Summary
Like all other drugs of abuse, the primary therapeutic objective for treating methamphetamine addiction research is the maintenance of abstinence and prevention of relapse to habitual drug-taking. Compounds with the potential to prevent relapse are often investigated in rats that are trained to self-administer intravenous methamphetamine, subjected to extinction training where responding is no longer reinforced, and then given tests for reinstatement of drug-seeking behavior triggered by methamphetamine injections or re-exposure to drug-paired cues. Experimental compounds are administered to the animals prior to the reinstatement tests to evaluate their potential for attenuating or preventing drug-seeking behavior. This article describes the common procedures of the extinction-reinstatement model in studies of this type, and identifies areas of discrepancy. This is followed by a comprehensive overview of the currently published anti-reinstatement effects of pharmacological compounds, classified by the most relevant neurological systems associated with these compounds. The article concludes with a brief discussion of how the study of anti-reinstatement effects can be expanded to further verify existing positive results or to find novel neurobiological targets.
Introduction
Compulsive abuse of and addiction to methamphetamine, a psychostimulant with reinforcing properties resembling those of cocaine, is a significant and rapidly growing global health problem [1]. After marijuana, methamphetamine is the most abused illicit drug in the world [2]. Currently no medications have been approved by the Federal Drug Administration for the treatment of addiction to psychostimulants, including methamphetamine. The conceptualization of addiction has been evolving towards that of a chronic disease, and consequently research efforts have focused on developing treatments to reduce the likelihood of relapse in abstinent individuals [3]. Relapse is preceded by drug craving, which is commonly brought about not only by re-exposure to the drug, but to environmental stimuli previously associated with past drug use [4]. In order to facilitate the development of anti-relapse treatments, preclinical models have been developed that represent craving as the reinstatement of previously methamphetamine-reinforced activity provoked by non-contingent drug exposure or cues conditioned to drug reward [5,6]. Studies using rats with a history of methamphetamine self-administration have been utilized to test the therapeutic potential of a range of compounds that span a wide variety of neurobiological systems [7].
The most popular and powerful procedure available to study drug craving in small animals is the extinction-reinstatement model. Typically used in rats, this model comprises of initial training where the subject acquires stable self-administration of the drug, followed by a period of extinction training and test sessions utilizing presentation of environmental stimuli previously associated with drug reinforcement [8]. Whether this technique provides a valid approximation of human craving and relapse to drug seeking is a topic of active debate [9–11]. This review is an overview of the use of conditioned reinstatement experiments to evaluate the therapeutic potential of various compounds toward the relief of methamphetamine addiction. Commentary is provided regarding the extent to which each of the major neurobiological systems has been investigated.
Common Training and Testing Procedures
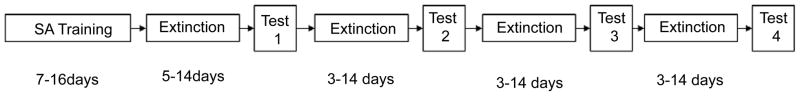
Although several variants of the extinction-reinstatement model have been developed for the study of cocaine and heroin seeking [12], the so-called between-session procedure [13,14] has been almost universally applied in recent experiments targeting methamphetamine reinstatement (Figure 1). The stages of training and testing described below mirror those of the majority of experiments testing for effects on cocaine reinstatement, and hence inherit their strengths and shortcomings.
Figure 1.
Standard experimental procedures for the operant extinction-reinstatement model. Self-administration sessions are typically conducted daily for limited (1–2 hr) exposure to methamphetamine reinforcement. This training typically lasts between seven [38,41] and 14 [76] days. This is followed by extinction training, which usually is continued until an extinction criterion is reached, usually requiring between five [49] and 14 [41,42] days, although the rate of extinction depends on whether cues are present or saline infusions are substituted for methamphetamine during this phase. Following the initial extinction period, a reinstatement test is performed following treatment by the test compound. Subsequent reinstatement tests are preceded by repeated daily extinction sessions, in order to reestablish the baseline responding.
Self-administration training
To condition rats to the reinforcing effects of methamphetamine, they are first trained to self-administer the drug by pressing a lever or exerting a nosepoke in the presence of response-contingent cues (usually a light or tone, or combination of the two). Methamphetamine is delivered via a surgically implanted intravenous catheter as reinforcement, using a dose usually ranging between 0.05 mg/kg and 0.1 mg/kg per infusion (Figure 2A). Training continues until a stable level of reinforced behavior is established, with a final reinforcement schedule ranging from FR 1 (where every active lever press is reinforced by methamphetamine, followed by a timeout period) to FR 5 (where reinforcement follows every five active lever presses). This period typically lasts from 10 to 16 days, using sessions of one to two hours in length.
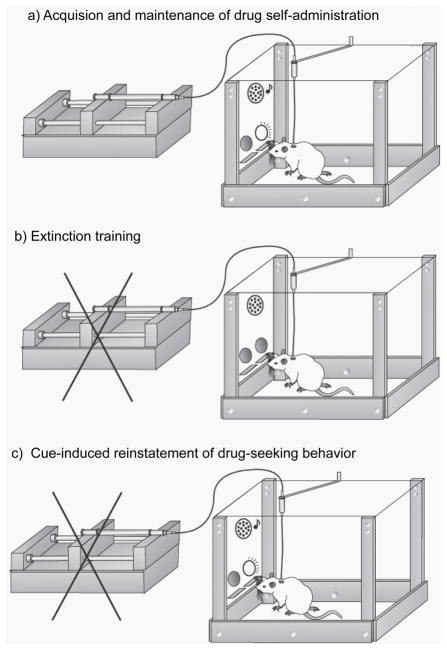
Figure 2.
