Abstract
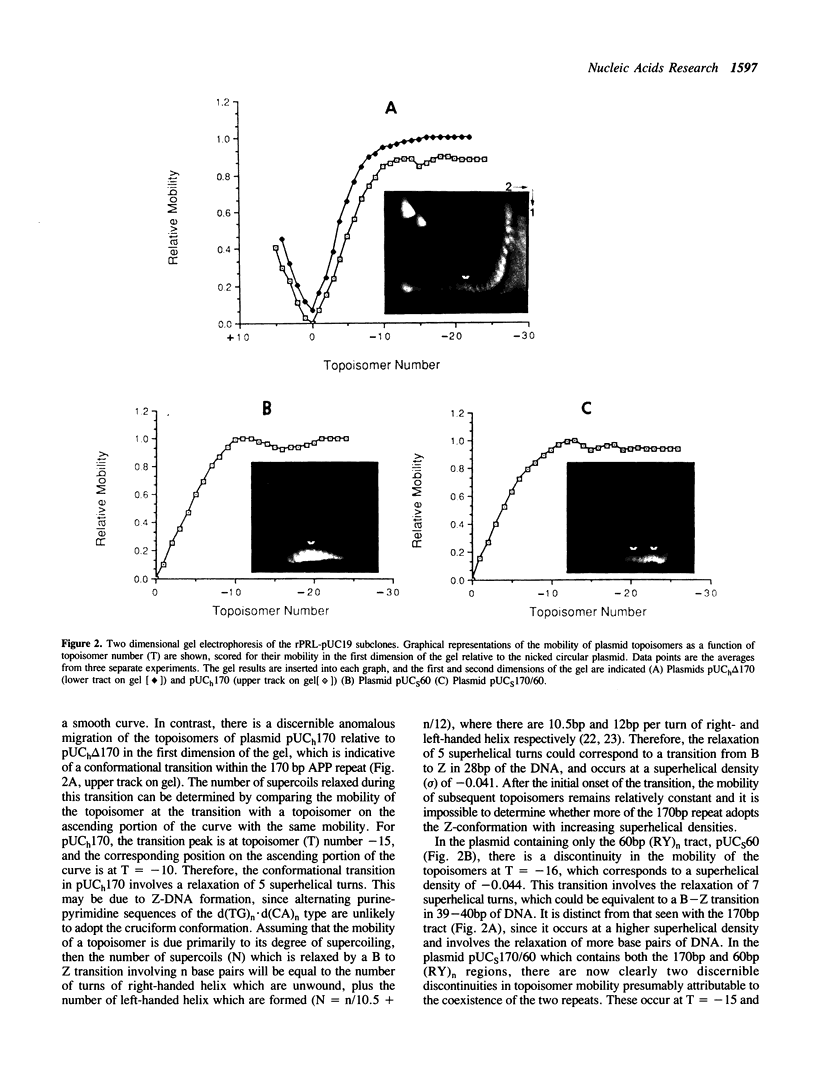
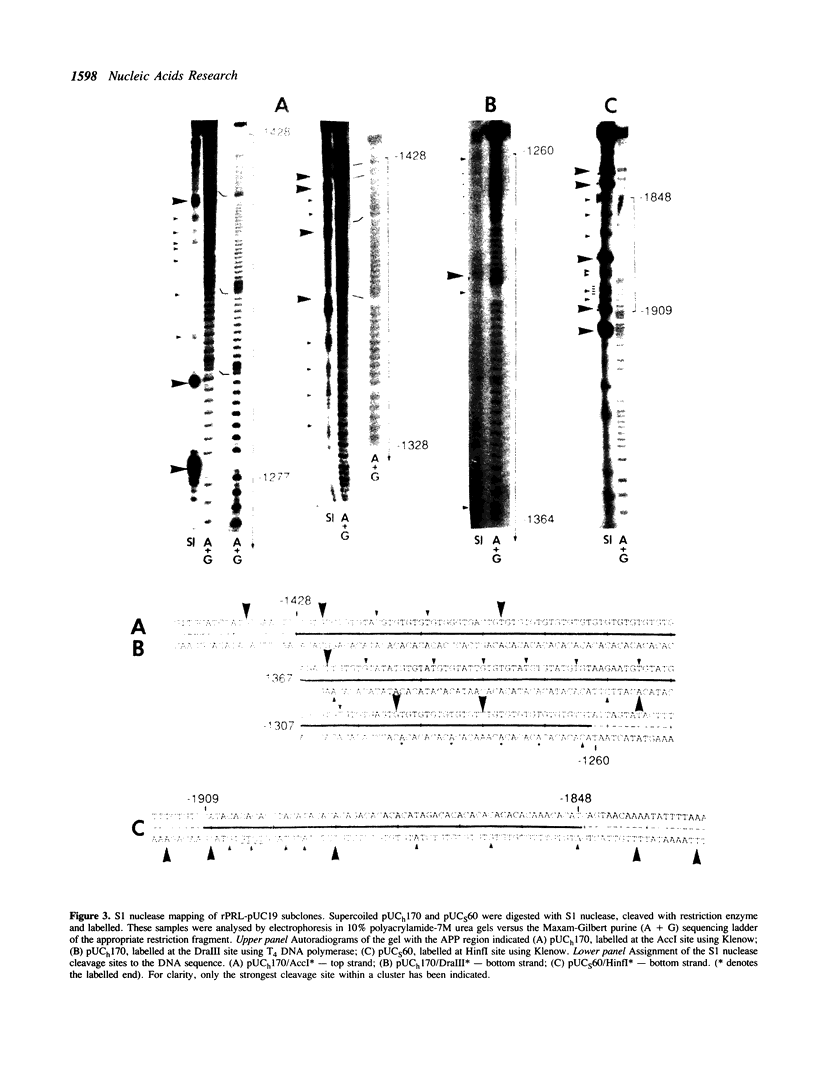
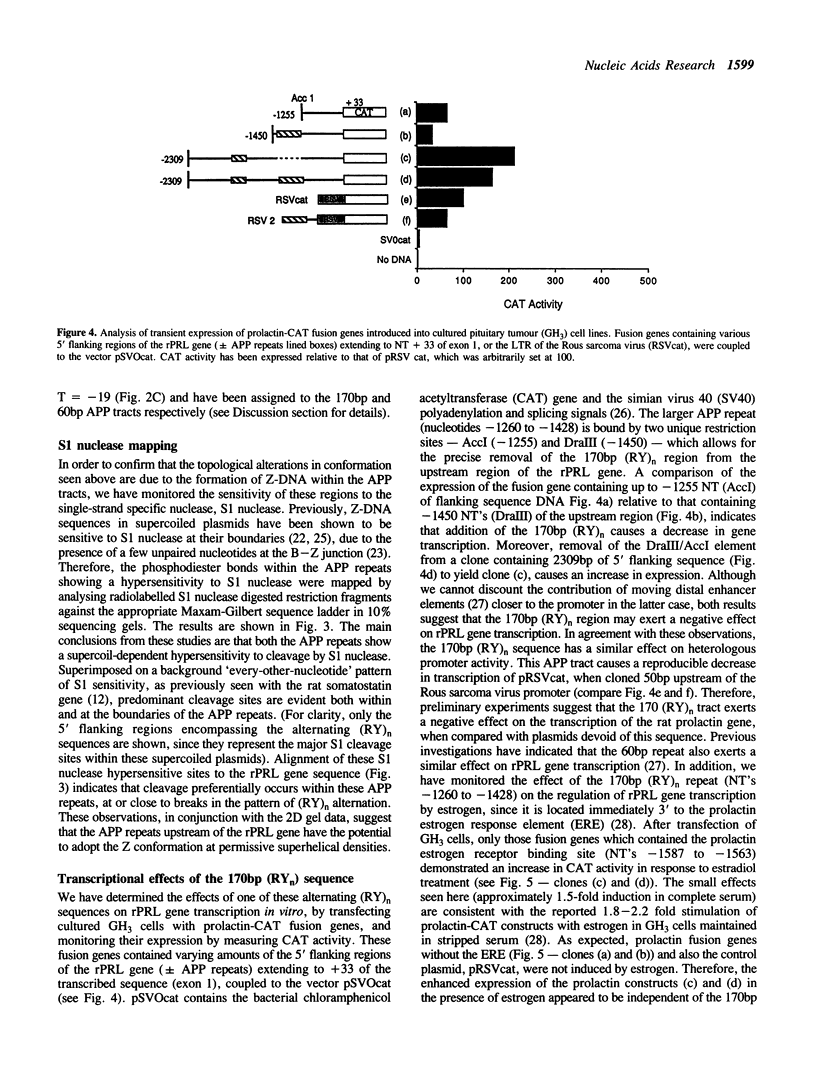
Two alternating purine-pyrimidine sequences of the d(TG)n.d(CA)n-type (170bp and 60 bp in length) lie upstream of the rat prolactin (rPRL) gene. Conformational studies of plasmids containing these sequences indicate that both form left-handed (Z) DNA, with transitions initiating at superhelical densities of -0.041 and -0.044 respectively. These alternating purine-pyrimidine (APP) sequences are hypersensitive to cleavage with S1 nuclease both at the boundaries and within these APP repeats, where there is a loss in APP alternation. We have investigated the function of one of these Z-DNA sequences in the regulation of rPRL transcription, by linking regions of the 5' flanking sequence of the rPRL gene to a reporter gene encoding chloramphenicol acetyltransferase (CAT), and transferring these plasmids into GH3 pituitary tumour cell lines. The major conclusion from these studies is that the 170bp repeat exerts a negative effect on the transcription of the rPRL gene, and also down-regulates the expression of the fusion gene pRSVcat when cloned 50bp upstream of the Rous sarcoma virus promoter. However, despite its proximity to an estrogen response element in prolactin, this sequence does not affect the responsiveness of the rPRL gene to estrogen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argentin S., Nemer M., Drouin J., Scott G. K., Kennedy B. P., Davies P. L. The gene for rat atrial natriuretic factor. J Biol Chem. 1985 Apr 25;260(8):4568–4571. [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Butzow J. J., Shin Y. A., Eichhorn G. L. Effect of template conversion from the B to the Z conformation on RNA polymerase activity. Biochemistry. 1984 Oct 9;23(21):4837–4843. doi: 10.1021/bi00316a004. [DOI] [PubMed] [Google Scholar]
