Abstract
Isolectin B4-positive [IB4(+)] primary afferent nociceptors challenged with an inflammatory or neuropathic insult develop a PKCε-dependent long-lasting hyperalgesic response to a subsequent challenge by the proinflammatory cytokine prostaglandin E2 (PGE2), a phenomenon known as hyperalgesic priming. Here we demonstrate that the neuroplasticity underlying nociceptor priming requires 72 h to be established; rats that have been challenged with the inflammatory mediator TNFα 24 or 48 h ahead of PGE2 do not show the enhanced and prolonged hyperalgesic response by which primed IB4(+)-nociceptors are being characterized. Moreover, as the underlying plasticity can be interrupted by the peripheral administration of the protein translation inhibitor anisomycin it is reflected by changes in the peripheral protein expression pattern. Finally, the induction of priming by the selective PKCε agonist, psi ε receptor for activated c kinase (ψεRACK) can be prevented, but not reversed by intrathecal injections of antisense oligodeoxynucleotides for the cytoplasmic polyadenylation element binding protein (CPEB) mRNA, a master regulator of protein translation that coimmunoprecipitated with PKCε and is almost exclusively expressed by IB4(+)-nociceptors. Our results suggest that CPEB is downstream of PKCε in the cellular signaling cascade responsible for the induction of priming, raising the intriguing possiblity that prion-like misfolding could be a responsible mechanism for the chronification of pain.
Introduction
Hyperalgesic priming has been defined as a long-lasting latent hyperresponsiveness of nociceptors to inflammatory mediators subsequent to an inflammatory or neuropathic insult (Aley et al., 2000; Parada et al., 2003b; Reichling and Levine, 2009). The neuroplasticity associated with the primed state can be detected behaviorally as an enhanced and prolonged hyperalgesic response to the proinflammatory cytokine prostaglandin E2 (PGE2) and be explained by a switch in the intracellular signaling pathway mediating PGE2-induced hyperalgesia (Aley et al., 2000; Khasar et al., 2008). In naive rats PGE2-induced hyperalgesia resolves within 4 h and is mediated by GαS-PKA-signaling (Aley et al., 2000). In contrast, in primed rats PGE2-induced hyperalgesia lasts for >24 h (Aley et al., 2000). The early phase of PGE2 hyperalgesia in primed rats is still mediated by GαS-PKA whereas the late phase is mediated by Gi/o-PKCε (Aley et al., 2000; Khasar et al., 2008; Dina et al., 2009). Moreover, the dose–response relationship for PGE2 hyperalgesia is shifted to the left by one order of magnitude (Parada et al., 2003b) suggesting that hyperalgesic priming also causes an increase in the magnitude of hyperalgesia.
Since hyperalgesic priming is still present months later (Aley et al., 2000; Parada et al., 2003b; Ferrari et al., 2010) it is likely associated with the formation of a molecular memory in primary afferent nociceptors. Given the continuous turnover of most cellular proteins it is difficult to conceive that a simple switch in the coupling of a G-protein coupled receptor is sufficient to explain the endurance of the plasticity associated with the “primed” nociceptor. As hyperalgesic priming is induced by PKCε activity and restricted to a subpopulation of small diameter C-fiber afferents, the so-called isolectin B4-positive [IB4(+)]-nociceptors (Aley et al., 2000; Parada et al., 2003b; Joseph and Levine, 2010), the search for molecular candidates that maintain the plastic changes associated with the priming and constitute the molecular memory should focus on molecules that are: (1) selectively expressed by IB4(+)-nociceptors and (2) downstream of PKCε in the signaling cascade mediating hyperalgesic priming.
Here we show that the plastic changes that maintain primed nociceptors in their long-lasting hyperexcitable state need ∼72 h to be established and that they are reflected by changes in the peripheral protein expression pattern. We also show that the cytoplasmic polyadenylation element binding protein (CPEB), an RNA-binding molecule that regulates the translation of otherwise dormant mRNAs (Richter, 2007; Villalba et al., 2011) is not only almost exclusively expressed by IB4(+)-nociceptors and can be coimmunoprecipitated with PKCε, the downregulation of its expression level by the intrathecal administration of antisense oligodeoxynucleotides (ODNs) targeting its mRNA can prevent, but not reverse, the induction of priming by the selective PKCε agonist ψεRACK (Dorn et al., 1999; Aley et al., 2000). Our results are compatible with the idea that a self-perpetuating conformer of CPEB constitutes a form of pain memory in the primary afferent nociceptor.
Materials and Methods
Animals.
All experiments were performed on adult male Sprague Dawley rats (250–400 g; Charles River Laboratories). Animals were housed, three per cage, under a 12 h light/dark cycle in a temperature- and humidity-controlled room in the animal care facility of the University of California, San Francisco. Food and water were available ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committee at University of California at San Francisco and adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All effort was made to minimize the number of animals used and their suffering.
Mechanical nociceptive threshold testing.
The nociceptive flexion reflex was quantified using an Ugo Basile analgesymeter (Stoelting), which applies a linearly increasing mechanical force to the dorsum of the rat's hindpaw. The nociceptive threshold was defined as the force in grams at which the rat withdrew its paw, and baseline paw-pressure threshold was defined as the mean of three readings before the test agents were injected. Each paw was treated as an independent measure and each experiment performed on a separate group of rats. Data are presented as mean change from baseline mechanical nociceptive threshold.
Drugs and their administration.
