Abstract
Background
Helicobacter pylori infected children from coastal Tumaco, Colombia have more parasitism, and adults have lower gastric cancer risk compared to high altitude Pasto/Tuquerres residents. Because helminth and Toxoplasma gondii infections alter helicobacter gastritis in rodent models, we determined if seropositivity to Ascaris lumbricoides or T. gondii was associated with Th2-IgG1 or Th1-IgG2 responses to H. pylori.
Methods
Sera (240) from the two populations were evaluated for A. lumbricoides and T. gondii seropositivity and results correlated with IgE and IgG isotype responses to H. pylori.
Results
Most Tumaco children and adults were seropositive for A. lumbricoides (89%, 66%), T. gondii (59%, 98%) or both (45%, 66%). In contrast, seropositivity among Pasto/Tuquerres children was much lower (9% A. lumbricoides, 11% T. gondii and 2% dual positive) but increased in adults (58% A. lumbricoides, 82% T. gondii and 41% dual positive). A. lumbricoides seropositivity correlated with elevated IgE and anti-inflammatory Th2-IgG1 responses to H. pylori, while Toxoplasma gondii seropositivity was linked to elevated IgE, pro-inflammatory Th1-IgG2, IgG3 and IgG4 responses to H. pylori. Individuals with high T. gondii titers had reduced Th1-IgG2, IgG3 and IgG4 responses to Helicobacter pylori.
Conclusions
Results support regional differences for childhood parasitism and indicate A. lumbricoides and T. gondii infections may impact inflammatory responses to H. pylori and partially explain differences in gastric cancer risk in Colombia.
Keywords: Helicobacter pylori, Ascaris lumbricoides, Toxoplasma gondii, immune response, gastric cancer, Colombia
Although gastric adenocarcinoma is initiated with Helicobacter pylori (Hp) infection (1), the basis for regional differences in the incidence of gastric cancer secondary to helicobacter infection remains unresolved (2). Hp-associated disease is most often limited to chronic but subclinical gastritis; other factors likely delay or promote the progression of gastritis to gastric adenocarcinoma (3). This multi-factorial etiology has been attributed to variations in Hp strain virulence (4) and origin (5), genetic polymorphisms of the human inflammatory response (6), environmental promoters such as smoking and dietary salt, environmental inhibitors including dietary antioxidants (7), and co-colonization of Hp-infected atrophic stomachs with enteric flora (8–9). Rodent models of helicobacter-induced chronic gastritis have provided evidence that concurrent helminth infections reduce gastric atrophy (10–11), a key premalignant lesion. Therefore, parasites may also reduce the risk for Hp-associated gastric cancer in humans.
Worldwide, approximately 50% of humans are infected with Hp but Hp prevalence is reported to be much higher in select populations, typically those of lower socioeconomic status (12–15). Several epidemiologic studies have evaluated gastric cancer risk for Colombian communities with a high prevalence (>90%) of Hp infection (16–18). Residents of coastal Tumaco have a lower incidence of Hp-associated atrophic gastritis (36.5%) and gastric cancer (6 per 100,000) compared to residents of Pasto/Tuquerres in the Andes Mountains, where the frequency of atrophic gastritis is 90.5% with a gastric cancer incidence of 150 per 100,000 (19–21).
Nematode infections are prevalent in areas of low socioeconomic status where individuals are commonly co-infected with Hp (18, 22). We reported that a higher prevalence of helminthiasis in Tumaco children, particularly Ascaris lumbricoides (Al), was associated with higher anti-inflammatory Th2-associated IgG1 isotype responses to Hp compared to Pasto/Tuquerres residents (18). These findings suggested that other parasites may influence the inflammatory response to Hp. Toxoplasma gondii (Tg), a tissue coccidian acquired by ingesting contaminated water, soil, or undercooked meat, (23) is a major health issue in Colombia (24–25) and worldwide (26). Tg stimulates Th1 host defenses (27) and promotes more severe gastritis and premalignant lesions with elevated gastric IFN-γ and IL-12 levels in gastritis-resistant BALB/c mice co-infected with H. felis (28). In contrast to rodent models of helicobacter gastritis in combination with helminth infections (10–11), co-infection with Tg could elevate the risk of Hp-associated gastric cancer in humans, although epidemiologic studies have not addressed this hypothesis.
Regional differences in gastric cancer incidence between adults living in Tumaco and Pasto/Tuquerres have persisted since the 1970s, despite nearly ubiquitous Hp infection. Given the prevalence of Hp infection and differences in parasite burden in Colombian children from these regions (18), we determined whether seropositivity for Al and Tg were associated with altered pro- or anti-inflammatory IgG isotype responses to Hp in a cross-sectional sampling of children and adults.
Methods
Study populations
Sera were obtained from volunteer, clinically healthy Colombians attending community health clinics in the regions of Tumaco and Pasto/Tuquerres. From Tumaco, 55 children aged 1–6 years and 41 adults aged 31–84 years were sampled, and serologic responses compared to 105 children aged 1–6 years and 39 adults aged 38–68 years from Pasto/Tuquerres. Individual serum IgE and IgG isotype responses to Hp and population-based fecal parasite screening in children were previously reported by our laboratory (18). In this follow-up study, sera that were IgG Hp seropositive were further screened for IgG to Al and IgM/IgG to Tg. Informed consent was obtained from adult participants and from parents of children, and the study was approved by the Massachusetts Institute of Technology Committee on Use of Human Experimental Subjects and the Ethics Committee of Universidad del Valle.
