Abstract
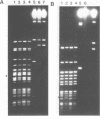
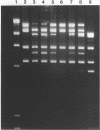
The DNA methylase M.Xbal was isolated from an E. coli recombinant clone. We deduce that the enzyme methylates at the sequence 5'-TCTAGm6A-3'. In combination with the methylation-dependent restriction endonuclease, DpnI (5'-Gm6A/TC-3'), DNA cleavage occurs at the sequence 5'-TCTAGA/TCTAGA-3'. This twelve-base-pair site should occur once every 16,000,000 base pairs in a random sequence of DNA. The exceptional rarity of the M.XbaI/DpnI sequence makes it an ideal candidate for transpositional integration of a unique cleavage site into bacterial genomes. Retrotransposition into mammalian genomes is also an attractive possibility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. J., Goodfellow J. M. Solvent interactions stabilising nucleic acid conformers. Nucleic Acids Res. 1989 Jan 11;17(1):389–404. doi: 10.1093/nar/17.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanish J., McClelland M. Activity of DNA modification and restriction enzymes in KGB, a potassium glutamate buffer. Gene Anal Tech. 1988 Sep-Oct;5(5):105–107. doi: 10.1016/0735-0651(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- McClelland M., Hanish J., Nelson M., Patel Y. KGB: a single buffer for all restriction endonucleases. Nucleic Acids Res. 1988 Jan 11;16(1):364–364. doi: 10.1093/nar/16.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
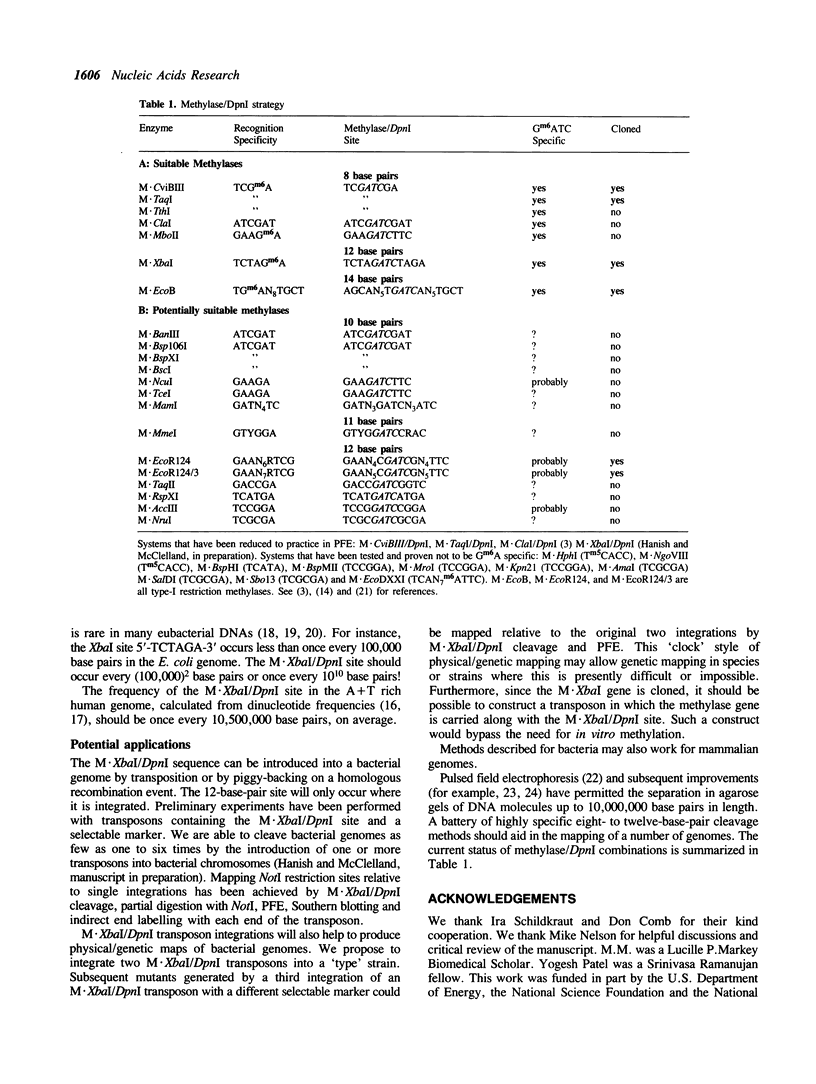
- McClelland M., Kessler L. G., Bittner M. Site-specific cleavage of DNA at 8- and 10-base-pair sequences. Proc Natl Acad Sci U S A. 1984 Feb;81(4):983–987. doi: 10.1073/pnas.81.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Nelson M. Enhancement of the apparent cleavage specificities of restriction endonucleases: applications to megabase mapping of chromosomes. Gene Amplif Anal. 1987;5:257–282. [PubMed] [Google Scholar]
- McClelland M. Selection against dam methylation sites in the genomes of DNA of enterobacteriophages. J Mol Evol. 1984;21(4):317–322. doi: 10.1007/BF02115649. [DOI] [PubMed] [Google Scholar]
- Nelson M., McClelland M. Purification and assay of type II DNA methylases. Methods Enzymol. 1987;155:32–41. doi: 10.1016/0076-6879(87)55007-8. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Vollrath D., Davis R. W., Yanofsky C. An electrophoretic karyotype of Neurospora crassa. Mol Cell Biol. 1988 Apr;8(4):1469–1473. doi: 10.1128/mcb.8.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Arnold J., Ivarie R. The effect of codon usage on the oligonucleotide composition of the E. coli genome and identification of over- and underrepresented sequences by Markov chain analysis. Nucleic Acids Res. 1987 Mar 25;15(6):2627–2638. doi: 10.1093/nar/15.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang B. Q., Schildkraut I. NotI and SfiI: restriction endonucleases with octanucleotide recognition sequences. Methods Enzymol. 1987;155:15–21. doi: 10.1016/0076-6879(87)55005-4. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Turmel C., Lalande M. Resolution of Schizosaccharomyces pombe chromosomes by field inversion gel electrophoresis. Nucleic Acids Res. 1988 May 25;16(10):4727–4727. doi: 10.1093/nar/16.10.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cott E. M., Wilson G. G. Cloning the FnuDI, NaeI, NcoI and XbaI restriction-modification systems. Gene. 1988 Dec 25;74(1):55–59. doi: 10.1016/0378-1119(88)90251-x. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M. D., McClelland M. Enzymatic cleavage of a bacterial genome at a 10-base-pair recognition site. Proc Natl Acad Sci U S A. 1989 Jan;86(1):51–55. doi: 10.1073/pnas.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G. Cloned restriction-modification systems--a review. Gene. 1988 Dec 25;74(1):281–289. doi: 10.1016/0378-1119(88)90304-6. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M., Crowther P. J., Jefferson S., Diver W. P. Methylation at dinucleotides other than CpG: implications for human maintenance methylation. Gene. 1988 Dec 25;74(1):151–152. doi: 10.1016/0378-1119(88)90273-9. [DOI] [PubMed] [Google Scholar]
- de la Campa A. G., Springhorn S. S., Kale P., Lacks S. A. Proteins encoded by the DpnI restriction gene cassette. Hyperproduction and characterization of the DpnI endonuclease. J Biol Chem. 1988 Oct 15;263(29):14696–14702. [PubMed] [Google Scholar]