Abstract
The divergence of distinct cell populations from multipotent progenitors is poorly understood, particularly in vivo. The gonad is an ideal place to study this process, because it originates as a bipotential primordium where multiple distinct lineages acquire sex-specific fates as the organ differentiates as a testis or an ovary. To gain a more detailed understanding of the process of gonadal differentiation at the level of the individual cell populations, we conducted microarrays on sorted cells from XX and XY mouse gonads at three time points spanning the period when the gonadal cells transition from sexually undifferentiated progenitors to their respective sex-specific fates. We analyzed supporting cells, interstitial/stromal cells, germ cells, and endothelial cells. This work identified genes specifically depleted and enriched in each lineage as it underwent sex-specific differentiation. We determined that the sexually undifferentiated germ cell and supporting cell progenitors showed lineage priming. We found that germ cell progenitors were primed with a bias toward the male fate. In contrast, supporting cells were primed with a female bias, indicative of the robust repression program involved in the commitment to XY supporting cell fate. This study provides a molecular explanation reconciling the female default and balanced models of sex determination and represents a rich resource for the field. More importantly, it yields new insights into the mechanisms by which different cell types in a single organ adopt their respective fates.
Author Summary
How cells diverge from a common progenitor and adopt specific fates is still poorly understood. We analyzed gene expression profiles in the distinct cell lineages of the gonad over the period when sex determination occurs. The undifferentiated progenitor cells expressed genes characteristic of both sexual fates, explaining the plasticity of the gonadal cells to differentiate as male or female cell types. The establishment of sex-specific fate in both the germ cells and somatic cells involved activation of some genes; but, importantly, we show that an active repression of genes associated with the alternative pathway is also a characteristic of cell fate commitment. Although germ cell progenitors expressed genes associated with both possible fates, genes characteristic of the male fate were over-represented in the progenitors, giving them a male bias. However, in somatic cell progenitors, which control sex determination, genes associated with the female fate were over-represented. These results suggest an explanation for why the female fate is the developmental default for the gonad, and they advance our understanding of how complex transcriptional networks regulate fate determination during organ development.
Introduction
Little is known about how the transcriptome of an undifferentiated progenitor is poised between two fates and resolves to one of two stable, differentiated outcomes during the process of cell fate determination. The bipotential gonad is a unique system for studying cell fate decisions during mammalian organ development. In mice, the gonads arise around embryonic day 10 (E10.0). For the first 36 hours the gonad primordia harbor the potential to become testes or ovaries irrespective of genetic sex. The process by which the bipotential gonad adopts the ovarian or testicular fate is known as primary sex determination, and involves a binary fate decision within cells of each gonadal lineage.
The early gonad is composed of four lineages: supporting cells, interstitial/stromal cells, germ cells, and endothelial cells. The determinant of gonadal fate in mammals is the Y-chromosome gene Sry. In XY individuals, expression of Sry in supporting cell progenitors triggers commitment to a testicular (“male”) fate, whereas the absence of Sry expression in XX supporting cells results in ovarian (“female”) development [1], [2]. Supporting cells and interstitial/stromal cells arise within the urogenital ridge from a common mesodermal progenitor, while the primordial germ cells and endothelial cells migrate into the developing gonad [3]–[6]. Despite their distinct origins, cells of each lineage in the early gonad are bipotential progenitors capable of adopting either a male or female fate, which they do in a coordinated manner to form a functional testis or ovary [7]–[9]. Temporal examination of the transcriptomes of these diverse progenitors as they make their parallel, binary fate decisions provides an opportunity to understand how cell fate decisions are made in the context of organ development.