TNFα was purchased from R&D Systems, anisomycin from EMD Biosciences, and λ-carrageenan and PGE2 from Sigma-Aldrich. The PKCε agonist ψεRACK (peptide-sequence: HDAPIGYD) was synthesized by Biomatik. The selection of the drug doses used in this study was based on dose–response curves determined during our previous studies (Taiwo and Levine, 1991; Aley et al., 2000; Parada et al., 2003a,b).
Stock solutions of TNFα (c = 1 μg/μl) in PBS containing 0.5% bovine serum albumin (Sigma-Aldrich) were further diluted (1:50 => cfinal = 20 ng/μl) with the same buffer immediately before injection. The injection volume was 5 μl. Stock solutions of PGE2 (c = 1 μg/μl) in abs. EtOH were diluted (1:50 => 20 ng/μl) with 0.9% NaCl immediately before injection. The EtOH concentration of the final PGE2 solution was ∼2% and the injection volume was 5 μl. Stock solutions of anisomycin (c = 10 μg/μl) in an 1:1 mixture of absolute EtOH and 0.9% NaCl were further diluted (1:25 => cfinal = 0.4 μg/μl) with 0.9% NaCl immediately before injection. The EtOH concentration of the final anisomycin solution was ∼2%. Carrageenan was dissolved and diluted in 0.9% NaCl (cfinal = 1%) immediately before injection. The injection volume was 5 μl. ψεRACK was dissolved and diluted in 0.9% NaCl (cfinal = 0.4 μg/μl). All drugs were administered via a beveled 30-gauge hypodermic needle attached to a Hamilton microsyringe (Hamilton) by polyethylene tubing. Anisomycin as well as ψεRACK were administered via hypotonic shock by which 2.5 μl of H2O, followed by 1 μl of air and 2.5 μl of the drug [cfinal (in both cases) = 0.4 μg/μl; dose = 1 μg] were administered sequentially into the rat hindpaw from the same syringe.
Immunohistochemistry.
Naive rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused through the left ventricle with ∼35 ml of PBS containing 100 U Heparin/ml PBS followed by 300 ml of methanol buffered 10% formalin (Fisher Scientific). L4 dorsal root ganglia (DRGs) were immediately dissected out and postfixed overnight in the same fixative before they were sequentially transferred and stored in PBS containing 10%, 20% or 30% sucrose and 0.02% NaN3 (Sigma Aldrich), for 12–24 h each. DRGs were embedded in Tissue Tek (OCT compound, Electron Microscopy Sciences), sectioned at 20 μm in a Leica cryostat, and transferred into PBS. The free-floating tissue sections were thoroughly rinsed with PBS and blocked and permeabilized under light agitation for 1 h at room temperature (RT) with PBS containing 0.3% Triton X-100 (PBS-T) and 5% normal rabbit serum (Jackson ImmunoResearch Laboratories). CPEB expression and IB4 reactivity were revealed by a 48 h incubation at 4°C with an 1:500 dilution of a goat anti-CPEB antibody (sc-48983, Santa Cruz Biotechnology) in PBS-T containing 5% normal rabbit serum followed by three washing steps with PBS-T and a 2 h incubation at RT and under light agitation with a 1:500 dilution of a Cy3 labeled anti-goat antibody (A21432, Invitrogen Life Technologies) and 5 μg of FITC-conjugated IB4 (I21411, Invitrogen)/ml PBS-T containing 5% normal rabbit serum and 0.1 mg of Ca2+ and Mg2+ ions/ml PBS-T. Tissue sections were washed three times with PBS-T containing 0.1 mg of Ca2+ and Mg2+ ions/ml PBS-T and mounted with DAPI containing mounting media (Invitrogen).
ODN preparation and administration.
The antisense ODN sequence for CPEB, 5′-CATACACCACTCCACCAAATAG-3′ (Invitrogen), was directed against a unique region of the rat mRNA sequence. The corresponding NCBI GenBank accession number and ODN position within the mRNA-sequence are NM_001106276.1 and 1354–1375. The mismatch ODN sequence 5′-AATAGAACACACCACCTGATAC-3′ corresponds to the antisense sequence with 7 bases mismatched (denoted in bold).
ODN was reconstituted in nuclease-free 0.9% NaCl (10 μg/μl) and stored at −20°C until use. For each injection, rats were anesthetized with 2.5% isoflurane. A dose of 40 μg (injection volume 20 μl) of CPEB antisense or mismatch ODN was administered using a 3/10 cc insulin syringe with a 29-gauge ultra fine ½-inch fixed hypodermic needle (Becton Dickinson) inserted intrathecally, on the midline between the fourth and fifth lumbar vertebrae, once daily over 3 consecutive days. Using this protocol others and we have previously demonstrated the downregulation of several different proteins including the TTX-resistant sodium channel, NaV1.8 (Lai et al., 2002), PLC β-3 (Joseph et al., 2007), gp130, a receptor subunit for IL-6 (Summer et al., 2008) or the mitochondrial fission regulator dynamin-related protein 1 (Ferrari et al., 2011).
Protein extraction and Western blot.
To confirm that the change in the nociceptive responses associated with antisense ODN treatment are due to a decrease in the peripheral protein expression level of CPEB, a Western blot analysis was performed. Saphenous nerves from anesthetized rats were ligated with silk surgical suture (4–0) 1 cm above the knee-level bifurcation of the nerves. A 5 mm section of the saphenous nerve proximal to the ligation was harvested 24 h after the last ODN injection and protein extraction and Western blot analysis were performed as previously described (Bogen et al., 2008; Summer et al., 2008).