Diagrams illustrating the stages of the standard operant extinction-reinstatement procedure. Self-administration training (A) is characterized by the availability of intravenous methamphetamine reinforcement that is delivered in the presence of discrete auditory and visual cues. Extinction training (B) is typically performed in the same operant chamber as self-administration, only no cues are presented and responding on the active lever (or nose-poke hole, not shown) results in no methamphetamine reinforcement. In test sessions for reinstatement elicited by cues (C), active lever responses (or nosepokes) result in presentation of the drug-paired cues, but not methamphetamine reinforcement. Alternatively, “primed” reinstatement of drug seeking is elicited by a non-contingent injection of methamphetamine.
The length of the self-administration experience in number of days, and in the number of hours per day, can have a profound effect on the physiology and behavior of rats exposed to many drugs of abuse, including methamphetamine [15,16]. When given longer access (six hr per session) to methamphetamine reinforcement, rats have been shown to gradually but significantly escalate their daily intake [17]. This increased intake of methamphetamine is a cardinal feature of the transition from drug abuse to addiction [18], and precipitates cognitive deficits as measured by novel object recognition and attention set-shifting [19,20]. Human chronic methamphetamine abusers are also known to significantly increase regular drug-taking following past experiences of unrestricted access [21], and demonstrate a variety of attention and cognitive impairments [22,23]. Exposing rodents to periods of extended drug access and/or intoxication has been termed the escalation model and has been developed in numerous studies of cocaine [24,25], morphine [26], heroin [27–29] and alcohol [30,31], all of which describe sustained enhancement in drug intake and evidence of dysregulated cognitive or stress-mediating systems that parallel clinical observations of drug and alcohol addicts [32]. The escalation model may therefore be a useful extension of the common short-access methamphetamine self-administration technique in preparing rats for the testing of potential therapeutic compounds [33,34].
Extinction training
All reinstatement experiments reviewed in this article describe a period of extinction training that follows self-administration, in which the rats are placed into the same operant chambers with the levers or nosepoke orifices available, but responses are not reinforced with drug delivery (Figure 2B). However, the conditions of extinction training beyond this definition vary considerably among studies. Methamphetamine infusions are either replaced by saline infusions [35] or no intravenous delivery of any kind [36]. Generally cues are not presented during extinction, but notable exceptions exist, where cues are presented during extinction training but not used for reinstatement testing [37,38] or are substituted with a set of inverse cue conditions signaling drug non-availability [39]. Most of all, variation exists in the conditions used for the termination of extinction training. In cases where a set number of extinction sessions are used, regardless of operant responding levels, this ranges from two days of 5-hr sessions [40] to 14 days of sessions using saline infusions [41]. Other studies used a criterion for operant responding to be achieved before terminating extinction training, usually a percentage (up to 20%) of the responses recorded at the end of self-administration training [36,39], or a set number of active lever presses per session [42,43]. These differences likely result in the length of the extinction period varying significantly among experiments, suggesting that rats in these studies were tested at different points of progress along what is properly considered a time-dependent learning process [44,45].
Reinstatement testing
After completion of extinction training, rats are tested for the reinstatement of methamphetamine-seeking behavior after administration of a pharmaceutical compound (Figure 2C). Reinstatement can be reliably induced by exposure to cues previously associated with drug reinforcement or non-contingent administration of the drug. In the studies reviewed here, these two triggers are most often used in separate experiments testing the same compound, with one exception where cues were used in combination with drug-priming injections [35]. This practice is a consequence of an accumulation of evidence that cue-elicited and drug-primed reinstatement behaviors are governed by distinct, yet overlapping, neurobiological substrates [46,12]. In fact, this notion has been confirmed in reports of different dose-response profiles for cue- and drug-elicited methamphetamine reinstatement [47,39]. These differences have been recently substantiated in the escalation model, where drug-induced but not cue-induced methamphetamine seeking was enhanced in rats with a history of prolonged exposure to drug availability [48].
In most of the studies reviewed here, the effects of pharmacologic compounds on reinstatement have been tested at various doses in a randomized within-subjects experimental design, ranging from two to six reinstatement tests with intervening periods of extinction retraining. This strategy provides an efficient and statistically potent assessment of the dose response of the anti-reinstatement effects, but is clearly vulnerable to the potential complicating factors of drug tolerance and behavioral fatigue. To our knowledge, the anti-reinstatement effect of a compound has been evaluated after chronic administration prior to the first reinstatement test in a small minority of studies [43,49,50]. This is an example of an acknowledged deficit in the field of drug seeking in animals, where potential therapeutic compounds are almost always tested once per subject per dose, despite the fact that clinical treatments are usually in the form of repeated treatments [51].
Besides non-contingent injections of methamphetamine and the exposure to drug-associated cues, stress-inducing experiences have also been found to result in reinstatement of methamphetamine-seeking behavior [37,52]. To our knowledge, exposure to intermittent footshock has only been utilized once to evaluate the anti-reinstatement effects of a compound in methamphetamine-trained rats [37], but this technique is known as the most reliable of stressful stimuli when inducing reinstatement of drug seeking [53,54]. However, since there is no human equivalent to footshock, the field of drug reinstatement has sought alternatives for inducing a more translatable stressful stimulus [55]. To date the most consistent stressor appears to be administration of yohimbine, an α2-adrenoreceptor antagonist that induces anxiety-like responses in humans and animals [56,57], and reinstates cocaine seeking in monkeys [58], and methamphetamine seeking in rats [52]. Given the importance of stress-mediating systems as motivating components in relapse [59], as well as the evidence of the stress response contributing to cue-induced drug seeking [60], use of yohimbine or other stressor in the extinction-reinstatement model could provide a vital probe into the therapeutic potential of various compounds in methamphetamine-dependent individuals.
Finally, it is worth noting that several studies using the extinction-reinstatement model incorporate a separate experiment using rats trained to respond for food or sucrose pellets in the presence of cues. The compounds found to attenuate reinstatement to methamphetamine seeking were then tested for effects on responding reinforced by food [35,39,47,49] or reinstatement of food-seeking behavior triggered by cues [36]. Measurement of food reinforcement or food seeking is often used in studies of drug-seeking behavior in order to establish specificity of a compound’s effects on drug versus non-drug mechanisms, but the caveat exists that the substrates of drug and food reinforcement heavily overlap [61].