ELISA for total IgG
Because the previous study focused on childhood exposure to Hp and intestinal parasites and sera had been previously thawed and re-frozen, a subset of sera (n=73) from children from both locations was randomly screened to confirm that adequate total IgG levels remained using the Total Human IgG Assay (AlerCHEK, Inc., Portland, ME, USA). According to manufacturer instructions, sera were diluted 1:100,000 and compared to a standard curve with a dynamic range of 0.156 – 1 μg/ml. IgG levels were within the reference range for children (29) and did not differ by geographic location (data not shown).
ELISA for IgG response to A. lumbricoides
Sera were assayed for qualitative IgG antibody levels to Al antigen using the Ascaris lumbricoides IgG ELISA kit (IBL International GMBH, Hamburg, Germany) with ≥ 95% sensitivity and specificity for Al. According to manufacturer instructions, diluted sera and kit-supplied positive, negative, and cut-off controls were incubated in 96 well plates pre-coated with Al antigens. Antigen-antibody complexes were detected by horseradish peroxidase-labeled Protein A conjugate reacting with tetramethylbenzidine substrate. Samples were considered negative if the absorbance was lower than 10% below the cut-off value, positive if the absorbance was higher than 10% over the cut-off value or equivocal if the absorbance value was 10% above or below the cut-off value.
ELISA for IgM and IgG response to T. gondii
Qualitative IgM and quantitative IgG levels to Tg antigen were determined using the Platelia Toxo IgM and IgG ELISA kits (Bio-Rad Laboratories) following manufacturer instructions. Standard control sera provided in the kit were calibrated by the manufacturer against the WHO standard (TOXM 185) (30) and cut-off values established by comparison of 200 sera to an indirect immunofluorescence and direct agglutination test. Diluted samples and controls were incubated in 96 well plates coated with antibody to either human IgM or IgG. Tg antigen derived from tachyzoites and horseradish peroxidase-conjugated monoclonal antibody to Tg were sequentially incubated in each well followed by peroxidase substrate and chromogen. Samples were categorized as negative for IgM to Tg if the optical reading was < 80% of the cutoff value, equivocal when ≥ 80% but < 100% of the cutoff value, and positive when ≥ 100% of the cutoff value. For quantitative IgG responses to Tg based on a standard curve, sera were categorized as seronegative if values were less than 6 IU/ml, equivocal for values ≥ 6 but < 9 IU/ml, and seropositive for values ≥ 9 but < 240 IU/ml; ≥ 240 IU/ml were categorized as high titer values.
Statistical analysis
Statistical analysis was performed using STATA (version 11.0; StataCorp LC, College Station, TX, USA) and Prism (GraphPad Inc., La Jolla, CA, USA). To achieve normal distribution as confirmed by probability plots, quantitative IgG levels to Tg and total IgE levels were log transformed, and optical density readouts for the IgG isotype responses to Hp were divided by the subject’s total IgG response to Hp. Sera with equivocal Al or Tg results were assigned a missing value in STATA. Chi square analysis, including Fisher’s exact test, of serology results generated odds ratios and 95% confidence intervals for evaluating relationships between age, sex, and geographic location and Al and Tg serologic status. Al/Tg seropositivity was further analyzed by ANOVA. Data were stratified by Al and Tg serology status to analyze differences in Hp-specific IgG isotype responses using the two-tailed Student’s t-test. Significance was set at p<0.05.
Results
Previous evaluation indicated that 95% of 251 Tumaco and Pasto/Tuquerres residents were seropositive to Hp (18). Although a variety of intestinal parasites were detected in feces from children from both regions, helminth infections were statistically more prevalent in Tumaco children, presumably due to the tropical coastal environment compared to the drier, colder Andes. This current study was restricted to Hp seropositive sera to further evaluate if serologic responses to Al and Tg were associated with select IgG isotype responses to Hp. Of 240 Hp seropositive sera used to establish the impact of location, sex, and age on exposure risk to Al and Tg (Table 1), 24 sera yielded equivocal results for IgG to Al or IgM/IgG to Tg; therefore sample size varied by assay as indicated in figure legends.
Table 1.
Impact of location, sex and age on exposure to A. lumbricoides (Al) and T. gondii (Tg) in H. pylori-seropositive children and adults from Tumaco and Pasto/Tuquerres
| A. lumbricoides | Odds ratiob | 95% CIc | p value | ||
|---|---|---|---|---|---|
| Location | Tumaco | 96 (76; 79%)a | 12.6 | 6.4–25.2 | <0.001 |
| Pasto/Tuquerres | 134 (31; 23%) | - | |||
| Sex | Male | 100 (45; 45%) | 0.9 | 0.5–1.6 | 0.7 |
| Female | 130 (62; 48%) | - | |||
| Age | Adult (31–84 yrs.) | 79 (49; 62%) | 2.6 | 1.4–4.8 | <0.001 |
| Child (1–6 yrs.) | 151 (45; 30%) | - | |||
| Concurrent IgM/IgG to Tg | Yes | 100 (80; 80%) | 5.2 | 2.8–9.7 | <0.001 |
| No | 116 (36; 31%) | - | |||
|
| |||||
| T. gondii | |||||
|
| |||||
| Location | Tumaco | 90 (68; 76%) | 8.0 | 4.2–15.4 | <0.001 |
| Pasto/Tuquerres | 136 (38; 26%) | - | |||
| Sex | Male | 95 (41; 43%) | 0.8 | 0.4–1.4 | 0.3 |
| Female | 131 (65; 50%) | - | |||
| Age | Adult (31–84 yrs.) | 74 (67; 92%) | 27.7 | 11.3–76.4 | <0.001 |
| Child (1–6 yrs.) | 152 (39; 27%) | - | |||
| Concurrent IgG to Al | Yes | 100 (80; 80%) | 5.2 | 2.8–9.7 | <0.001 |
| No | 116 (36; 31%) | - | |||
number of sera evaluated, number and percent positive
odds ratio in comparison to baseline (−) condition
95% CI, 95% confidence interval
Seropositivity to A. lumbricoides and T. gondii was more common in coastal Tumaco compared to high altitude Pasto/Tuquerres
Serology data indicate a significant environmental risk for human exposure to Al and Tg infections, particularly in Tumaco (Table 1). Tumaco residents were nearly 13 times more likely to be seropositive for Al (p<0.001) and 8 times more likely to be seropositive for Tg (p<0.001) compared to Pasto/Tuquerres residents. Males and females were at similar risk, but adults were nearly 3 times more likely than children to be Al seropositive (p<0.001) and 28 times more likely to be Tg seropositive (p<0.001). Concurrent seropositivity for Al and Tg was 5 times more likely than for either parasite alone (p<0.001).