CPEB immunoreactivity was detected with a 1:500 dilution of affinity-purified goat anti-CPEB antibody (sc-48983, Santa Cruz Biotechnology) followed by incubation with a 1:5000 dilution of horseradish peroxidase-conjugated mouse anti-goat antibody (31400, Thermo Fisher Scientific). A 1:1000 dilution of affinity-purified rabbit anti-β-actin antibody (ab8227; Abcam) followed by a 1:5000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit antibody (NA-934; GE Healthcare Biosciences) was used to normalize the immunoreactivities of the protein samples. Immunoreactivities were visualized by using the enhanced chemiluminescence detection system (Thermo Fisher Scientific). Results were analyzed by computer-assisted densiometry (AlphaEaseFC software, Alpha Innotech Corporation) and levels of CPEB immunoreactivity were normalized with respect to the β-actin levels in each sample. The percentage decrease in protein expression level was calculated using the formula: [normalized density for antisense-normalized density for mismatch]/normalized density for mismatch × 100.
Coimmunoprecipitation experiments.
L4/L5 DRGs dissected out of rats that were primed by a single intradermal injection of 5 μl of a 1% carrageenan solution 3 d prior were transferred into PBS-T supplemented with a complete protease inhibitor cocktail (Roche Diagnostics) and homogenized with an ultrasonic tissue homogenizer. The homogenized DRGs were incubated during shaking at 1400 rpm for 4 h at RT in an Eppendorf shaker and solubilized proteins extracted by centrifugation at 20,000 × g for 15 min at RT. After adding of 5 μl of rabbit anti-PKCε (sc-214, Santa Cruz Biotechnology) or 5 μl of goat anti-CPEB (sc-48983, Santa Cruz Biotechnology) antibodies protein extracts were incubated under shaking for 30 min at RT before 50 μl of preequilibrated protein A (Sigma-Aldrich) or G (GE Healthcare Biosciences) Sepharose beads were added and the extracts were further incubated for additional 4 h at RT under continuous rotation. The protein A or G Sepharose beads were spun down and washed twice with PBS-T under vigorous shaking at 1000 rpm in an Eppendorf shaker and eluted by boiling in sample buffer (3% (w/v) SDS, 10% (v/v) Glycerol, 5% (v/v) β-Mercaptoethanol, 0.025% (w/v) Bromophenol blue, 62.5 mm Tris-HCl, pH 6.8). The protein precipitates were separated by SDS-PAGE and transferred to a nitrocellulose membrane as described previously (Bogen et al., 2008; Summer et al., 2008). Protein extracts coimmunoprecipitated with the rabbit anti-PKCε antibody/protein A Sepharose were probed with an 1:500 dilution of the anti-CPEB antibody followed by a 1:2000 dilution of an horseradish peroxidase (HRP)-conjugated anti-goat antibody (Thermo Fisher Scientific) whereas protein extracts coimmunoprecipitated with the goat anti-CPEB antibody/Protein G Sepharose were probed with an 1:500 dilution of the anti-PKCε antibody followed by an 1:2000 dilution of an HRP-conjugated anti-rabbit antibody (GE Healthcare Biosciences) and immunoreactivities were visualized by using the enhanced chemiluminescence detection system (Thermo Fisher Scientific).
Characterization of the anti-CPEB and anti-PKCε antibodies.
The goat anti-CPEB antibody that we used in this study had not been characterized before. Therefore, we have validated the specificity of the antibody by competitive Western blotting of rat recombinant CPEB under reducing/denaturating as well as nonreducing/naive conditions using the commercially available antigenic peptide (sc-48983-P, Santa Cruz Biotechnology) as a blocking peptide. The antibody shows a specific immunoreactivity to the rat recombinant CPEB at the apparent molecular weight of 64 kDa, which can be competitively blocked by increasing amounts of the antigenic peptide added to the primary antibody solution (data not shown).
The specificity of the rabbit anti-PKCε antibody that we used in the present study has been demonstrated previously (Khasar et al., 1999; Dina et al., 2005).
Statistics.
Two-way repeated-measures ANOVAs with up to eight within-subjects factor (time) and one between-subjects factor (treatment) were used to determine whether significant differences between experimental groups were observed. For within-subjects effects the Mauchly criterion was used to determine whether the assumption of sphericity was met; if not, Greenhouse-Geiser p-values are presented. Statistical significance (i.e., the α-level) was set at p < 0.05.
Results
Onset of hyperalgesic priming
To identify molecules that are responsible for the neuroplasticity that maintains primed nociceptor latent hyperexcitability to a subsequent challenge by proinflammatory mediators we first sought to analyze the time course for the onset of priming. Most of the molecules that are known to induce priming e.g., carrageenan, GDNF, NGF, ψεRACK and IL-6 also induce long-lasting mechanical hyperalgesia (Aley et al., 2000; Bogen et al., 2008; Dina et al., 2008; Ferrari et al., 2010; Joseph and Levine, 2010) which make them less suitable for determination of the onset of priming (Aley et al., 2000; Parada et al., 2003b). However, TNFα not only induces priming (Parada et al., 2003a) it also induces mechanical hyperalgesia that lasts <24 h (Parada et al., 2003a). We therefore used TNFα to analyze the time course for the onset of priming.