- Durand R., Job C., Zarling D. A., Teissère M., Jovin T. M., Job D. Comparative transcription of right- and left-handed poly[d(G-C)] by wheat germ RNA polymerase II. EMBO J. 1983;2(10):1707–1714. doi: 10.1002/j.1460-2075.1983.tb01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrin L. K., Gorski J. The prolactin gene hypersensitive sites are present early in development and are not induced by estrogen administration. Endocrinology. 1985 Nov;117(5):2098–2105. doi: 10.1210/endo-117-5-2098. [DOI] [PubMed] [Google Scholar]
- Giaever G. N., Wang J. C. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 1988 Dec 2;55(5):849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. S., Huang S. Y., Garrard W. T. Chromatin structure of the potential Z-forming sequence (dT-dG)n X (dC-dA)n. Evidence for an "alternating-B" conformation. J Mol Biol. 1985 May 25;183(2):251–265. doi: 10.1016/0022-2836(85)90218-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Kakunaga T. Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature. 1982 Jul 22;298(5872):396–398. doi: 10.1038/298396a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T., Seidman M., Stollar B. D. Characterization of genomic poly(dT-dG).poly(dC-dA) sequences: structure, organization, and conformation. Mol Cell Biol. 1984 Dec;4(12):2610–2621. doi: 10.1128/mcb.4.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. The in-vivo occurrence of Z DNA. J Biomol Struct Dyn. 1983 Dec;1(3):593–609. doi: 10.1080/07391102.1983.10507467. [DOI] [PubMed] [Google Scholar]
- Hayes T. E., Dixon J. E. Z-DNA in the rat somatostatin gene. J Biol Chem. 1985 Jul 5;260(13):8145–8156. [PubMed] [Google Scholar]