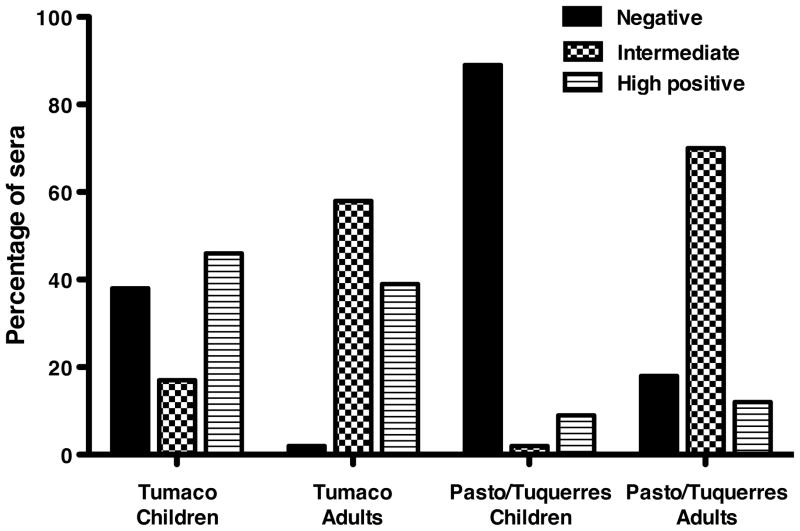
A high percentage of Tumaco children (89%) were seropositive for Al and 59% were seropositive for Tg; 45% were dual seropositive and no Tumaco children were seronegative for both parasites (Table 2). Nine children in Tumaco (17%) had both IgM and IgG titers to Tg, and 21 children had mature Tg IgG titers without IgM. High titers to Tg (>240 IU/ml) were measured in 37% of sera from Tumaco children (Figure 1). In contrast, only 9% of Pasto/Tuquerres children were Al seropositive, 11% were Tg seropositive with the majority (9 of 11) of these having high Tg titers. Just 2% were seropositive for both parasites.
Table 2.
A. lumbricoides and T. gondii serology status of H. pylori-positive children and adults from Tumaco and Pasto/Tuquerres
| Ascaris lumbricoides (Al) | Toxoplasma gondii (Tg) | Al & Tg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgG | IgG | ||||||||||
| Neg | Pos | % Pos | Neg | Pos | % Pos | Neg | Pos | % Pos | Dbl Neg | Dbl Pos | % Dbl Pos | ||
| Tumaco | Children n=55* | 6 | 49 | 89% | 44 | 9 | 17% | 21 | 30 | 59% | 0 | 25 | 45% |
| Adults n=41** | 14 | 27 | 66% | 40 | 0 | 0% | 1 | 40 | 98% | 1 | 27 | 66% | |
|
|
|||||||||||||
| Pasto Tuquerres | Children n=105*** | 87 | 9 | 9% | 105 | 0 | 0% | 92 | 11 | 11% | 77 | 2 | 2% |
| Adults**** n=39 | 16 | 22 | 58% | 36 | 1 | 3% | 6 | 27 | 82% | 2 | 16 | 41% | |
Neg = negative serology; Pos = positive serology; Dbl = double; % = percent. All data represent absolute numbers and percentage of samples that yielded non-equivocal serology status for Al and Tg.
No equivocal results for IgG to Al, 2 sera equivocal for IgM, 6 equivocal for IgG to Tg;
No equivocal results for IgG to Al, 1 serum equivocal for IgM, none equivocal for IgG to Tg;
9 equivocal results for IgG to Al, none equivocal for IgM, but 2 sera equivocal for IgG to Tg;
1 equivocal result for IgG to Al, 2 sera equivocal for IgM, 6 for IgG to Tg. Final sample size was 216 sera with unequivocal results.
Figure 1.
Percentage of sera with negative (<6 IU/ml), intermediate (> 9 but < 240 IU/ml) or high Tg titers (>240 IU/ml). In children and adults from Tumaco, 37% and 39% of sera, respectively, were categorized as high titer. Although only 11% of Pasto/Tuquerres children were Tg seropositive, most had high titers. High titers to Tg were observed in only 12% of Pasto/Tuquerres adults. Absolute sample numbers were as follows; Tumaco children negative n=21, intermediate n=8, high titer n=22; Tumaco adults negative n=1, intermediate n=24, high titer n=16; Pasto/Tuquerres children negative n=92, intermediate n=2, high titer n=9; Pasto/Tuquerres adults negative n=6, intermediate n=23, high titer n=4.