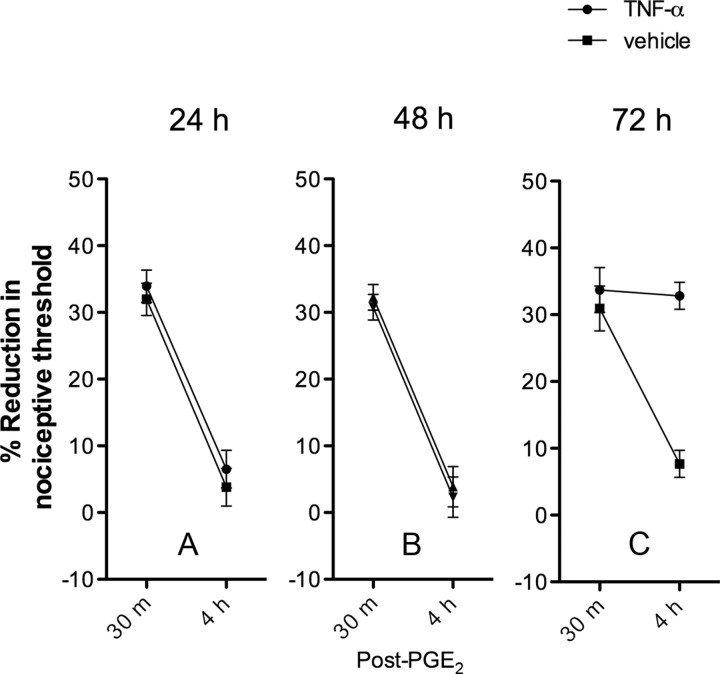
As shown in Figure 1, rats treated with a single intradermal injection of 100 ng of rat recombinant TNFα, 24 or 48 h ahead of a subsequent injection of 100 ng of PGE2 into the same site, are not primed. The PGE2-induced mechanical hyperalgesia lasts <4 h (Fig. 1A,B). However, rats that received TNFα 72 h before PGE2 are primed, as PGE2 mechanical hyperalgesia is still present at 4 h (Fig. 1C) (Aley et al., 2000; Parada et al., 2003b). These results suggest that the plastic changes that render primed nociceptors hyperexcitable to proinflammatory mediators need ∼72 h to be established.
Figure 1.
Time to onset of priming. TNFα (100 ng) or vehicle was administered intradermally into the hindpaws of separate groups of rats. As previously demonstrated, TNFα-induced hyperalgesia returned to baseline within 24 h following administration (Parada et al., 2003a). To test for the existence of priming, PGE2 (100 ng) was administered into the same site 1, 2, or 3 d following TNFα administration (A–C, respectively). PGE2-induced hyperalgesia returned to baseline within 4 h on days 1 and 2 following TNFα administration and on all 3 d following vehicle administration. However, PGE2-induced hyperalgesia remained elevated at 4 h on day 3 in the TNFα-treated rats, indicating the onset of hyperalgesia priming (Aley et al., 2000; Parada et al., 2003b). A, Day 1. The two-way repeated-measures ANOVA showed no significant differences between the TNFα- and the vehicle-treated groups, either with respect to the group × time interaction (F(1,10) = 0.033, p = 0.861) or main effect of group (F(1,10) = 0.575, p = 0.466). B, Day 2. The two-way repeated-measures ANOVA showed no significant differences between the TNFα- and the vehicle-treated groups, either with respect to the group × time interaction (F(1,10) = 0.007, p = 0.985) or main effect of group (F(1,10) = 0.236, p = 0.638). C, Day 3. The two-way repeated-measures ANOVA showed significant differences between the TNFα- and the vehicle-treated groups, the group × time interaction was F(1,10) = 55.487, p < 0.001, and the main effect of group was F(1,10) = 14.828, p = 0.003, indicating that the onset of hyperalgesic priming is not immediate but occurs after a 3 d delay after TNFα administration.
Hyperalgesic priming depends on protein translation in the periphery
Given that the plastic changes associated with primed nociceptors need ∼72 h to be established (Fig. 1), a time span too short for the transcription and anterograde transport of new gene products to the periphery (Alvarez et al., 2000; Giuditta et al., 2002) and too long to be explained by the covalent modification of proteins (Hucho and Levine, 2007), the insertion of mechanosensitive ion channels into the cell membrane (Zhang et al., 2005; Di Castro et al., 2006) or the crosstalk of complex signaling networks in peripheral nociceptive terminals (Bhalla and Iyengar, 1999) we next tested whether peripheral administration of the commonly used protein translation inhibitor anisomycin (Dale et al., 1987; O'Leary et al., 1995; Manseau et al., 1998) could prevent priming. As shown in Figure 2, hyperalgesic priming induced by a single intradermal injection of carrageenan (5 μl of a 1% solution) can be prevented by intradermal injections of anisomycin (1 μg per day over 3 d) into the same site. This suggests that the enhanced and prolonged hyperalgesic response of primed nociceptors to a subsequent inflammatory stimulus is reflected by changes in the peripheral protein expression pattern.
Figure 2.
Carrageenan-induced priming can be prevented by anisomycin. Anisomycin (1 μg) or its vehicle was injected intradermally once each day in separate groups. A single dose of carrageenan (5 μl of a 1% solution) was administered to all rats on the first day of anisomycin administration. Carrageenan-induced hyperalgesia returned to baseline by day 4 in both anisomycin- and vehicle-treated rats (data not shown). To test for hyperalgesic priming, PGE2 (100 ng) was administered into the same sites and tested at 30 min, 4 h and 24 h. PGE2-induced hyperalgesia remained elevated to the 24 h time point in vehicle-treated rats, indicating the presence of hyperalgesic priming, but hyperalgesia was dissipated by 4 h in anisomycin-treated rats, demonstrating its ability to block of hyperalgesic priming. The two-way repeated-measures ANOVA showed a significant time × group interaction (F(3,30) = 61.731, p < 0.001) as well as a significant main effect of group (F(1,10) = 193.272, p < 0.001).