Investigation of the Anti-Reinstatement Potential of Specific Neurochemical Substrates
Dopamine
The characteristic neurobiological effects of methamphetamine are exerted on the dopamine system; as an analogue of amphetamine, it exerts its reinforcing properties via occupation and reversal of the dopamine transporter [62,63]. A well-established consequence of chronic methamphetamine exposure is a depression of brain monoamine levels, where repeated high doses of methamphetamine result in reduced amounts of serotonin and dopamine that persist for several months [64,65]. These observations were confirmed in primates weeks after the completion of high-dose methamphetamine regimens [66] as well as doses comparable to human abuse patterns [67]. Methamphetamine binds to transporter proteins for dopamine, serotonin and norepinephrine, reducing their capability to manage synaptic catecholamine release [62,68,69]. In addition, methamphetamine is internalized by the presynaptic cell and accumulated in synaptic vesicles, where it disrupts the electrochemical gradient required for dopamine sequestration [70]. Consequently, dopamine accumulates in the presynaptic cell and is eventually released into the synapse via reverse dopamine transport [62]. Methamphetamine exposure also results in the formation of reactive oxygen and reactive nitrogen species, contributing to changes in dopamine sequestration and other dopamine-related functional deficiencies [71,72]. Human imaging studies have shown reduced levels of dopamine transporters and D2 receptors in the early stages of methamphetamine withdrawal [73,74], the latter phenomenon having a correlation with post-scan incidence of relapse [75].
The discovery of pharmaceutical compounds that serve to stabilize dopamine function in animals with a history of drug exposure has been a focus of research. In particular, a recent study found the treatment with the D3 receptor antagonist PG01037 attenuated cue-induced reinstatement of methamphetamine seeking as well as methamphetamine reward [76]. The D1 agonist SKF-81297 was found to dose-dependently attenuate both cue- and drug-primed reinstatement, with both effects reversible by pretreatment with the D1 antagonist SCH-23390 [50]. Additionally, the synthetic compound (−)-BPAP, an enhancer of electrically stimulated monoamine release that does not itself release catecholamines, was also found to attenuate cue-induced methamphetamine seeking, presumably by activating D1 receptors, but these effects were not reversed by either SCH-23390 or the D2 agonist amisulpride [50]. Another study also found that pretreatment by SCH-23390 resulted in attenuated methamphetamine-primed methamphetamine seeking, but pretreatment by the D2 antagonist eticlopride failed to exert a comparable effect [77]. This result was consistent with prior observations that eticlopride had no significant effect on self-administration of methamphetamine [78], but is at variance with the anti-reinstatement effects found for eticlopride and other D2 antagonists in cocaine- [79,80]and heroin-seeking behavior [81]. Together, these results exhibit candidate treatments for methamphetamine relapse tailored to act upon dopamine receptor subtypes D1 and D3.
The dopamine receptor family appears to be comprised of prime candidates for investigation with rats given extended access to methamphetamine in order to produce an escalation of daily intake [17]. This increased rate of self-administration is accompanied by enhanced sensitivity to the effects of dopamine receptor ligands, including the antipsychotic aripiprazole, which among its various neurotransmitter actions [82] is a dopamine (D2) receptor antagonist [83], and antagonist of the D3 receptor [84]. In methamphetamine self-administering rats, dopamine transporter deficiencies were only detected the dorsal striatum and forebrain of animals exposed to extended access sessions [85]. Thus, the anti-reinstatement performance of dopamine-stabilizing drugs such as aripiprazole may be significantly altered if the escalation model is incorporated into the experimentation.
Currently best known for its association with the biological mechanisms of pair-bonding and maternal behaviors, [86] the neuropeptide oxytocin is also thought to inhibit long-term habitual behaviors associated with drug reinforcement by inhibiting the dopaminergic activity of mesolimbic neurons [87]. Recently, systemic injections of oxytocin were shown to reduce levels of methamphetamine self-administration, as well as drug-primed reinstatement of methamphetamine seeking [38].
The drug pergolide, an existing treatment of Parkinson’s disease, is a direct agonist of D1 and D2 receptors, as well as a partial serotonin 5-HT2 receptor antagonist [88]. Administration of pergolide in combination with the serotonin 5-HT3 receptor antagonist ondanestron prior to extinction training resulted in reduced drug-primed methamphetamine-seeking behavior, but the lack of an extended extinction period (2 days) and subsequent baseline make these data difficult to conclusively interpret as motivational effects [40].
Glutamate
The effects of methamphetamine in the central nervous system extend beyond changes in the dopamine system, and include dramatic increases in the release of other monoamines, including glutamate. Glutamate pathways are ubiquitous throughout the brain and interact with drug-induced dopamine imbalances in a complex fashion involving a variety of glutamate receptor families [89], including links between changes in NMDA function and D1 receptor activation [90], and between D2 receptor activation and glutamate responses to psychostimulants [91]. The direct and conditioned reinforcing effects of methamphetamine are also sensitive to glutamate manipulation [92–94], suggesting that potential therapeutic compounds may be developed by targeting glutamate neurotransmission. Moreover, the enthusiasm toward finding glutamate receptor ligands for mediation of drug abuse stems from the recently developed links between persistent imbalances of synaptic and extrasynaptic glutamate and the loss of control over drug-seeking behaviors [95,96]. Research on cocaine and alcohol addiction using animal models has definitively tied glutamate mechanisms to reinstatement triggered by cues, drug priming and stress [97].