Positive serology for A. lumbricoides and T. gondii increased with age, and prevalence converged between adult populations
Most adults (66%) in Tumaco had antibodies to both parasites (Table 2). One third of adults (34%) were seropositive for Tg only with an overall Tg seroprevalence of 98%, all IgM negative and IgG positive with 39% categorized as high titer to Tg. Only one of 41 Tumaco adults was seronegative for both Al and Tg. In Pasto/Tuquerres, low seroprevalence for Al and Tg in children contrasted with much higher prevalence in adults. Serology data from the adults indicated 58% were seropositive for Al and 82% for Tg with 12% of Tg seropositive individuals having high IgG titers. Titers to both parasites were noted in 41% of the adults from Pasto/Tuquerres. Thus, seroprevalence in adult residents of Tumaco and Pasto/Tuquerres were much more similar than regional differences between children. Indeed, Al seroprevalence was so common in adults that regional differences were not statistically significant but Tg seroprevalence was significantly lower in Pasto/Tuquerres adults (p<0.05).
IgG responses to A. lumbricoides and T. gondii were associated with elevated serum IgE levels
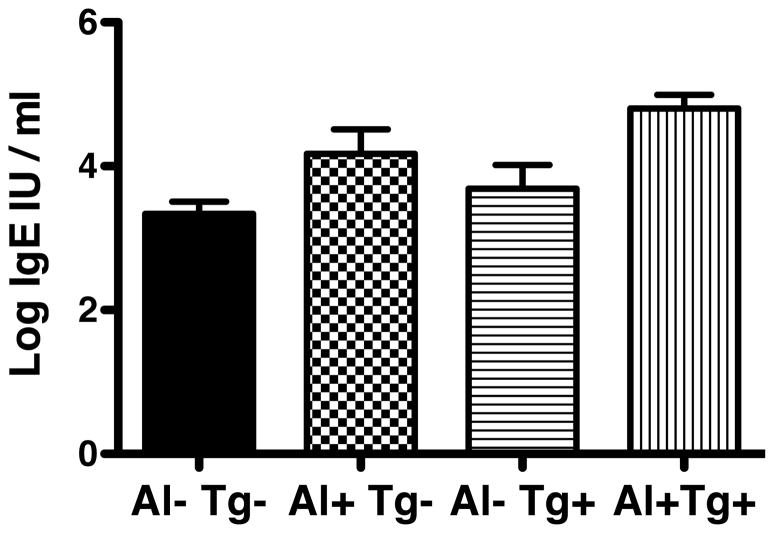
We previously reported that serum IgE levels were elevated in Tumaco residents compared to Pasto/Tuquerres residents and attributed the difference to higher helminthiasis in Tumaco children compared to Pasto/Tuquerres children (18), acknowledging that parasite test results were not available for adults. Current data enabled sorting IgE levels based on serologic evidence for parasite exposure. Seropositivity for Al or Tg was similarly associated with elevated IgE (p<0.01 and p=0.01, respectively; Figure 2), supporting that IgE levels developed principally in response to parasitism.
Figure 2.
Serum IgE levels in Colombians that were seronegative (−) or positive (+) for Al and Tg were compared by ANOVA. Seropositivity for Al or Tg (p<0.001, p=0.01) were significantly associated with elevated IgE. Sample numbers were as follows Al−Tg− n=80; Al+Tg− n= 36; Al−Tg+ n= 30, Al+Tg+ n= 70. Bars represent standard error of the mean.
IgG to A. lumbricoides was associated with enhanced Th2-IgG1 and IgG to T. gondii with higher Th1-IgG2 responses to H. pylori
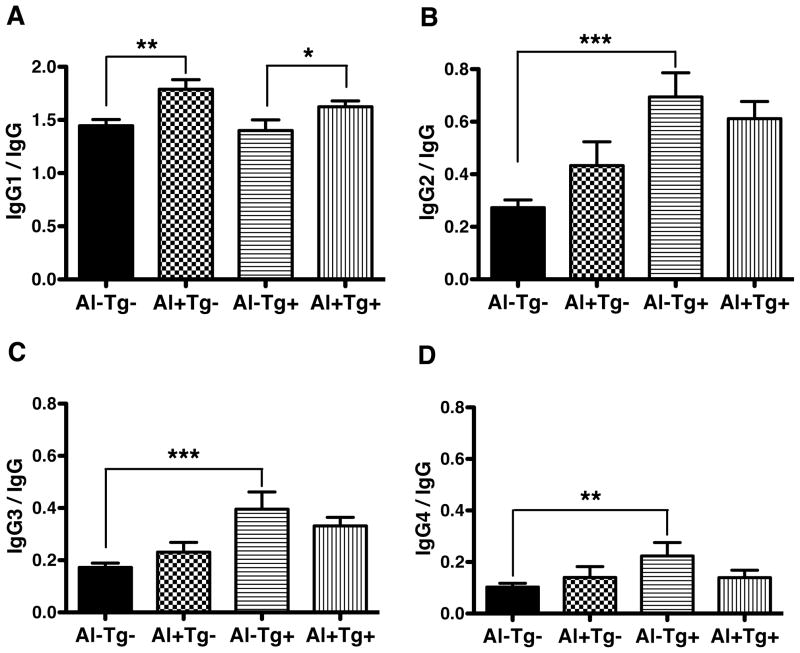
Serum levels of Hp-specific IgG1, IgG2, IgG3, and IgG4 from Hp-positive children and adults were stratified by seronegative or seropositive status for Al and Tg and compared by the two-tailed Student’s t-test. IgG titers to Al were associated with enhanced anti-inflammatory Th2-associated IgG1 responses to Hp in both Tg seronegative and seropositive individuals (p<0.01 and p<0.05, respectively) (Figure 3A) with no significant association with IgG2, IgG3, or IgG4 responses to Hp (Figure 3B–D). IgG to Tg did not impact Th2-IgG1 responses to Hp but was associated with enhanced Th1-associated IgG2 responses to Hp (p<0.0001), as well as elevated IgG3 (p<0.0001) and IgG4 responses (p<0.01), but only in Al seronegative sera. Notably, Th2-IgG1 responses to Hp were unaffected by high IgG titers to Tg (data not shown). For unexplained reasons, the Th1-IgG2, as well as IgG3 and IgG4 responses to Hp were all reduced in high titer Tg sera (p<0.0001, p<0.001, p<0.003, respectively, data not shown).