CPEB is necessary for the induction of hyperalgesic priming
That priming can be induced by peripheral administration of a selective PKCε activator (Aley et al., 2000; Parada et al., 2003b) and be blocked by the peripheral administration of a protein translation inhibitor (Fig. 2) suggests that the plastic changes that constitute the memory of the primed nociceptor are: (1) dependent on protein biosynthesis and (2) located in the peripheral nociceptive terminals. In our search for molecules that regulate the translation of gene products that are responsible for the latent and long-lasting hyperexcitability of primed nociceptors we focused on CPEB. CPEB is an RNA-binding molecule that initiates the translation of mRNAs that carry a cytoplasmic polyadenylation element at their 3′ end (Richter, 2007; Villalba et al., 2011) and has been shown to contribute to long-term facilitation in sensory neurons, in the marine mollusk Aplysia californica (Si et al., 2003a,b; Bailey et al., 2004).
To analyze for a possible role of CPEB in the process of hyperalgesic priming we first sought to find out whether CPEB is expressed by sensory neurons in the DRG. As shown in Figure 3A, anti-CPEB-immunoreactivity can be detected in a large subpopulation of primarily small-to-medium diameter sensory neurons. To further analyze whether the CPEB-expressing neurons are nociceptors we next sought to determine whether the CPEB-expressing neurons can be colabeled with IB4, a well characterized marker for GDNF-responsive, Ret-expressing nociceptors (Molliver et al., 1997; Stucky and Lewin, 1999; Fang et al., 2006). As shown in Figure 3A, almost all CPEB-expressing neurons are IB4-positive suggesting that, indeed, most of the CPEB-expressing neurons give rise to nociceptive afferents of the class that mediates hyperalgesic priming (Ferrari et al., 2010; Joseph and Levine, 2010). To evaluate for a functional role of CPEB in the process of hyperalgesic priming we next tested whether carrageenan-induced priming can be prevented by the intrathecal administration of antisense ODN to CPEB mRNA. As shown in Figure 3B, rats treated with 40 μg of antisense ODN for CPEB mRNA for 7 consecutive days (starting 3 d before carrageenan challenge and for the 4 d of carrageenan-induced hyperalgesia) do not show the enhanced and prolonged hyperalgesic response following peripheral administration of 100 ng of PGE2 that characterizes primed nociceptors (Aley et al., 2000; Parada et al., 2003b), while rats treated with mismatch ODN do. To confirm that the attenuation of the PGE2-induced hyperalgesia in carrageenan-treated rats is due to the decrease in the peripheral protein expression level of CPEB we performed a Western blot analysis of protein extracts of the saphenous nerve of a separate group of rats. As shown in Figure 3C, rats that were treated with antisense ODN for CPEB mRNA showed a 28 ± 8% (p < 0.05) reduction in the protein expression level of CPEB in the saphenous nerve compared with those that were treated with mismatch ODN.
Figure 3.

A, Colocalization of anti-CPEB immunoreactivity and IB4-reactivity in rat L4 DRGs. Anti-CPEB immunoreactivity (left) can be detected in a large subpopulation of small to medium sized sensory neurons. As the majority of cells that express CPEB immunoreactivity can also be labeled with IB4 (middle and right), a marker for a subset of nociceptive C-fiber afferents, many of them are supposed to be nociceptors that innervate the upper epidermal layer of the skin, at the side of nociceptive testing. Red fluorescence, Goat anti-CPEB antibody/Cy3-conjugated anti-goat antibody. Green fluorescence, IB4-FITC conjugate. Blue, DAPI counterstain. Scale bar, 100 μm. B, Antisense ODNs for CPEB mRNA can prevent carrageenan-induced priming. Naive rats were treated with antisense or mismatch ODN for CPEB mRNA (40 μg/day) for 7 consecutive days. A single dose of carrageenan (5 μl of a 1% solution) was administered to all rats on day 4 of ODN administration. On day 8, 1 d after the last ODN administration, carrageenan-induced hyperalgesia had diminished to baseline levels, and PGE2 was administered into the same site, as was carrageenan before. To test for hyperalgesic priming, paw-withdrawal thresholds were measured 30 min, 4 h and 24 h after PGE2 administration. PGE2-induced hyperalgesia remained elevated to the 24 h time point in mismatch-treated rats, indicating the presence of hyperalgesic priming, but hyperalgesia was dissipated by 4 h in antisense-treated rats, demonstrating its ability to block of hyperalgesic priming. A two-way repeated-measures ANOVA showed a significant main effect of time (F(2,20) = 486.280; p < 0.001), indicating that, overall, nociceptive thresholds changed over the times tested, but no significant group × time interaction (F(2,20) = 1.199; p = 0.322) and no significant main effect of group (F(1,10) = 3.341; p = 0.098). These results indicate that the ODN groups did not differ significantly from each other up until the time of PGE2 injection. A second two-way repeated-measures ANOVA that included the three post-PGE2 time points showed a significant group × time interaction (F(5,50) = 68.856; p < 0.001), a significant main effect of group (F(1,10) = 120.561; p < 0.001), as well as a significant main effect of time (F(5,50) = 250.686; p < 0.001). Together these results indicate that the groups differed significantly only after PGE2 administration. C, Downregulation of the peripheral CPEB expression level by antisense ODN for CPEB mRNA. Protein extracts derived from the saphenous nerve of animals that have been treated with intrathecal antisense ODN for CPEB mRNA for 3 consecutive days demonstrate a 28 ± 8% decrease in the anti-CPEB immunoreactivity compared with those that were treated with mismatch ODN (p < 0.05; unpaired Student's t test; N = 6 each for antisense- and mismatch-treated rats). Note that the two bands visualized by anti-CPEB immunoreactivity are most likely due to a phosphorylated (upper band) and nonphosphorylated (lower band) variant. The calculated molecular weight of CPEB is 62 kDa (according to UniProtKB/Swiss-Prot database-entry P0C279).