Some gonadal lineages have been studied at the transcriptome level in independent experiments [10]–[15], resulting in the identification of genes that are up-regulated in a sex- or (in some cases) lineage-specific manner. However, the molecular relationship between the somatic lineages (i.e., supporting cells versus interstitial/stromal cells) has never been examined, as these lineages were not separated in previous studies. Previous studies did not fully characterize the undifferentiated progenitors or the temporal sequence for the divergence of the multiple progenitors to their sexual fates. Furthermore, other potentially important transcriptional patterns associated with differentiation and fate commitment, such as the specific transcript depletion previously noted in the Arabidopsis root and in early primordial germ cells [16], [17], have not been characterized.
As part of the GenitoUrinary Molecular Anatomy Project (GUDMAP, http://www.gudmap.org/), we undertook a comprehensive transcriptome analysis of the four principle gonadal lineages in XX and XY gonads at three time points, spanning the period from the undifferentiated bipotential stage until the cells adopt sex-specific fates. While this type of comprehensive transcriptome analysis has been performed in other developing systems [16], [18], the relative simplicity of the gonad and the theoretical framework for sex determination allowed us to extend our analysis to test distinct models for the process of cell fate determination, and to evaluate the fit of these models to the theories of sex determination that have been proposed in the past 50 years.
To explore how the cells of the gonad adopt their sex-specific fates, we considered the various theories that have been proposed for gonadal sex determination. It has been suggested that the female fate is the “default” state because expression of Sry is required to “divert” the cells to the male fate [19]–[21]. The concept of a female default state originated in the secondary sex determination literature [19], but that language crept into the field of primary sex determination and became a way to conceptualize female gonad development [20], [21]. Others proposed that a female-promoting “Z” gene normally blocks an underlying male developmental program and that Z is itself blocked by Sry [22]. Still others have proposed that both the female and male programs require an active switch to initiate differentiation from their initially “bipotential” state (i.e., that there is an “ovary-determining gene” as well as a “testis-determining gene”) [23]. More recently, it has been suggested that the gonad is balanced between the male and female fates by antagonistic signaling pathways [24]–[27].
We wanted to determine whether one of these models could describe the differentiation of the gonadal cells at the level of the transcriptome. Therefore, we re-framed and logically extended these models so that we could test them against our transcriptional data, and we used the concept of lineage priming to do so. Studies suggest that multipotent cells are not a “blank slate”, but rather are “lineage primed” by expressing markers of all potential fates they can adopt [28]–[35]. During their differentiation, multipotent cells repress markers of specific fates that were not adopted while maintaining gene expression associated with the fate that was adopted [28]–[35]. A similar phenomenon has also been observed in the early embryo, where individual blastomeres express transcripts that later become restricted to the specific lineages of the blastocyst [36]–[38]. It is possible for progenitor cells to be equally “balanced” between their multiple fates, expressing similar numbers of genes associated with each alternative differentiated fate. However, the progenitors need not have all differentiation programs equally represented [28]. Instead a progenitor may show “biased priming” if markers characteristic of one of its possible fates predominate, indicating the closer relationship of the progenitor to that fate.
We investigated the transcriptional profiles of the gonadal cell lineages as they differentiate. Interestingly, we identified different variations of biased lineage priming: while germ cells showed male-biased priming, supporting cells showed female-biased priming. This study provides a molecular explanation reconciling the female default and balanced models of sex determination and represents a rich resource for the field. In addition, it affords insight into the mechanisms by which different cell types in a single organ adopt their respective fates.