Figure 3.
Mean ratios of serum Hp-specific IgG1 (A), IgG2 (B), IgG3 (C) and IgG4 (D) from Hp-positive children and adults from Tumaco and Pasto/Tuquerres were stratified by seronegative (−) or positive (+) status for Al and Tg and compared by the two-tailed Student’s t-test. IgG titers to Al were associated with enhanced anti-inflammatory Th2-asssociated IgG1 responses to Hp in both Tg seronegative (** p<0.01) and Tg seropositive individuals (* p<0.05). Tg seropositivity was linked to higher pro-inflammatory Th1-IgG2 (*** p<0.0001), as well as IgG3 (*** p<0.001) and IgG4 responses to Hp (** p<0.01) in Al negative sera. Sample numbers were as follows Al−Tg− n=80; Al+Tg− n= 36; Al−Tg+ n= 30, Al+Tg+ n= 70. Bars represent standard error of the mean.
Discussion
This study is the first to establish regional differences in A. lumbricoides (Al) and T. gondii (Tg) seroprevalence in Colombians residing in Tumaco and Pasto/Tuquerres and is supported by results from our previous fecal screening for parasitic ova in children living in these two areas (18). Although differences in childhood seroprevalence to Al and Tg were dramatic, increasing age was a significant risk factor for Al (odds ratio 2.6) and even more dramatically for Tg exposure (odds ratio 27.7). Al seroprevalence was so common in adults that regional differences were not statistically significant but Tg seroprevalence was significantly lower in Pasto/Tuquerres adults. Importantly, the matched data for Hp-, Al-, and Tg-induced antibody responses within these Colombian populations validate that Al and Tg infections were common and may have biased inflammatory response to Hp infection. Despite high seroprevalence of Al and Tg in adults from both areas, it was possible to detect a significant association between Al and Tg seropositivity and higher Th2-IgG1 and Th1-IgG2 responses to Hp, respectively. Consistent with the hygiene hypothesis (31), these results suggest that pediatric infections with Hp, Al, and Tg may be representative of a spectrum of illnesses that condition a balanced host immune response to exogenous antigens. Infections early in life may be protective by inducing regulatory networks capable of preventing or delaying onset of sequelae such as gastric cancer, that develop after decades of Hp-associated gastritis.
Previously, only population-based fecal parasitology data was available for Tumaco and Pasto/Tuquerres children without epidemiologic evidence for either fecal shedding or serologic evidence of parasites in adults (18). Compared to Pasto/Tuquerres, Tumaco children had higher fecal shedding of Al, Trichuris trichiura (whipworms), and Giardia amongst a spectrum of other parasites including hookworms, strongyles, pinworms, tapeworms, and 8 different protozoa (18). Because parasite ova shedding can be intermittent and Colombians with gastrointestinal symptoms living in underdeveloped areas are often treated with anthelminthics (32), seroprevalence rates indicate higher helminth exposure than we reported by fecal testing and likely represent a more accurate estimate of environmental exposure. The children and adults evaluated in this study were attending rural health clinics and may have received anthelminthics. Although helminth eradication has been shown to not restore expression of select innate immunity genes to control levels (33), anthelminthic therapy is unlikely to have impacted seroprevalence of Al or Tg. The serology data clearly indicate that Tumaco children were at much greater risk for Al and Tg exposure compared to Pasto/Tuquerres children, likely due to the colder and drier environment in Pasto/Tuquerres. Tropical conditions in Tumaco are comparable to other coastal areas of Colombia with similar problematic parasitic infections (34). Tumaco residents also likely have significant contact with contaminated water sources as shown for Tg infections in Brazil (35).
The shift from comparatively low seroprevalence of Al and Tg in Pasto/Tuquerres children to higher prevalence in adults may be due to low but chronic exposure to fewer infective oocysts in the environment of the Andes compared to the coast. A low but chronic exposure to oocysts may also explain the lower prevalence of high Tg titers in Pasto/Tuquerres adults compared to Tumaco. Compared to children, increased seroprevalence in Pasto/Tuquerres adults may potentially reflect greater exposure of adults to Tg-contaminated meat. Importantly, lower exposure of Pasto/Tuquerres children to Al and Tg may be key to the higher gastric cancer risk in adults compared to Tumaco where childhood parasitism was endemic. Al and Tg infections in children are maintained by parasite-specific immune responses shown to have significant effects on dendritic cells (36) and may alter responses to unrelated antigens such as vaccines (37–38). Indeed, the hygiene hypothesis attributes the increasing trend of allergic, autoimmune, and other inflammatory diseases in industrialized countries to decreased antigenic stimulation during childhood, resulting in more polarized immune responses when individuals are eventually exposed to a specific microorganism (31).