Untreated drug seeking in cocaine and methamphetamine addiction is characterized by increased synaptic glutamate, but simply reducing glutamate levels by blocking ionotropic receptors is known to produce a range of undesirable side effects including psychotomimesis [98]. Recent research efforts have therefore focused on the manipulation of metabotropic glutamate receptors (mGluRs) to modulate and exert control over glutamate release and neurotransmission [99]. A seminal study using knockout mice demonstrated that the type 5 mGluR receptor (mGluR5) in particular was key in the development of cocaine-induced hyperlocomotion and reinforcement [100]. Blockade of postsynaptic mGluR5 receptors was found to attenuate cue-, drug- and stress-induced reinstatement of cocaine and alcohol seeking [101–105]. The selective mGluR5 antagonist MTEP was found to reduce cue- and drug-primed reinstatement of methamphetamine seeking, using doses that had no effect on reinstatement to food-seeking [36]. Persistent cognitive deficits resulting from escalated methamphetamine self-administration were reversed by positive allosteric modulation of mGluR5 by the compound CDPPB [20]. Given the recent evidence that mGluR control of drug-motivated behavior is altered in rats with a history of extended access to cocaine [106] or chemical dependency on alcohol [107,108], investigation of mGluR ligands with the methamphetamine reinstatement model is clearly warranted.
Recently the non-amphetamine stimulant modafinil has been investigated in clinical studies of cocaine and methamphetamine abuse, exhibiting promising trends toward increased rates of abstinence [109–111]. The exact neurobiological targets of modafinil are complex, but its effects include a combination of dopaminergic and glutamatergic activities [112]. In a recent preclinical study, chronic administration of modafinil resulted in the attenuation of cue-induced and drug-primed reinstatement [43]. Reinstatement elicited by a return to the self-administration chamber, following extinction training in a different operant chamber [113], was also reduced by modafinil treatment [42]. In addition, the stimulant properties of modafinil did not induce reinstatement to drug seeking [42], inviting the possibility that this drug could relieve some of the debilitative effects of psychostimulant withdrawal and maintain abstinence for an extended period of time [111,114].
Modafinil was also recently investigated in separate groups of male and female rats; to our knowledge representing the only study of gender differences in anti-reinstatement effects pertaining to methamphetamine [115]. Female rats exhibited greater levels of reinstatement of drug seeking after pretreatment by vehicle, were equally sensitive to the attenuating effects of modafinil but were more sensitive to the effects of the neurosteroids all opregnanelone [115]. These results represent initial evidence of neurobiological explanations behind the different methamphetamine use patterns associated with gender [116].
Opioids
Naltrexone is perhaps the best example of a successful treatment for drug relapse emerging from translational research incorporating reinstatement studies involving humans and animals. Naltrexone, a competitive opioid receptor antagonist, has been found to reduce alcohol-seeking in rodents [117,118] and self-reported craving for alcohol in humans [119]. It is currently an approved medication for the treatment of relapse to alcohol consumption in alcoholics, and its potential use as an anti-relapse therapy against other drugs of abuse is of prime interest [120–122]. However, the clinical benefits of this treatment are known to be limited by the lack of compliance in alcoholic patients [123], and its anti-craving effects are markedly reduced in rats physically dependent on alcohol [124]. In rats trained to self-administer methamphetamine, naltrexone treatment effectively reduced subsequent cue-induced drug seeking but not drug-primed seeking [47]. To our knowledge, the anti-reinstatement effects of naltrexone have not yet been tested in rats with a history of methamphetamine self-administration. Furthermore, a regimen of repeated naltrexone injections was found to decrease cue-induced cocaine seeking [125] but has not been tested for cues associated with methamphetamine.
Despite the reported effectiveness of buprenorphine and methadone in attenuating cocaine reinstatement and their clinical availability [126,127], no published reports of opioid receptor-targeting compounds beyond naltrexone have been tested on methamphetamine reinstatement. A recent report on the attenuating effect of buprenorphine on responding to sucrose-associated cues indicates some nonspecific consequences for this treatment [128], but the opioid system remains a relatively unexplored area in the field of methamphetamine craving.
Serotonin
The effects of methamphetamine exposure and dependence on serotonin neurotransmission are well documented [129], making the many receptor subfamilies of this monoamine intriguing targets for investigation with the extinction-reinstatement model. Acute administration of methamphetamine results in a dramatic increase in striatal serotonin release [130], and chronic exposure to the drug is accompanied by long-lasting reductions in serotonin production [6,131] and serotonin transporter expression [70]. Several experiments have found that injections of 5-HT2 receptor antagonists or 5-HT3 receptor agonists reduce cocaine-seeking behavior elicited by cues, following extensive self-administration and extinction training [132–134]. Treatment with the atypical antidepressant mirtazapine, which has actions on the 5-HT2A/C, 5-HT3, histamine and α2-norepinephrine receptors, was found to reduce cue-induced methamphetamine seeking [181]. However, besides the study investigating the 5-HT3 receptor antagonist ondansetron in combination with a dopamine agonist pergolide that was discussed earlier [40], to date there have been no further investigations of reinstatement-blocking potential of serotonergic compounds in the rat model. Given the regulatory role 5-HT3 receptors have on the dopaminergic reward- and motivation-mediating pathways of the brain and the evidence linking serotonin function with cocaine-seeking behavior [135], their contribution to methamphetamine seeking would seem to be worth investigating in a model that matches the standard procedures established by the cocaine literature.
In addition to the relative paucity of animal studies, the clinical application of serotonin pharmacology to methamphetamine dependence problems has resulted in mainly negative findings. In a recent study, treatment with the selectively serotonin reuptake inhibitor (SSRI) sertraline resulted in an increased risk for relapse to methamphetamine use in abstinent individuals [136]. Other investigations found that the SSRIs paroxetine and fluoxetine had no significant effect on reducing methamphetamine use [137,138].