Results
Sorted cell microarrays accurately reflect known gene expression patterns
We quantified global gene expression in four lineages of the XX and XY developing mouse gonad at E11.5, E12.5, and E13.5. To isolate individual lineages, we utilized mouse lines expressing fluorescent cell-specific markers (Figure 1A, Figure S1). The cells from separately pooled XX and XY gonads were isolated by fluorescence-activated cell sorting (FACS). Sry-EGFP [7] and Sox9-ECFP [39] were used as markers for supporting cells (see Materials and Methods for a full explanation). XY interstitial cells and XX stromal cells were isolated using Mafb-EGFP [40], [41]. The XY interstitial cells are excluded from testis cords and give rise to steroidogenic Leydig cells [41]. The XX stroma is not defined morphologically, but for the purposes of this analysis, is defined as the population labeled with Mafb-EGFP. Germ cells were isolated using Oct4-EGFP [42], and endothelial cells were isolated using Flk1-mCherry [43], [44]. In general, cells were pooled from gonads of multiple embryos on non-inbred genetic backgrounds (see Materials and Methods). RNA purified from each XX and XY cell population was used to measure transcript abundance with Affymetrix Mouse Genechip Gene 1.0 ST Arrays. We produced 3 biological replicates for each population. The data are available in GEO (accession number GSE27715) and at http://www.gudmap.org/. RMA normalized values used in our analysis are provided with the capability to generate an expression graph for any gene, as a user-friendly resource for the community (Dataset S1).
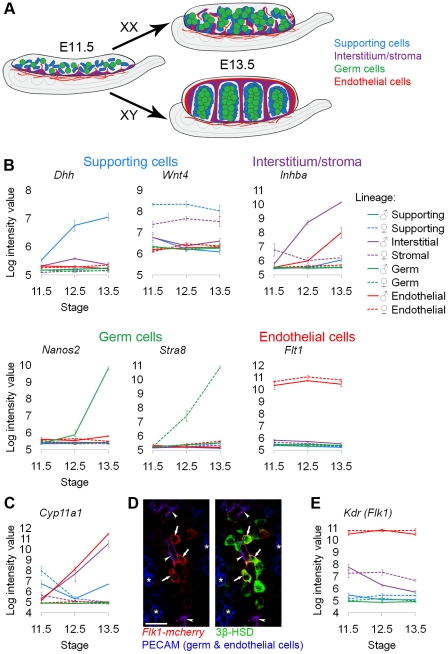
Figure 1. Sorted cell lineages and microarray validation.
(A) Illustration of the developing XX and XY gonad with supporting cells (blue), interstitial/stromal cells (purple), germ cells (green), and endothelial cells (red). (B, C, and F) Graphs of the log-transformed, normalized intensity values from the microarrays for control genes known to be specific to each lineage. The color for each lineage is conserved in all figures and matches the illustration (A), with XX (♀) values shown as dashed lines, and XY (♂) values shown as solid lines. The error bars are standard error of the mean (“standard error”) of the log transformed values. The Y-axis scale differs for each graph because each transcript cluster has its own intensity range. (B) The control genes were found in the expected lineage, except for (C) genes characteristic of Leydig cells. Leydig cell genes were highly expressed in both the interstitium (as expected) and the endothelial cell fraction. (D) Immunofluorescence of E13.5 XY gonads with Flk1-mCherry (red), PECAM1 (germ and endothelial cells, blue), and 3β-HSD (Leydig cells, green). Arrowheads indicate Flk1-mCherry and PECAM1 double positive endothelial cells. Arrows indicate Flk1-mCherry positive, PECAM1 negative cells that were positive for 3β-HSD, confirming aberrant reporter expression in some Leydig cells. Asterisks indicate germ cells positive for PECAM1 alone. Scale bar = 25 µm. (E) The XY interstitial cells have very low expression of the endogenous Flk1 (Kdr) transcript at E13.5, supporting our conclusion that the Flk1-mCherry transgene is aberrantly expressed in Leydig cells.
We also isolated cells from the αSma-EYFP transgenic mouse [45] with the expectation that this reporter would label a larger population of cells in the interstitium and stroma (Figure S2A). While this population transcriptionally resembled the interstitial/stromal population isolated with the Mafb-EGFP line (data not shown), unlike the Mafb-EGFP positive cells, the αSma-EYFP cells also expressed high levels of Sry at E11.5, a gene predicted to be specific to XY supporting cells [46] (Figure S2B). Indeed, E11.5 αSma-EYFP cells stained positive for both SRY and SOX9 protein (Figure S2C). Consequently, at least early in development, it appears that αSma-EYFP labels a heterogeneous population of cells containing supporting cells as well as interstitial/stromal cells. While this finding may have biological significance, it could also result from leaky expression of the αSma-EYFP transgene. Thus, we excluded the αSma-EYFP data from this analysis but have made the data available with the rest of the microarrays.