Al seropositive individuals had enhanced IgE and higher anti-inflammatory Th2-IgG1 responses to Hp, consistent with prior reports that children in undeveloped areas frequently become infected with Hp and parasites within the first few years of life and respond with a Th2 immune response (13, 18, 39). Robust IgE and other Th2-predominant immune responses to helminth infection are promoted by Th2-associated cytokines, including IL-4 and IL-5, and commonly persist in children despite anthelminthic treatment (40). Hp-induced gastritis in adults living in low socioeconomic conditions is also commonly characterized as Th2-predominant (13, 18, 39). In contrast, pro-inflammatory Th17/Th1 responses are more typical of helicobacter gastritis in developed areas of the world (41–43). Unfortunately, suitable samples for evaluating cytokine responses to Hp in these populations were not collected.
Tg was evaluated because epidemiologic evidence supports its high prevalence in Colombian human and animal populations (24–25). The predominance of IgG titers to Tg in the absence of IgM, particularly in adults from both areas, indicates primary exposure was not recent and likely reflects a mature immune response to ongoing re-exposure from the environment. Human and animal studies have demonstrated that Th1-skewed immune responses driven by Tg can counter Th2 responses induced by fluke and nematode infections, respectively (44–45). This study links Tg seroprevalence to higher Th1-IgG2 responses to Hp. Tg seroprevalence, especially high titers in children, also impacted IgG3 and IgG4 titers to Hp. The significance of IgG3 and IgG4 responses to Hp are poorly understood but IgG3 has been associated with peptic ulcer disease and antral inflammation in Hp infected children (46). IgG4 production is promoted by Th2 anti-inflammatory cytokines such as IL-10 and is believed to be most relevant to non-microbial, allergy conditions (47) and helminth infections in humans (48). Elevated IgE was associated with seropositivity to both parasites. Although IgE reduces Al numbers in the gut, IgG4 can block IgE directed against Al, decreasing resistance to infection particularly in children (49).
Rodent models (10–11) and epidemiologic studies (18, 50) support the hypothesis that concurrent helminth infection delays progression of Hp-induced premalignant lesions to gastric cancer. Our study demonstrates that Colombians living in Tumaco, and less so in Pasto/Tuquerres, are likely continually exposed to Hp, Al, Tg, and a broader spectrum of enteric pathogens including parasitic infections as previously detected by fecal testing (18). T. trichiura is a candidate for future study because it was prevalent in Tumaco children (18), and Al and T. trichiura infections in poor Brazilian children were associated with enhanced IL-5 and IL-10 production by peripheral blood mononuclear cells along with hyporesponsiveness to mitogen stimulation in culture (51). Our data are also consistent with elevated serum levels of IL-5, IL-10, and TNFα with an elevated IL-10/IL-12 ratio in asymptomatic African refugee children concurrently infected with Hp, malaria and helminths (52).
This study was limited to a cross-sectional analysis by serology, but future work will focus on a longitudinal analysis pairing parasite infection status, anthelmintic therapy, and cytokine responses with gastric pathology in Hp-infected adults from these same regions. Recent gastric biopsy data indicated eosinophil density was significantly higher in men from Tumaco compared to Pasto/Tuquerres, and eosinophil numbers increased in parallel with lesion progression to atrophic gastritis, while mast cell density increased in parallel with lesion progression in both populations (53). Eosinophils were decreased in Tumaco men with intestinal metaplasia and dysplasia compared to Pasto/Tuquerres men with similar lesions. This disparate association may be an indicator of anti- or pro-inflammatory response to Hp, since eosinophils not only increase in Hp gastritis but are a key responding cell type in parasitic diseases.
These results support regional differences in Colombia for childhood parasitism as one of the risk factors for gastric cancer in adults against a background of other potentially important epidemiological factors. These include differences in diet with fish as the predominant food source along with more antioxidants in fresh fruits and vegetables in Tumaco and higher grain and salt intake in the Pasto/Tuquerres region; the significance of some of these factors along with smoking have remained controversial (54). There has been no analysis of genetic polymorphisms related to gastric cancer as done elsewhere (55) and ethnicity between regions is quite different. The Tumaco population is largely mixed in African and European ancestry and Pasto/Tuquerres ‘Mestizos’ are of Amerindian descent with European admixture (7, 11, 16). Notably, more is known about the genetics of the Hp strains infecting residents of Tumaco and Pasto/Tuquerres (5) than the indigenous genetic polymorphisms that likely also contribute to the disparity in gastric cancer risk. Consistent with human migration to these areas, multi-locus sequencing of 64 cagA and vacA s1m1 positive Hp strains were of European origin in high risk Pasto/Tuquerres and in low risk Tumaco, most residents (66%) were infected with African Hp strains, with the remainder infected with European strains. Thus, as with exposure to Al and Tg, environmental exposure to specific Hp strains likely contributes to gastric cancer risk. Our study provides further insight into the complex relationship between parasites, Hp, and the inflammatory response and therefore may partially explain regional differences in gastric cancer risk for Colombian populations with similar high infection rates with Hp.
Acknowledgments
The authors thank Steven Tsai for invaluable assistance in the preparation of this manuscript.
Funding:
This work was supported by the National Institutes of Health grants T32RR07036, PO1CA028842-23 and P30ES02109.
Footnotes
Conflict of Interest Statement:
The authors do not have any associations that might pose a conflict of interest.
Previous Presentation of Data:
Portions of this data were presented at Digestive Disease Week in June 2009 in Chicago, IL (abstract 587616).