Stress-mediating systems
A major conceptualization of addiction is that of a stress surfeit disorder, in which dependent individuals experience chronic craving and relapse in response to drug-associated or stressful stimuli [59]. As mentioned earlier, stressful experiences form a plausible trigger for reinstatement to drug-seeking in animals and craving in humans [139,54]. Additionally, reinstatement to psychostimulant-seeking behavior triggered by cues is also mediated by components of the stress response system, including CRF1 receptors and corticosterone [140]. Both the CRF1 receptor antagonist CP-154,526 and the glucocorticoid receptor inhibitor ketoconazole were effective in attenuating reinstatement triggered by a small methamphetamine priming injecton, but not by drug-paired cues, when systemically administered [39]. However, centrally injected (into the cerebral ventricle) CP-154,526 attenuated cue-induced reinstatement, signaling a critical role for CRF1 signaling but not corticosterone production for this behavior. Although the rats in this experiment were training in limited-access (2 hr) sessions, the total drug exposure was apparently sufficient to induce withdrawal marked by a negative affective state reversible by CRF1 antagonism. This is in contrast to cocaine exposure, where the CRF1 antagonist antalarmin reduced cocaine intake in rats with a history of extended access and cocaine escalation, but was ineffective in changing the behavior of nondependent, short-access rats [141]. More studies focusing on CRF and other substrates of the stress response are needed, but the present results may represent the role of negative affect in methamphetamine-conditioned behaviors [142].
The first drug shown to conclusively reduce operant responding during a stress-induced reinstatement session is the anti-inflammatory drug AV411 (ibudilast), principally known for its analgesic and neuroprotective properties [143,144]. A study utilizing 15 min intermittent footshock and 1 mg/kg methamphetamine priming injection in different cohorts demonstrated that ibudilast significantly reduced reinstatement behavior in both tests [37]. The anti-reinstatement effects of ibudilast were presumably the result of its neuroregulatory actions, which include attenuation of glial activity, production of the growth factor GDNF and the inhibition of phosphodiesterase activity [145]. This compound has no known direct interactions with dopamine, glutamate, opioid, serotonin or cannabinoid receptors, or with dopamine or serotonin transporters [143], and thus represents a distinct target for managing the consequences of methamphetamine exposure on stress systems.
Nicotine and cannabinoid receptors
The cannabinoid neurotransmission system is widespread throughout the brain and has been linked with reward processing and drug-seeking activity [146,147]. Not only does the stimulation of endocannabinoid receptors (primarily CB1 receptors) result in cannabinoid seeking in rats previously trained to self-administer cannabinoid agonists [148], but also elicits drug-seeking in other drug-trained rats as well [149]. Conversely, the CB1 antagonist rimonabant has been shown to interfere with drug-primed, cue-primed but not stress-elicited reinstatement of various drugs, including cocaine [150]. The stimulation of CB1 by the drug Δ9-tetrahydrocannabinol (THC) and blocking of CB1 by rimonabant were tested for effects on reinstatement of methamphetamine seeking [49]. THC augmented reinstatement elicited by cues combined with 1 mg/kg methamphetamine, but reduced drug-seeking behavior when co-administered with a high priming dose (3.2 mg/kg) of methamphetamine, indicating a modulatory effect on the drug-primed motivational circuits [49]. In contrast, another study failed to show CB1-antagonist effects on reinstatement induced by a small priming dose of methamphetamine (0.1 mg/kg) [151]. Although the drugs used in these two conflicting studies were different (rimonabant and AM251, a CB1 inverse agonist), the limited amount of data does not conclusively support a critical role for CB1 function in the reinstatement of methamphetamine seeking.
Nicotine has been found to exert protective effects against Parkinson’s-like symptoms associated with methamphetamine toxicity [152]. The contribution of nicotinic acetylcholinergic neurotransmission to methamphetamine-conditioned behavior was investigated in a series of reinstatement tests [35,50]. Systemic injections of nicotine as well as the acetylcholinesterase inhibitor donepezil resulted in suppression of cue- and drug-induced reinstatement of methamphetamine seeking, via selective activation of nicotinic acetylcholine and not muscarinic receptors. In each case the anti-reinstatement effects were specific to methamphetamine seeking and not food seeking [35]. However, a recent report of nicotine eliciting methamphetamine-seeking in rats with prior exposure to nicotine reinforcement demonstrated that in subjects with a history of exposure to both drugs, nicotine can act as a trigger for reinstatement instead of a therapeutic agent [153]. Considering the extremely high rate of nicotine useamong regular methamphetamine abusers [154,155], the possibility of nicotine being utilized as a protective treatment is admittedly difficult to appreciate.
The naturally occurring nicotinic receptor ligand lobeline has been known to decrease methamphetamine-induced stereotypy as well as methamphetamine self-administration [156–158], properties associated with possible dopamine and stimulant effects. Unlike stimulants, however, lobeline was found not to support self-administration on its own, and also had no effect on drug-primed reinstatement of methamphetamine seeking [41]. Additionally, this compound did not reinstate extinguished methamphetamine seeking or have any observable effect on central dopamine release. Thus, the therapeutic value of lobeline appears to be restricted to the reduction of methamphetamine intake without having abuse potential of its own, rather than preventing relapse of drug craving [41].
Despite the equivocal or negative outcomes in establishing anti-relapse properties of CB1 ligands and nicotine, the ability of THC or nicotine to induce or augment reinstatement to methamphetamine seeking illustrates an important triggering mechanism. Depending on the time course of exposure and abstinence, neuroadaptive changes induced by chronic nicotine, cannabis, alcohol and other drugs can conceivably exert either protective effects against reinstatement or confer vulnerability to greater methamphetamine seeking. This question remains underinvestigated in the research of not only methamphetamine but also cocaine and other important drugs of abuse [9].
Conclusions
The studies of the anti-reinstatement properties of a large variety of compounds demonstrate that promising candidate treatments for methamphetamine abuse exist across a spectrum of neurobiological substrates. The most developed series of experiments appear to be those concerning modafinil and dopamine receptor ligands, but there are many other promising results to build upon. To date, almost all of the other positive anti-reinstatement findings remain as single published studies. The generally consistent approach in incorporating the extinction-reinstatement model for methamphetamine addiction grants the benefits of standardization and increased comparability of results from different studies, but also bestows the theoretical and practical shortcomings of this paradigm. In particular, the reinstatement test is a behavioral interpretation of craving, which in addicts is a subjective response [55]. Self-reports of craving in a laboratory setting have generally been poor predictors of actual relapse among study participants [159,160]. The in-laboratory measurement of craving as a single variable [161] as well as the use of retrospective reports of craving and other triggers of relapse remain controversial [162]. In spite of these problems, the triggers for reinstatement of drug-seeking behavior in the rat bear many similarities to the stimuli that provoke craving in addicts [163–166]. The lack of predictive validity associated with cocaine and methamphetamine reinstatement animal models is primarily due to the fact that clinical studies of treatments have focused on reduction in drug intake among patients and not the maintenance of abstinence [9]. It can also be argued that in vivo functional magnetic resonance imaging studies of human stimulant abusers have adequately demonstrated measureable brain responses to drug priming and drug-associated audiovisual cues that temporally match elevated levels of self-reported craving [167–169]. Though obstacles remain in establishing the extinction-reinstatement model as a fully valid approach to the study of relapse in the minds of all investigators, its flexibility and relevance toward all major drugs of abuse make it a viable strategy for researching the mechanisms of addiction.