CPEB is downstream of PKCε in the signaling cascade mediating priming
Given that priming can be induced by the peripheral administration of a selective PKCε agonist (Aley et al., 2000; Parada et al., 2003b) and mammalian CPEB activity is regulated by phosphorylation (Huang et al., 2002; Atkins et al., 2004) we next sought to analyze whether CPEB could be a potential PKCε substrate. Protein extracts derived from dorsal root ganglia of rats primed by a single intradermal injection of carrageenan (5 μl of a 1% solution) 3 d ahead were first immunoprecipitated with an anti-PKCε or anti-CPEB antibody and the respective protein precipitates afterward analyzed by Western blotting using the appropriate anti-CPEB or anti-PKCε antibodies. As shown in Figure 4A, PKCε as well as CPEB could be coimmunoprecipitated with the respective anti-CPEB or anti-PKCε antibodies compatible with the suggestion that priming in nociceptors is due to a PKCε-induced activation of CPEB. To further confirm that CPEB is downstream in the signaling cascade responsible for the plastic changes underlying priming we next sought to analyze whether priming induced by the intradermal administration of the selective PKCε agonist, ψεRACK (Dorn et al., 1999; Aley et al., 2000) can be prevented by the downregulation of CPEB expression at the periphery. As shown in Figure 4B, priming induced by the intradermal injection of 1 μg of ψεRACK can be prevented by the intrathecal administration of antisense ODN for CPEB mRNA for 7 consecutive days further substantiating the idea that priming is due to a PKCε-induced activation of CPEB. Finally, to determine whether CPEB activity constitutes the memory that maintains primed nociceptors hyperexcitable to the subsequent challenge by proinflammatory mediators we analyzed whether hyperalgesic priming can be reversed by antisense ODN for CPEB mRNA. As shown in Figure 4C, intrathecal administration of antisense ODN for CPEB mRNA for three consectutive days does not reverse the priming induced by intradermal administration of 1 μg of ψεRACK.
Figure 4.

A, PKCε can be coimmunoprecipitated with CPEB (and vice versa). Protein extracts derived from DRGs of carrageenan-primed rats were first immunoprecipitated with an anti-CPEB or anti-PKCε antibody and the precipitates subsequently analyzed by Western blotting using the respective anti-PKCε or anti-CPEB antibodies as described in Materials and Methods. Note that the calculated molecular weight of PKCε is 84 kDa (according to UniProtKB/Swiss-Prot database-entry P09216). B, ψεRACK-induced priming can be prevented by antisense ODN for CPEB mRNA. Naive rats were treated with antisense or mismatch ODN for CPEB mRNA (40 μg/day) for 7 consecutive days. A single dose of ψεRACK (1 μg) was administered to all rats on day 4 of ODN administration. On day 8, 1 d after the last ODN administration PGE2 was administered into the same sites as ψεRACK before. To test for hyperalgesic priming, paw-withdrawal thresholds were measured 30 min, 4 h and 24 h after PGE2 administration. PGE2-induced hyperalgesia remained elevated to the 24 h time point in mismatch-treated rats, indicating the presence of hyperalgesic priming, but hyperalgesia was dissipated by 4 h in antisense-treated rats, demonstrating its ability to block of hyperalgesic priming. A two-way repeated-measures ANOVA showed a significant main effect of time (F(4,40) = 283.485; p < 0.001), indicating that, overall, nociceptive thresholds changed over the times tested, but no significant group × time interaction (F(4,40) = 0.389; p = 0.815) and no significant main effect of group (F(1,10) = 0.019; p = 0.892). These results indicate that the ODN groups did not differ significantly from each other up until the time of PGE2 injection. A second two-way repeated-measures ANOVA that included the three post-PGE2 time points showed a significant group × time interaction (F(7,70) = 66.212; p < 0.001), a significant main effect of group (F(1,10) = 70.272; p < 0.001), as well as a significant main effect of time (F(7,70) = 241.187; p < 0.001). Together these results indicate that the groups differed significantly only after PGE2 administration. C, ψεRACK-induced priming cannot be reversed by antisense ODN for CPEB mRNA. Naive rats received a single dose of ψεRACK (1 μg) on day 1 of the experiment. Beginning on day 5, rats were treated with antisense or mismatch ODN for CPEB mRNA (40 μg/day) for 3 consecutive days. On day 8, 1 d after the last ODN administration, PGE2 was administered into the same site as ψεRACK before. To test for hyperalgesic priming, paw-withdrawal thresholds were measured 30 min, 4 h and 24 h after PGE2 administration. PGE2-induced hyperalgesia remained elevated to the 24 h time point in mismatch- and antisense-treated rats, indicating the presence of hyperalgesic priming in both groups. Thus, antisense for CPEB mRNA was not able to reverse ψεRACK-induced priming. The two-way ANOVA showed that neither the time × group interaction nor the main effect of group were significant (F(3,30) = 0.50; p < 0.985 and F(1,10) = 0.066; p < 0.803, respectively), indicating that the decrease in the CPEB expression level fails to reverse hyperalgesic priming induced by ψεRACK.