- Hentschel C. C. Homocopolymer sequences in the spacer of a sea urchin histone gene repeat are sensitive to S1 nuclease. Nature. 1982 Feb 25;295(5851):714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- Hipskind R. A., Clarkson S. G. 5'-flanking sequences that inhibit in vitro transcription of a xenopus laevis tRNA gene. Cell. 1983 Oct;34(3):881–890. doi: 10.1016/0092-8674(83)90545-7. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Hsieh W. T., Blaho J. A., Larson J. E., Wells R. D. Left-handed DNA in vivo. Science. 1987 Nov 6;238(4828):773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lilley D. Bacterial chromatin. A new twist to an old story. Nature. 1986 Mar 6;320(6057):14–15. doi: 10.1038/320014a0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Estradiol regulates the transcription of the prolactin gene. J Biol Chem. 1982 Mar 10;257(5):2133–2136. [PubMed] [Google Scholar]
- Maurer R. A., Notides A. C. Identification of an estrogen-responsive element from the 5'-flanking region of the rat prolactin gene. Mol Cell Biol. 1987 Dec;7(12):4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Selective binding of the estradiol receptor to a region at least one kilobase upstream from the rat prolactin gene. DNA. 1985 Feb;4(1):1–9. doi: 10.1089/dna.1985.4.1. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Wells R. D. The role of sequence in the stabilization of left-handed DNA helices in vitro and in vivo. Biochim Biophys Acta. 1988 Sep 7;950(3):243–254. doi: 10.1016/0167-4781(88)90120-0. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. D., Dixon G. H., Rattner J. B., van de Sande J. H. Assembly and characterization of nucleosomal cores on B- vs. Z-form DNA. Biochemistry. 1985 Jan 1;24(1):102–109. doi: 10.1021/bi00322a015. [DOI] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Nickol J., Behe M., Felsenfeld G. Effect of the B--Z transition in poly(dG-m5dC) . poly(dG-m5dC) on nucleosome formation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1771–1775. doi: 10.1073/pnas.79.