The various compounds discussed above could be tested using extensions of the extinction-reinstatement model, including stress-induced reinstatement tests, incorporation of the escalation model to evaluate the effects of physical dependence, and inclusion of female rats to begin to assess the impact of gender on methamphetamine craving. Adaptations of the stress response incorporate a large number of neurotransmitter and neuropeptide systems, including most of those reviewed above and alteration of stress-mediating brain circuitsis thought to be critical in the transition from habitual drug use to drug dependence [32,59]. Additionally, the scope of investigation of anti-relapse targets can be expanded beyond the existence of available ligands by utilizing the extinction-reinstatement model in transgenic mice [170,171].
An alternative procedure for assessing drug-motivated behavior is testing for the reinstatement of extinguished conditioned place preference (CPP), [172,173]. Rats are first exposed to two neutral environments (distinguished from each other by olfactory, visual and/or tactile cues) in separate repeated conditioning sessions, with one environment being paired with injections of methamphetamine and the other environment paired with a non-drug state (i.e., saline). Following sufficient conditioning, the rats, when given an opportunity to choose between the environments in a non-drug state, express a preference toward the methamphetamine-paired environment. This CPP behavior is then extinguished by repeated extinction sessions where the rats are exposed to both environments without methamphetamine. CPP toward the previously drug-paired environment is then reinstated by a priming injection of methamphetamine, providing an indirect assessment of the persistent reinforcing effects of methamphetamine. Drug-induced reinstatement of methamphetamine CPP has been shown to be attenuated by pretreatment with the nitrous oxide inhibitor 7-nitroindazole [174] or the GABA transaminase inhibitor vigabatrin [175] in rats, and by bee venom in mice [176]. Though it has been utilized infrequently for methamphetamine research [177] and concerns persist about the standardization of its procedure [178], reinstatement of CPP offers an alternative strategy for investigation into the biological substrates of conditioned methamphetamine seeking.
Finally, it is worth noting that the compounds evaluated so far represent a partial overlap of the total number of drugs actively being researched in clinical studies for the same ultimate purpose [179]. The current enthusiasm for “repurposing” medications that are currently approved for other clinical uses [180] has potential for motivating reinstatement experiments directly relevant to the scope of available treatments.
Acknowledgments
This work was supported by grant DA025606 from the National Institute on Drug Abuse.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The authors declare that they have no conflicts of interest.
References
- 1.Rutkowski BA, Maxwell JC. Epidemiology of methamphetamine use. In: Roll JM, Rawson RA, Ling W, editors. Methamphetamine addiction: from basic science to treatment. Guilford Press; New York: 2009. pp. 6–29. [Google Scholar]
- 2.WHO. Substance Abuse Dept. World Health Organization (WHO); Geneva, Switzerland: 1996. Amphetamine-like stimulants: a report from the WHO meeting an amphetamines, MDMA and other psychostimulants. [Google Scholar]
- 3.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 4.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, et al. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 5.Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- 6.Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, et al. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 7.Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- 8.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 9.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 11.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 12.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- 14.Stretch R, Gerber GJ, Wood SM. Factors affecting behavior maintained by response-contingent intravenous infusions of amphetamine in squirrel monkeys. Can J Physiol Pharmacol. 1971;49:581–589. doi: 10.1139/y71-075. [DOI] [PubMed] [Google Scholar]
- 15.Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- 16.Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press; San Diego, CA: 2006. [Google Scholar]
- 17.Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 19.Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011a;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culbertson C, De La Garza R, 2nd, Costello M, Newton TF. Unrestricted access to methamphetamine or cocaine in the past is associated with increased current use. Int J Neuropsychopharmacol. 2009;1(2):677–685. doi: 10.1017/S1461145708009668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe C, Bush KR, Straits-Troster K, Meredith C, Romwall L, et al. A comparison of methamphetamine-dependent inpatients childhood attention deficit hyperactivity disorder symptomatology. J Addict Dis. 2005;24:133–152. doi: 10.1300/J069v24n03_11. [DOI] [PubMed] [Google Scholar]
- 23.Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 25.Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 26.Kruzich PJ, Chen AC, Unterwald EM, Kreek MJ. Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPgammaS binding. Synapse. 2003;47:243–249. doi: 10.1002/syn.10173. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 28.Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, et al. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 29.Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 32.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 34.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beardsley PM, Shelton KL, Hendrick E, Johnson KW. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol. 2010;637:102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- 40.Davidson C, Gopalan R, Ahn C, Chen Q, Mannelli P, et al. Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Eur J Pharmacol. 2007;565:113–118. doi: 10.1016/j.ejphar.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 41.Harrod SB, Dwoskin LP, Green TA, Gehrke BJ, Bardo MT. Lobeline does not serve as a reinforcer in rats. Psychopharmacology (Berl) 2003;165:397–404. doi: 10.1007/s00213-002-1289-6. [DOI] [PubMed] [Google Scholar]
- 42.Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reichel CM, See RE. Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. Int J Neuropsychopharmacol. 2011b:1–11. doi: 10.1017/S1461145711000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouton ME. Context and ambiguity in the extinction of emotional learning: implications for exposure therapy. Behav Res Ther. 1988;26:137–149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 45.Gass JT, Cleva RM, Wischerath KC, Olive MF. Neurobiological substrates underlying the extinction of drug-seeking behavior. Res Adv in Addiction Biology. 2010;1:1–23. [Google Scholar]