References
- 1.Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010 Dec;24(4):853–69. vii. doi: 10.1016/j.idc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33(4):429–31. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010 Mar;105(3):493–8. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres S, Schmidt HM, Mitchell H, Rhen M, Maeurer M, Engstrand L. Helicobacter pylori defines local immune response through interaction with dendritic cells. FEMS Immunol Med Microbiol. 2010 Nov 16; doi: 10.1111/j.1574-695X.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 5.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011 Sep;60(9):1189–95. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macarthur M, Hold GL, El Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. AmJPhysiol GastrointestLiver Physiol. 2004;286(4):G515–G20. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez CA, Lopez-Carrillo L. Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scand J Gastroenterol. 2010;45(1):6–14. doi: 10.3109/00365520903401959. [DOI] [PubMed] [Google Scholar]
- 8.Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011 Jan;140(1):210–20. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006 Jan 17;103(3):732–7. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000 May;6(5):536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 11.Martin HR, Shakya KP, Muthupalani S, Ge Z, Klei TR, Whary MT, et al. Brugia filariasis differentially modulates persistent Helicobacter pylori gastritis in the gerbil model. Microbes Infect. 2010 Sep;12(10):748–58. doi: 10.1016/j.micinf.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correa P, Fox J, Fontham E, Ruiz B, Lin YP, Zavala D, et al. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66(12):2569–74. doi: 10.1002/1097-0142(19901215)66:12<2569::aid-cncr2820661220>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell HM, Ally R, Wadee A, Wiseman M, Segal I. Major differences in the IgG subclass response to Helicobacter pylori in the first and third worlds. Scand J Gastroenterol. 2002 May;37(5):517–22. doi: 10.1080/00365520252903044. [DOI] [PubMed] [Google Scholar]
- 14.Tanih NF, Dube C, Green E, Mkwetshana N, Clarke AM, Ndip LM, et al. An African perspective on Helicobacter pylori: prevalence of human infection, drug resistance, and alternative approaches to treatment. Ann Trop Med Parasitol. 2009 Apr;103(3):189–204. doi: 10.1179/136485909X398311. [DOI] [PubMed] [Google Scholar]
- 15.Vorobjova T, Ren Z, Dunkley M, Clancy R, Maaroos HI, Labotkin R, et al. Response of IgG1 and IgG2 subclasses to Helicobacter pylori in subjects with chronic inflammation of the gastric mucosa, atrophy and gastric cancer in a country with high Helicobacter pylori infection prevalence. APMIS. 2006 May;114(5):372–80. doi: 10.1111/j.1600-0463.2006.apm_392.x. [DOI] [PubMed] [Google Scholar]
- 16.Camargo MC, Yepez MC, Ceron C, Guerrero N, Bravo LE, Correa P, et al. Age at acquisition of Helicobacter pylori infection: comparison of two areas with contrasting risk of gastric cancer. Helicobacter. 2004;9(3):262–70. doi: 10.1111/j.1083-4389.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodman KJ, Correa P, Tengana Aux HJ, Ramirez H, DeLany JP, Guerrero PO, et al. Helicobacter pylori infection in the Colombian Andes: a population-based study of transmission pathways. AmJ Epidemiol. 1996;144(3):290–9. doi: 10.1093/oxfordjournals.aje.a008924. [DOI] [PubMed] [Google Scholar]
- 18.Whary MT, Sundina N, Bravo LE, Correa P, Quinones F, Caro F, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005 Jun;14(6):1464–9. doi: 10.1158/1055-9965.EPI-05-0095. [DOI] [PubMed] [Google Scholar]
- 19.Correa P, Cuello C, Duque E, Burbano LC, Garcia FT, Bolanos O, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976 Nov;57(5):1027–35. doi: 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 20.Bravo LE, van Doom LJ, Realpe JL, Correa P. Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? AmJGastroenterol. 2002;97(11):2839–42. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998 Jun;114(6):1169–79. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 22.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009 May 2;373(9674):1570–5. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 23.Pereira KS, Franco RM, Leal DA. Transmission of Toxoplasmosis (Toxoplasma gondii) by Foods. Adv Food Nutr Res. 2010;60:1–19. doi: 10.1016/S1043-4526(10)60001-0. [DOI] [PubMed] [Google Scholar]
- 24.Rosso F, Les JT, Agudelo A, Villalobos C, Chaves JA, Tunubala GA, et al. Prevalence of infection with Toxoplasma gondii among pregnant women in Cali, Colombia, South America. Am J Trop Med Hyg. 2008 Mar;78(3):504–8. [PubMed] [Google Scholar]
- 25.Dubey JP, Cortes-Vecino JA, Vargas-Duarte JJ, Sundar N, Velmurugan GV, Bandini LM, et al. Prevalence of Toxoplasma gondii in dogs from Colombia, South America and genetic characterization of T. gondii isolates. Vet Parasitol. 2007 Apr 10;145(1–2):45–50. doi: 10.1016/j.vetpar.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Innes EA. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health. 2010 Feb;57(1):1–7. doi: 10.1111/j.1863-2378.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- 27.Yap GS, Shaw MH, Ling Y, Sher A. Genetic analysis of host resistance to intracellular pathogens: lessons from studies of Toxoplasma gondii infection. Microbes Infect. 2006 Apr;8(4):1174–8. doi: 10.1016/j.micinf.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Stoicov C, Whary M, Rogers AB, Lee FS, Klucevsek K, Li H, et al. Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J Immunol. 2004 Sep 1;173(5):3329–36. doi: 10.4049/jimmunol.173.5.3329. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs D, Altman DG, Tidmarsh CE, Valman HB, Webster AD. Serum immunoglobulin concentrations in preschool children measured by laser nephelometry: reference ranges for IgG, IgA, IgM. Journal of clinical pathology. 1983 Oct;36(10):1193–6. doi: 10.1136/jcp.36.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiter-Owona I, Petersen E, Joynson D, Aspock H, Darde ML, Disko R, et al. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull World Health Organ. 1999;77(11):929–35. [PMC free article] [PubMed] [Google Scholar]