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passananti C., Davies B., Ford M., Fried M. Structure of an inverted duplication formed as a first step in a gene amplification event: implications for a model of gene amplification. EMBO J. 1987 Jun;6(6):1697–1703. doi: 10.1002/j.1460-2075.1987.tb02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Santoro C., Costanzo F., Ciliberto G. Inhibition of eukaryotic tRNA transcription by potential Z-DNA sequences. EMBO J. 1984 Jul;3(7):1553–1559. doi: 10.1002/j.1460-2075.1984.tb02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Kilpatrick M. W., Wells R. D. S1 nuclease recognizes DNA conformational junctions between left-handed helical (dT-dG n. dC-dA)n and contiguous right-handed sequences. J Biol Chem. 1984 Feb 10;259(3):1963–1967. [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Thomae R., Beck S., Pohl F. M. Isolation of Z-DNA-containing plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5550–5553. doi: 10.1073/pnas.80.18.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Kiang D. T. A twenty-two-fold increase in the relative affinity of estrogen receptor to poly (dA-dC).poly (dG-dT) in the presence of polyamines. Nucleic Acids Res. 1988 May 25;16(10):4705–4720. doi: 10.1093/nar/16.10.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]
- Wells R. D. Unusual DNA structures. J Biol Chem. 1988 Jan 25;263(3):1095–1098. [PubMed] [Google Scholar]
- White B. A., Preston G. M. Evidence for a role of topoisomerase II in the Ca2+-dependent basal level expression of the rat prolactin gene. Mol Endocrinol. 1988 Jan;2(1):40–46. doi: 10.1210/mend-2-1-40. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Jaworski A., Larson J. E., Wells R. D. The B- to Z-DNA equilibrium in vivo is perturbed by biological processes. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7069–7073. doi: 10.1073/pnas.85.19.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