Discussion
Primary afferent nociceptors challenged with an inflammatory or neuropathic insult develop a long-lasting latent hyperresponsiveness to a subsequent challenge with proinflammatory cytokines, a phenomenon known as hyperalgesic priming (Aley et al., 2000; Parada et al., 2003b; Reichling and Levine, 2009). Although the intracellular signaling pathways that explain the enhanced and prolonged hyperalgesic response of primed animals have begun to be elucidated (Aley et al., 2000; Khasar et al., 2008; Dina et al., 2009) an explanation for the plastic changes that constitute the memory that maintains primed nociceptors hyperexcitable to proinflammatory mediators has remained elusive. To identify the molecular basis for the underlying neuroplastic changes in primed nociceptors we first analyzed the time course for the onset of priming. Our results show (Fig. 1C) that only rats that had been treated with TNFα 3 d before PGE2 demonstrate the enhanced and prolonged hyperalgesic response by which primed nociceptors are being characterized (Aley et al., 2000; Parada et al., 2003a,b). Given that the neuroplastic changes that were induced by TNFα need ∼72 h to be established (Fig. 1), a time span that correlates best with the idea that the underlying neuroplasticity is due to changes in the protein expression pattern in the primary afferent nociceptor we next sought to analyze whether hyperalgesic priming could be prevented by the commonly used protein translation inhibitor anisomycin (Dale et al., 1987; O'Leary et al., 1995; Manseau et al., 1998). As shown in Figure 2, carrageenan-induced priming could be prevented by the peripheral administration of anisomycin suggesting that the plastic changes that constitute the memory in primed nociceptors are due to changes in the peripheral protein expression pattern. Our results are not just consistent with the idea that long-lasting plastic changes underlying the formation of a molecular memory are induced by the synthesis of new proteins (Dale et al., 1987; Martin et al., 1997; Costa-Mattioli et al., 2009) but also with the finding that peripheral protein translation contributes to long-lasting nociceptor hyperexcitability (Di Castro et al., 2006; Jiménez-Díaz et al., 2008; Melemedjian et al., 2010).
It has recently been shown that a functionally homologous mechanism to hyperalgesic priming in rats, namely long-term facilitation (LTF), in the marine mollusk A. californica, is mediated by the synthesis of a subset of proteins that is directed by translational dormant mRNAs (Si et al., 2003b; Bailey et al., 2004). The translation of those proteins is initiated by polyadenylation of their respective mRNAs and regulated by CPEB, an RNA-binding protein that recruits all the molecular components necessary to catalyze the elongation of their poly(A) tail (McGrew et al., 1989; Richter, 2007). To determine whether CPEB function could contribute to the plastic changes that explain the latent and long-lasting hyperexcitability of primed nociceptors we first analyzed whether CPEB is expressed by sensory neurons in the DRGs. As shown in Figure 3A, not only was anti-CPEB immunoreactivity detected in a large subpopulation of small to medium sized sensory neurons, the majority of them could also be colabeled with IB4, a well characterized marker for a subpopulation of nociceptors (Molliver et al., 1997; Stucky and Lewin, 1999; Fang et al., 2006). Of note in this regard is the recent finding by Joseph and Levine who showed that animals that have been pretreated with the selective neurotoxin IB4-saporin cannot be primed (Vulchanova et al., 2001; Joseph et al., 2008; Joseph and Levine, 2010).
To evaluate whether CPEB function contributes to the neuroplasticity that constitutes the molecular memory of primed nociceptors we determined whether carrageenan-induced priming could be prevented by the knockdown of the CPEB expression level in primary sensory neurons. As shown in Figure 3B, rats treated with intrathecal antisense ODN to CPEB mRNA do not show the enhanced and prolonged hyperalgesic response to PGE2 that characterizes primed nociceptors (Aley et al., 2000; Reichling and Levine, 2009). By using Western blotting we confirmed that the behavioral changes in the nociceptive response of animals treated with antisense ODN for CPEB mRNA correlate with a reduction of the CPEB expression in the peripheral neuron (Fig. 3C).
Given that a selective PKCε agonist (Dorn et al., 1999) can induce hyperalgesic priming (Aley et al., 2000) and CPEB activity in mammals has been reported to depend on phoshorylation (Huang et al., 2002; Atkins et al., 2004) we next sought to analyze whether CPEB could be a potential PKCε substrate. As shown in Figure 4A, PKCε could be coimmunoprecipitated with the anti-CPEB antibody and vice versa, suggesting that the plastic changes underlying hyperalgesic priming in IB4(+)-nociceptors are due to a PKCε-induced activation of CPEB. Interestingly, CPEB contains at least one potential PKCε phosphorylation site K394MSSRR399 (Nishikawa et al., 1997; Numazaki et al., 2002).
To further substantiate that CPEB is a potential PKCε substrate we examined whether the induction of priming by the selective PKCε agonist ψεRACK (Dorn et al., 1999; Aley et al., 2000) could be prevented by the intrathecal administration of antisense ODN for CPEB mRNA. As shown in Figure 4B, ψεRACK-induced priming could be prevented by the downregulation of the CPEB expression level in primary afferent nociceptors. Interestingly, the effect of the CPEB antisense seems to be restricted to the neuroplastic changes that are induced by the priming stimulus as the antisense itself affects neither the baseline nociceptive threshold of naive rats nor the onset, amplitude or time course of carrageenan- or ψεRACK-induced hyperalgesia (Figs. 3B, 4B).