- 31.Ewald PW. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: symbionts and immunopathology in chronic diseases: insights from evolution. Clin Exp Immunol. 2010 Apr;160(1):27–34. doi: 10.1111/j.1365-2249.2010.04127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harhay MO, Horton J, Olliaro PL. Epidemiology and control of human gastrointestinal parasites in children. Expert Rev Anti Infect Ther. 2010 Feb;8(2):219–34. doi: 10.1586/eri.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acevedo N, Mercado D, Vergara C, Sanchez J, Kennedy MW, Jimenez S, et al. Association between total immunoglobulin E and antibody responses to naturally acquired Ascaris lumbricoides infection and polymorphisms of immune system-related LIG4, TNFSF13B and IRS2 genes. Clin Exp Immunol. 2009 Aug;157(2):282–90. doi: 10.1111/j.1365-2249.2009.03948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaudaux JD, Muccioli C, James ER, Silveira C, Magargal SL, Jung C, et al. Identification of an atypical strain of Toxoplasma gondii as the cause of a waterborne outbreak of toxoplasmosis in Santa Isabel do Ivai, Brazil. J Infect Dis. 2010 Oct 15;202(8):1226–33. doi: 10.1086/656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho L, Sun J, Kane C, Marshall F, Krawczyk C, Pearce EJ. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology. 2009 Jan;126(1):28–34. doi: 10.1111/j.1365-2567.2008.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper PJ, Chico ME, Gaus D, Griffin GE. Relationship between bacille Calmette-Guerin vaccination, Mantoux test positivity, and geohelminth infection. Trans R Soc Trop Med Hyg. 2003 Jul–Aug;97(4):473–6. doi: 10.1016/s0035-9203(03)90094-0. [DOI] [PubMed] [Google Scholar]
- 37.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001 Mar;69(3):1574–80. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres J, Perez GP, Ximenez C, Munoz L, Camorlinga-Ponce M, Ramos F, et al. The association of intestinal parasitosis and H. pylori infection in children and adults from a Mexican community with high prevalence of parasitosis. Helicobacter. 2003 Jun;8(3):179–85. doi: 10.1046/j.1523-5378.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 39.Wright VJ, Ame SM, Haji HS, Weir RE, Goodman D, Pritchard DI, et al. Early exposure of infants to GI nematodes induces Th2 dominant immune responses which are unaffected by periodic anthelminthic treatment. PLoS Negl Trop Dis. 2009;3(5):e433. doi: 10.1371/journal.pntd.0000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998 Mar;114(3):482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 41.D’Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Helicobacter pylori, T cells and cytokines: the “dangerous liaisons”. FEMS Immunol Med Microbiol. 2005 May 1;44(2):113–9. doi: 10.1016/j.femsim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Khamri W, Walker MM, Clark P, Atherton JC, Thursz MR, Bamford KB, et al. Helicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytes. Infect Immun. 2010 Feb;78(2):845–53. doi: 10.1128/IAI.00524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller CM, Smith NC, Ikin RJ, Boulter NR, Dalton JP, Donnelly S. Immunological interactions between 2 common pathogens, Th1-inducing protozoan Toxoplasma gondii and the Th2-inducing helminth Fasciola hepatica. PLoS One. 2009;4(5):e5692. doi: 10.1371/journal.pone.0005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan IA, Hakak R, Eberle K, Sayles P, Weiss LM, Urban JF., Jr Coinfection with Heligmosomoides polygyrus fails to establish CD8+ T-cell immunity against Toxoplasma gondii. Infect Immun. 2008 Mar;76(3):1305–13. doi: 10.1128/IAI.01236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dzierzanowska-Fangrat K, Raeiszadeh M, Dzierzanowska D, Gladkowska-Dura M, Celinska-Cedro D, Crabtree JE. IgG subclass response to Helicobacter pylori and CagA antigens in children. Clin Exp Immunol. 2003 Dec;134(3):442–6. doi: 10.1111/j.1365-2249.2003.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009 Apr;39(4):469–77. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 47.Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol. 2010 Sep;104(6):455–64. doi: 10.1179/136485910X12786389891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005 Jun;7(7–8):990–6. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 49.Geiger SM, Massara CL, Bethony J, Soboslay PT, Carvalho OS, Correa-Oliveira R. Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunol. 2002;24(11–12):499–509. doi: 10.1046/j.1365-3024.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 50.Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010 Apr 19; doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherian S, Burgner DP, Cook AG, Sanfilippo FM, Forbes DA. Associations between Helicobacter pylori infection, co-morbid infections, gastrointestinal symptoms, and circulating cytokines in African children. Helicobacter. 2010 Apr;15(2):88–97. doi: 10.1111/j.1523-5378.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- 52.Piazuelo MB, Camargo MC, Mera RM, Delgado AG, Peek RM, Jr, Correa H, et al. Eosinophils and mast cells in chronic gastritis: possible implications in carcinogenesis. Hum Pathol. 2008 Sep;39(9):1360–9. doi: 10.1016/j.humpath.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camargo MC, Burk RF, Bravo LE, Piazuelo MB, Hill KE, Fontham ET, et al. Plasma selenium measurements in subjects from areas with contrasting gastric cancer risks in Colombia. Arch Med Res. 2008 May;39(4):443–51. doi: 10.1016/j.arcmed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003 May;124(5):1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]