To validate the cell sorting and microarray data, we examined the expression of genes known to be specific to each of the cell populations (Figure 1B). The expression of each control gene was consistent with previous reports: Dhh (desert hedgehog) was enriched in XY supporting cells [10], Wnt4 (wingless-related MMTV integration site 4) was enriched in XX supporting cells [10], Inhba (inhibin beta-A) was enriched in the XY interstitium [47], [48], Nanos2 (nanos homolog 2) was enriched in XY germ cells [49], Stra8 (stimulated by retinoic acid gene 8) was enriched in XX germ cells [50], and Flt1 (FMS-like tyrosine kinase 1, VEGFR1) was enriched in endothelial cells [51]. Therefore, the microarrays on the sorted cell populations accurately reflected gene expression patterns that were previously characterized for each of the cell lineages.
An unexpected expression pattern was observed in XY Flk1-mCherry-positive cells (Figure 1C). We found that genes expressed in Leydig cells, such as Cyp11a1 (Scc) [52], were enriched in both XY interstitial (containing steroidogenic Leydig cells) and Flk1-mCherry-positive “endothelial” populations. This was surprising given that steroidogenic enzymes have not previously been reported in endothelial cells [41]. When we immunostained the Flk1-mCherry-positive cells purified by FACS for a Leydig cell marker, we found that Leydig cells were present in this sorted population (data not shown). To investigate the basis for this finding, we stained E13.5 Flk1-mCherry gonads with the vascular marker PECAM1 and an antibody against the Leydig cell marker 3β-HSD. We found Flk1-mCherry-positive cells that were negative for the vascular marker, but positive for the Leydig marker (Figure 1D). This suggests that the Flk1-mCherry transgene was ectopically expressed in some Leydig cells. Because the corresponding Flk1 transcript (Kdr) was expressed at low levels in the XY interstitial cells at E13.5 (Figure 1E), we do not believe that the Flk1-mCherry-positive, 3β-HSD-positive cells express endogenous Kdr/Flk1. We concluded that the source of the Leydig cell contamination was leaky expression of the Flk1-mCherry transgene in Leydig cells. While this result complicated our analysis of the endothelial population, it provided strong evidence that the array data accurately reflected gene expression in the sorted populations.
Lineage, sex, and stage all influence gene expression
To investigate the effects of cell lineage, sex, and stage on global gene expression and to validate the consistency of expression measurements in biological replicates, we clustered all 72 individual microarrays based on the expression of transcript clusters that met our inclusion criteria outlined in the Materials and Methods (Figure 2A). With the exception of one E11.5 XY endothelial array (see Materials and Methods), biological replicates, and even samples of different sexes (early XX and XY germ cells) or stages (late XX supporting cells) that were expected to be similar, showed consistent expression patterns as indicated by the tight clustering of those samples (Figure 2A). This again validates the quality of the microarray data.
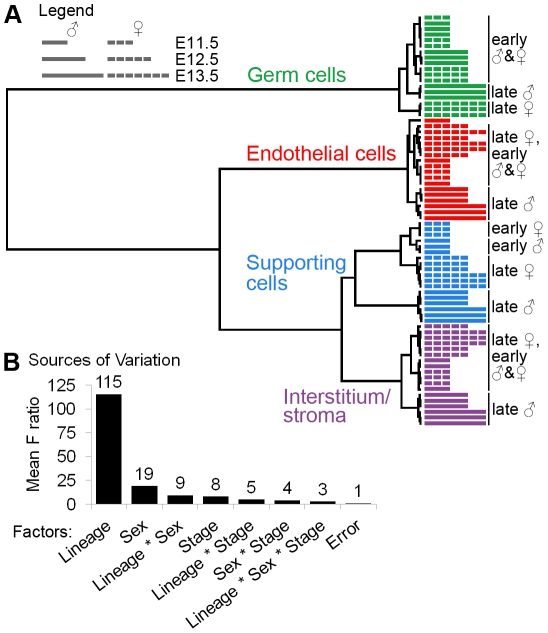
Figure 2. Gene expression was affected by lineage, sex, and stage.