- 46.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T. Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward. Brain Res. 2004b;1021:272–276. doi: 10.1016/j.brainres.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 48.Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004a;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- 50.Hiranita T, Yamamoto T, Nawata Y. A tryptamine-derived catecholaminergic enhancer, (−)-1-(benzofuran-2-yl)-2-propylaminopentane [(−)-BPAP], attenuates reinstatement of methamphetamine-seeking behavior in rats. Neuroscience. 2010;165:300–312. doi: 10.1016/j.neuroscience.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 51.Yahyavi-Firouz-Abadi N, See RE. Anti-relapse medications: preclinical models for drug addiction treatment. Pharmacol Ther. 2009;124:235–247. doi: 10.1016/j.pharmthera.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 53.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, et al. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 54.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 55.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 57.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 58.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 59.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22 :7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 62.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 63.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 64.Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- 65.Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- 66.Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- 67.Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, et al. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaneko Y, Kashiwa A, Ito T, Ishii S, Umino A, et al. Selective serotonin reuptake inhibitors, fluoxetine and paroxetine, attenuate the expression of the established behavioral sensitization induced by methamphetamine. Neuropsychopharmacology. 2007;32:658–664. doi: 10.1038/sj.npp.1301111. [DOI] [PubMed] [Google Scholar]
- 69.Yui K, Goto K, Ikemoto S. The role of noradrenergic and dopaminergic hyperactivity in the development of spontaneous recurrence of methamphetamine psychosis and susceptibility to episode recurrence. Ann N Y Acad Sci. 2004;1025:296–306. doi: 10.1196/annals.1316.037. [DOI] [PubMed] [Google Scholar]
- 70.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Anderson KL, Itzhak Y. Methamphetamine-induced selective dopaminergic neurotoxicity is accompanied by an increase in striatal nitrate in the mouse. Ann N Y Acad Sci. 2006;1074:225–233. doi: 10.1196/annals.1369.021. [DOI] [PubMed] [Google Scholar]
- 72.Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 74.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Higley AE, Kiefer SW, Li X, Gaal J, Xi ZX, et al. Dopamine D(3) receptor antagonist SB-277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. Eur J Pharmacol. 2011;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carati C, Schenk S. Role of dopamine D1- and D2-like receptor mechanisms in drug-seeking following methamphetamine self-administration in rats. Pharmacol Biochem Behav. 2011;98:449–454. doi: 10.1016/j.pbb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Brennan KA, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administration in rats. Behav Pharmacol. 2009;20:688–694. doi: 10.1097/FBP.0b013e328333a28d. [DOI] [PubMed] [Google Scholar]
- 79.Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- 80.Schenk S, Gittings D. Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN 35,428 in rats. Psychopharmacology (Berl) 2003;168:118–123. doi: 10.1007/s00213-002-1276-y. [DOI] [PubMed] [Google Scholar]
- 81.Self DW. Neural substrates of drug craving and relapse in drug addiction. Ann Med. 1998;30:379–389. doi: 10.3109/07853899809029938. [DOI] [PubMed] [Google Scholar]
- 82.Stark AD, Jordan S, Allers KA, Bertekap RL, Chen R, et al. Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT 2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology (Berl) 2007;190:373–382. doi: 10.1007/s00213-006-0621-y. [DOI] [PubMed] [Google Scholar]
- 83.Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addict Biol. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, et al. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 87.McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Langtry HD, Clissold SP. Pergolide. A review of its pharmacological properties and therapeutic potential in Parkinson’s disease. Drugs. 1990;39:491–506. doi: 10.2165/00003495-199039030-00009. [DOI] [PubMed] [Google Scholar]
- 89.Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci. 2007;27:6823–6831. doi: 10.1523/JNEUROSCI.0013-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 92.Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, et al. Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci. 2005;22:2744–2754. doi: 10.1111/j.1460-9568.2005.04467.x. [DOI] [PubMed] [Google Scholar]
- 93.Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 94.Osborne MP, Olive MF. A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann N Y Acad Sci. 2008;1139:206–211. doi: 10.1196/annals.1432.034. [DOI] [PubMed] [Google Scholar]
- 95.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 96.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- 99.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 101.Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- 102.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 103.Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, et al. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin-Fardon R, Weiss F. (−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl] piperidine (MTEP) similarly attenuate stress-induced reinstatement of cocaine seeking. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR2/3 agonist LY379268 and increased functional activity of mGluR2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.174. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 111.McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, et al. Open-label pilot study of modafinil for methamphetamine dependence. J Clin Psychopharmacol. 2009;29:488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 113.Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shearer J, Darke S, Rodgers C, Slade T, van Beek I, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 115.Holtz NA, Lozama A, Prisinzano TE, Carroll ME. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- 117.Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- 118.Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, et al. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- 119.Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, et al. Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. J Abnorm Psychol. 2000;109:738–742. [PubMed] [Google Scholar]
- 120.Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess. 2007;11:iii–iv. 1–85. doi: 10.3310/hta11060. [DOI] [PubMed] [Google Scholar]
- 121.Schmitz JM, Lindsay JA, Green CE, Herin DV, Stotts AL, et al. High-dose naltrexone therapy for cocaine-alcohol dependence. Am J Addict. 2009;18:356–362. doi: 10.3109/10550490903077929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 123.Rohsenow DJ. What place does naltrexone have in the treatment of alcoholism? CNS Drugs. 2004;18:547–560. doi: 10.2165/00023210-200418090-00001. [DOI] [PubMed] [Google Scholar]
