without potentially introducing potential confounding side effects. First, the vast majority of behavioral studies conducted with CDPPB have shown effectiveness at doses between 10 and 60 mg/kg; therefore, we do not believe the 60 mg/kg dose used in the present study to be a lower threshold dose. Second, it has been demonstrated that high doses (100 mg/kg and greater) of ADX47273, an mGluR5 PAM with similar potency and dose-response relationships as CDPPB, decrease extracellular levels of dopamine in the nucleus accumbens [41], which potentially reflects decreased brain reward function and/or decreased limbic-motor integration, and also suppress vertical locomotor activity (i.e., rearing) [42], suggesting motor side effects. Thus, we do not believe doses of CDPPB higher than 60 mg/kg would produce effects without introducing potential side effects that would confound the interpretation of reductions of responding during extinction and/or reinstatement. As mentioned earlier, however, testing of mGluR5 PAMs with greater potency, brain penetrance, and fewer side effects at higher doses is clearly warranted.
Other possible factors that might have contributed to the lack of effects of CDPPB on extinction and contextual reinstatement of methamphetamine-seeking behavior are the reported effects of methamphetamine on the expression and subcellular distribution of mGluR5 receptors in key extinction and reinstatement-related brain regions. For example, it has been recently demonstrated that place preference conditioning with only 3 administrations of methamphetamine produces a loss of cell surface expression of mGluR5 receptors in the prefrontal cortex (PFC) [43]. If the same phenomenon were to be observed following active methamphetamine self-administration, it could be postulated that the sequestration of mGluR5 receptors away from the cell surface would render these receptors less available for positive allosteric modulation by CDPPB, thereby reducing its ability to facilitate extinction learning. In addition, repeated administration of amphetamine has been shown to reduce levels of mGluR5 mRNA in the nucleus accumbens (NAc) [44]. Since the PFC becomes dysregulated following chronic methamphetamine exposure in humans and animals [45–49], and PFC-NAc glutamatergic pathways are important mediators of extinction, relapse-related behaviors and numerous other addiction-related behaviors [50–52], it is therefore plausible that methamphetamine-induced alterations in the levels and/or sub cellular distribution of mGluR5 receptors in these regions may have contributed to the observed lack of efficacy of CDPPB in the present study.
In summary, we observed that positive allosteric modulation of mGluR5 receptors with CDPPB failed to alter extinction learning or reduce contextual reinstatement of methamphetamine-seeking behavior. While these findings may initially suggest that mGluR5 PAMs may not be of potential use in the treatment of methamphetamine addiction as adjunctive therapies to cue exposure-based approaches, additional studies with other mGluR5 PAMs with improved pharmacological properties are clearly warranted before this conclusion can be substantiated.
Acknowledgments
This work was supported by Public Health Service grant DA024355 from the National Institute on Drug Abuse.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, et al. Limbic activation during cueinduced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 3.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 4.Havermans RC, Jansen AT. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict Behav. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- 5.Martin T, LaRowe SD, Malcolm R. Progress in cue extinction therapy for the treatment of addictive disorders: a review update. Open Addiction Journal. 2010;3:92–101. [Google Scholar]
- 6.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 7.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 8.Myers KM, Carlezon WA., Jr Extinction of drug- and withdrawal-paired cues in animal models: relevance to the treatment of addiction. Neurosci Biobehav Rev. 2010;35:285–302. doi: 10.1016/j.neubiorev.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olausson P, JR, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56 (Suppl):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Lalumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, et al. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57BL/c mice. Behav Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011;65:938–944. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torregrossa MM, Sanchez H, Taylor JR. D-cycloserine reduces the context specificity of Pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W, Kalivas PW. N-Acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleva RM, Gass JT, Widholm JJ, Olive MF. Glutamatergic targets for enhancing extinction learning in drug addiction. Curr Neuropharmacol. 2010;8:394–408. doi: 10.2174/157015910793358169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleva RM, Olive MF. Positive allosteric modulators of type 5 metabotropic glutamate receptors (mGluR5) and their therapeutic potential for the treatment of CNS disorders. Molecules. 2011;16:2097–2106. doi: 10.3390/molecules16032097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantak KM, Nic Dhonnchadha BA. Pharmacological enhancement of drug cue extinction learning: translational challenges. Ann N Y Acad Sci. 2011;1216:122–137. doi: 10.1111/j.1749-6632.2010.05899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nic Dhonnchadha BA, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav. 2011;99:229–244. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by exteded access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, et al. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, et al. Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem. 2011;95:73–79. doi: 10.1016/j.nlm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits flexibility induced by NMDA receptor cognitive blockade. Eur J Pharmacol. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. Eur J Pharmacol. 2010;639:17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, et al. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci. 2011;125:10–19. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 36.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 37.Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 38.Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care. 2011;38:41–58. doi: 10.1016/j.pop.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, et al. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;1:96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu F, Grauer S, Kelley C, Navarra R, Graf R, et al. ADX47273: a novel metabotropic glutamate receptor 5 selective positive allosteric modulator with preclinical antipsychotic-like and pro-cognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- 42.Schlumberger C, Pietraszek M, Gravius A, Danysz W. Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacol Biochem Behav. 2010;95:23–30. doi: 10.1016/j.pbb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Herrold AA, Voigt RM, Napier TC. Brain region-selective cellular redistribution of mGlu5 but not GABAB receptors following methamphetamine-induced associative learning. Synapse. 2011;65:1333–1343. doi: 10.1002/syn.20968. [DOI] [PubMed] [Google Scholar]
- 44.Mao L, Wang JQ. Differentially altered mGluR1 and mGluR5 mRNA expression in rat caudate nucleus and nucleus accumbens in the development and expression of behavioral sensitization to repeated amphetamine administration. Synapse. 2001;41:230–240. doi: 10.1002/syn.1080. [DOI] [PubMed] [Google Scholar]
- 45.Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;1:5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 46.Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Neurol Clin. 2011;29:641–655. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- 51.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]