(A) Clustering dendrogram of individual microarray samples. The E11.5, E12.5, and E13.5 samples are represented by short, intermediate, and long bars, respectively. The dashed bars indicate XX samples, and the solid bars indicate XY samples. Ward's method with squared Euclidean distance as the distance metric was used. The arrays cluster primarily by lineage, and secondarily by sex and stage. (B) Analysis of the sources of variation confirmed that the primary source of variation is lineage, and secondarily sex and stage.
The dendrogram indicates that cell lineage is the most significant factor affecting gene expression. The same general patterns were observed using other clustering methods (Figure S3A, S3B). This result was confirmed by an analysis of the sources of variation (ANOVA), in which lineage was identified as the most significant factor influencing gene expression variation (Figure 2B, Figure S3C). Interstitial/stromal cells were distinct from supporting cells at all stages, indicating that, despite their shared origin [3], these are separate lineages by E11.5.
Sex and stage were also sources of expression variation in the gonadal cell populations, albeit to a lesser extent than lineage (Figure 2A, 2B). XY and XX supporting cells clustered in distinct groups at E11.5, confirming that these cells embark on their sex-specific differentiation by E11.5 [10], [11]. There was no distinction between the sexes in the other cell types until E12.5 or E13.5. While the late stage XY endothelial cells clustered away from the early XX and XY endothelial cells, this could be due to the sex-specific contamination by Leydig cells. In summary, this analysis confirms the high quality of the data and shows that each lineage is distinct from E11.5 onwards.
Each lineage has uniquely expressed enriched and depleted genes that provide insight into the biology of the cells
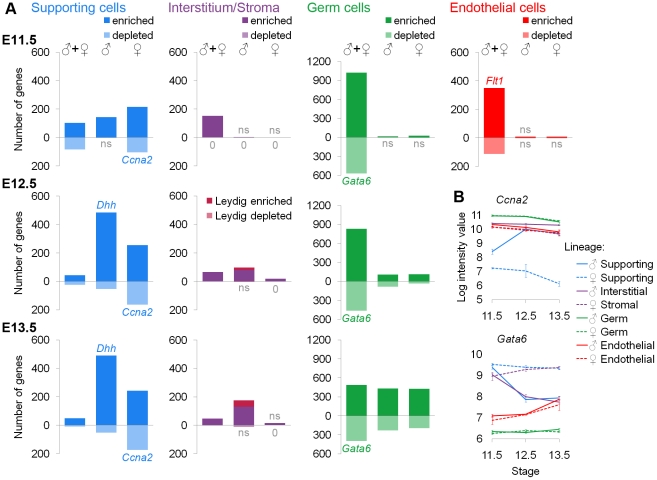
To explore the differences between the cell types apparent in the dendrogram, we identified “lineage specific” genes that were specifically enriched and depleted in each lineage relative to the other lineages at each stage. We then determined whether these genes were expressed in a “sex-specific” (expression was different between XY and XX samples) or a “sex-independent” (expression was similar in XY and XX samples) manner. We identified these genes by performing multiple pairwise comparisons on the normalized array values (similar to previously described methods [53]) with a p-value cutoff of 0.05 and a fold change cutoff of 1.5. “Enriched” genes were more highly expressed than in other lineages, and “depleted” genes were less highly expressed than in other lineages. Examples of genes showing these different patterns include the sex-specific enrichment of Dhh in XY supporting cells (Figure 1B, Figure 3A), the sex-independent enrichment of Flt1 in XX and XY endothelial cells (Figure 1B, Figure 3A), the sex-specific depletion of Ccna2 (cyclin A2) in XX supporting cells (Figure 3A, 3B), and the sex-independent depletion of Gata6 (GATA binding protein 6) in germ cells (Figure 3A, 3B). A full description of our statistical methods is provided in the Materials and Methods and a list of all genes identified appears in Dataset S2A.