Finally, to determine whether CPEB activity represents the molecular memory that maintains primed nociceptors hyperresponsive to the subsequent challenge by proinflammatory mediators we analyzed whether ψεRACK-induced priming could also be reversed by the downregulation of the peripheral expression of CPEB. As shown in Figure 4C, ψεRACK-induced priming could not be reversed by the intrathecal administration of antisense ODN for CPEB mRNA. According to findings by the Kandel group CPEB in Aplysia is a protein with prion-like properties that exists in two different conformations (Si et al., 2003b). One of its conformers is catalytically inactive whereas the other one not only promotes the synthesis of proteins that are responsible for the plastic changes underlying LTF in Aplysia (Si et al., 2003a) it additionally self-replicates its active conformation by inducing a conformational switch in their nonactive counterpart (Si et al., 2003b). If a similar mechanism exists in IB4(+)-nociceptors it might not be surprising that a 28% decrease in the peripheral CPEB expression level after intrathecal administration of antisense ODN for CPEB mRNA cannot reverse the plastic changes underlying primed nociceptors (Fig. 4B). This decrease could just be too low to impact the conformational self-replication of CPEB that might maintain primed IB4(+)-nociceptors hyperexcitable to a subsequent challenge by inflammatory mediators.
LTF in Aplysia has also been attributed to the formation of new varicosities along sensory branches in sensory-motor neuron cocultures (Miniaci et al., 2008). The formation and stabilization of varicosities is a protein synthesis-dependent process that needs ∼72 h to take place (Miniaci et al., 2008) a time span that correlates well with our findings (Fig. 1). Moreover, as varicosities give rise to new synaptic connections that would boost the neurotransmitter-release in response to an incoming action potential their formation could not only explain the enhanced and prolonged hyperalgesic response of primed nociceptors but also their underlying latent and long-lasting plasticity (Aley et al., 2000; Parada et al., 2003b; Ferrari et al., 2010). However, as priming can be induced by the peripheral administration of a selective PKCε agonist (Fig. 4A) and be prevented by the peripheral administration of a protein synthesis inhibitor (Fig. 2), the enhanced neurotransmitter-release at the central terminals of primed IB4(+)-nociceptors must be somehow controlled by a PKCε-dependent mechanism at the peripheral nociceptive terminals.
In conclusion, we show that the neuroplastic changes underlying hyperalgesic priming a model for the chronification of inflammatory and neuropathic pain are induced by a PKCε-dependent activation of CPEB in the peripheral terminals of IB4(+)-nociceptors. Our results raise the intriguing possibility that a prion-like mechanism could be responsible for the chronification of certain pain syndromes.
Footnotes
This study was supported by the NIH.
References
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Nozaki N, Shigeri Y, Soderling TR. Cytoplasmic polyadenylation element binding protein-dependent protein synthesis is regulated by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2004;24:5193–5201. doi: 10.1523/JNEUROSCI.0854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Kandel ER, Schacher S. Serotonin produces long-term changes in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. 1987;7:2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro A, Drew LJ, Wood JN, Cesare P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A. 2006;103:4699–4704. doi: 10.1073/pnas.0508005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Hucho T, Yeh J, Malik-Hall M, Reichling DB, Levine JD. Primary afferent second messenger cascades interact with specific integrin subunits in producing inflammatory hyperalgesia. Pain. 2005;115:191–203. doi: 10.1016/j.pain.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160:501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, Csukai M, Wu G, Lorenz JN, Mochly-Rosen D. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. Role of drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci. 2011;31:11404–11410. doi: 10.1523/JNEUROSCI.2223-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuditta A, Kaplan BB, van Minnen J, Alvarez J, Koenig E. Axonal and presynaptic protein synthesis: new insights into the biology of the neuron. Trends Neurosci. 2002;25:400–404. doi: 10.1016/s0166-2236(02)02188-4. [DOI] [PubMed] [Google Scholar]
- Huang YS, Jung MY, Sarkissian M, Richter JD. N-methyl-d-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and alpha CaMKII mRNA polyadenylation at synapses. EMBO J. 2002;21:2139–2148. doi: 10.1093/emboj/21.9.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Jiménez-Díaz L, Géranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS One. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9:463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Manseau F, Sossin WS, Castellucci VF. Long-term changes in excitability induced by protein kinase C activation in Aplysia sensory neurons. J Neurophysiol. 1998;79:1210–1218. doi: 10.1152/jn.1998.79.3.1210. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Dworkin-Rastl E, Dworkin MB, Richter JD. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989;3:803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniaci MC, Kim JH, Puthanveettil SV, Si K, Zhu H, Kandel ER, Bailey CH. Sustained CPEB-dependent local protein synthesis is required to stabilize synaptic growth for persistence of long-term facilitation in Aplysia. Neuron. 2008;59:1024–1036. doi: 10.1016/j.neuron.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- O'Leary FA, Byrne JH, Cleary LJ. Long-term structural remodeling in Aplysia sensory neurons requires de novo protein synthesis during a critical time period. J Neurosci. 1995;15:3519–3525. doi: 10.1523/JNEUROSCI.15-05-03519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003a;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003b;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the Aplysia CPEB has prion-like properties. Cell. 2003a;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in Aplysia. Cell. 2003b;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- Villalba A, Coll O, Gebauer F. Cytoplasmic polyadenylation and translational control. Curr Opin Genet Dev. 2011;21:452–457. doi: 10.1016/j.gde.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]