- 124.Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- 125.Gerrits MA, Kuzmin AV, van Ree JM. Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. Eur Neuropsychopharmacol. 2005;15:297–303. doi: 10.1016/j.euroneuro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Leri F, Zhou Y, Goddard B, Levy A, Jacklin D, et al. Steady-state methadone blocks cocaine seeking and cocaine-induced gene expression alterations in the rat brain. Eur Neuropsychopharmacol. 2009;19:238–249. doi: 10.1016/j.euroneuro.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- 128.Hood S, Sorge RE, Stewart J. Chronic buprenorphine reduces the response to sucrose-associated cues in non food-deprived rats. Pharmacol Biochem Behav. 2007;86:566–575. doi: 10.1016/j.pbb.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 129.Hanson GR, Fleckenstein AE. Basic neuropharmacological mechanisms of methamphetamine. In: Roll JM, Rawson RA, Ling W, Shoptaw S, editors. Methamphetamine addiction. Guilford Press; New York: 2009. pp. 30–60. [Google Scholar]
- 130.Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trulson ME, Trulson VM. Reduction in brain serotonin synthesis rate following chronic methamphetamine administration in rats. Eur J Pharmacol. 1982;83:97–100. doi: 10.1016/0014-2999(82)90290-4. [DOI] [PubMed] [Google Scholar]
- 132.Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123:382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, et al. Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Engleman EA, Rodd ZA, Bell RL, Murphy JM. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol Disord Drug Targets. 2008;7:454–467. doi: 10.2174/187152708786927886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shoptaw S, Huber A, Peck J, Yang X, Liu J, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 137.Batki SL, Moon J, Delucchi K, Bradley M, Hersh D, et al. Methamphetamine quantitative urine concentrations during a controlled trial of fluoxetine treatment. Preliminary analysis. Ann N Y Acad Sci. 2000;909:260–263. doi: 10.1111/j.1749-6632.2000.tb06688.x. [DOI] [PubMed] [Google Scholar]
- 138.Piasecki MP, Steinagel GM, Thienhaus OJ, Kohlenberg BS. An exploratory study: the use of paroxetine for methamphetamine craving. J Psychoactive Drugs. 2002;34:301–304. doi: 10.1080/02791072.2002.10399967. [DOI] [PubMed] [Google Scholar]
- 139.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goeders NE, Clampitt DM. Potential role for the hypothalamopituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 141.Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, et al. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 143.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- 144.Mizuno T, Kurotani T, Komatsu Y, Kawanokuchi J, Kato H, et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 145.Huang Z, Liu S, Zhang L, Salem M, Greig GM, et al. Preferential inhibition of human phosphodiesterase 4 by ibudilast. Life Sci. 2006;78:2663–2668. doi: 10.1016/j.lfs.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 146.Ameri A, Wilhelm A, Simmet T. Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br J Pharmacol. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 148.Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212–2 in rats. Psychopharmacology (Berl) 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- 149.Fattore L, Spano MS, Deiana S, Melis V, Cossu G, et al. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007;53:1–16. doi: 10.1016/j.brainresrev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 150.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 151.Boctor SY, Martinez JL, Jr, Koek W, France CP. The cannabinoid CB1 receptor antagonist AM251 does not modify methamphetamine reinstatement of responding. Eur J Pharmacol. 2007;571:39–43. doi: 10.1016/j.ejphar.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maggio R, Riva M, Vaglini F, Fornai F, Molteni R, et al. Nicotine prevents experimental parkinsonism in rodents and induces striatal increase of neurotrophic factors. J Neurochem. 1998;71:2439–2446. doi: 10.1046/j.1471-4159.1998.71062439.x. [DOI] [PubMed] [Google Scholar]
- 153.Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106:72–78. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 155.Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97:861–869. doi: 10.1046/j.1360-0443.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- 156.Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298:172–179. [PubMed] [Google Scholar]
- 157.Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, et al. Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol. 2007;571:33–38. doi: 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Lobeline attenuates methamphetamine-induced stereotypy in adolescent mice. Neurochem Res. 2006;31:1359–1369. doi: 10.1007/s11064-006-9180-1. [DOI] [PubMed] [Google Scholar]
- 159.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 160.Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000b;95(Suppl 2):S145–153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- 161.Tiffany ST, Carter BL, Singleton EG. Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction. 2000a;95(Suppl 2):S177–187. doi: 10.1080/09652140050111753. [DOI] [PubMed] [Google Scholar]
- 162.McKay JR, Franklin TR, Patapis N, Lynch KG. Conceptual, methodological, and analytical issues in the study of relapse. Clin Psychol Rev. 2006;26:109–127. doi: 10.1016/j.cpr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 163.Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- 164.See RE, Grimm JW, Kruzich PJ, Rustay N. The importance of a compound stimulus in conditioned drug-seeking behavior following one week of extinction from self-administered cocaine in rats. Drug Alcohol Depend. 1999;57:41–49. doi: 10.1016/s0376-8716(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 165.Stewart J, De Wit H. Reinstatement of drug-taking behavior as a method for assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 211–227. [Google Scholar]
- 166.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, et al. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 168.Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 169.Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 170.Yan Y, Yamada K, Nitta A, Nabeshima T. Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res. 2007a;177:261–268. doi: 10.1016/j.bbr.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 171.Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. FASEB J. 2007b;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- 172.Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–134. doi: 10.1016/S0893-133X(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 173.Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- 174.Li SM, Ren YH, Zheng JW. Effect of 7-nitroindazole on drugpriming reinstatement of D-methamphetamine-induced conditioned place preference. Eur J Pharmacol. 2002;443:205–206. doi: 10.1016/s0014-2999(02)01580-7. [DOI] [PubMed] [Google Scholar]
- 175.DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, et al. Racemic gamma vinyl-GABA (R,S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse. 2009;63:87–94. doi: 10.1002/syn.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kwon YB, Li J, Kook JA, Kim TW, Jeong YC, et al. Bee venom suppresses methamphetamine-induced conditioned place preference in mice. Neurol Res. 2010;32(Suppl 1):101–106. doi: 10.1179/016164109X12537002794408. [DOI] [PubMed] [Google Scholar]
- 177.Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 178.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 179.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, et al. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 181.Graves SM, Napier TC. Mirtazapine alters cue-associated methamphetamine seeking in rats. Biol Psychiatry. 2011;69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]