Figure 3. Lineage-specific enriched and depleted genes revealed distinct differentiation programs.
(A) Graphs of the number of genes specific to each lineage. The gene lists and permutation tests are provided in Dataset S2. The “♂” and “♀” symbols indicate lineage-specific and sex-specific genes, while the “♂+♀” symbol indicates genes that are lineage-specific and sex-independent. Pale bars below the axis indicate genes that are depleted relative to other lineages. The E11.5 graphs are on the top row, the E12.5 graphs are in the middle, and the E13.5 graphs are on the bottom row. The germ cell Y-axis is scaled to accommodate the larger number of genes specific to this lineage. Leydig cell genes (burgundy) were separately identified by cross-referencing the endothelial and interstitial data and added to the bars for the XY interstitium. Lists with >20% false positives are indicated by “ns”. Lists with no genes are marked with “0”. Some bars also have a colored gene name exemplifying the pattern within that category (the graphs for Dhh and Flt1 appear in Figure 1B). (B) Graphs of the log-transformed, normalized intensity values for genes that are sex-specifically (Ccna2) and sex-independently (Gata6) depleted. The error bars are standard error. Three lineages showed specific gene depletion in addition to enrichment. Each lineage had transcriptionally distinct progenitors as indicated by “♂+♀” genes at E11.5. Supporting cells were already in the midst of their sex-specific differentiation by E11.5 as indicated by genes in the “♂” or “♀” columns at E11.5, but the other cell types were sexually undifferentiated at E11.5.
Due to the sex-specific Leydig cell contamination of the endothelial population after E11.5 (Figure 1C–1E), we present the gene lists (Dataset S2A) but not the graphs (Figure 3A) because we could not determine whether sex-specific gene expression was due to an artifact of the contamination or genuine differences in endothelial cells. We deduced a list of Leydig cell genes (Figure 3A, Dataset S2A) by identifying genes specifically over-expressed or under-expressed in both XY endothelial cells and interstitial cells at E12.5 and E13.5 compared to other lineages. These genes were removed from XY endothelial gene lists at E12.5 and E13.5 (Dataset S2A).
To evaluate these lists, we first performed permutation testing (Dataset S2B), and considered those with a false positive rate <20% as acceptable (lists that did not pass this test are marked “ns”) (Figure 3A). Second, we determined whether positive control genes with known expression patterns, such as the expression of Sox9 in XY supporting cells, were found in the expected lists (Dataset S2A). Third, we interrogated all significant lists in Figure 3A (not “ns” or “0”) for enrichment of BioCarta and KEGG pathways (Dataset S2C) as well as GO terms (Dataset S2D). As evidence of the high quality of the sorted cell microarray data, functional annotation of the enriched/depleted lists identified expected terms for each cell type: germ cell development in the germ cell lists, steroid production in interstitial and Leydig lists, vascular development in the endothelial list, and sex determination in supporting cell lists (Dataset S2C, S2D). All enriched terms for significant lists are provided to facilitate the discovery of new pathways involved in the functions of these cells (Dataset S2C, S2D).
This analysis identified several novel expression features of these cells. For example, all of the lineages had sex-specific and sex-independent cohorts of depleted genes, with the exception of interstitial/stromal cells (Figure 3A). The genes identified as depleted appear biologically relevant based on individual genes and enriched categories of genes. Both XY and XX germ cells repressed Gata6 (Figure 3B, Dataset S2A), which can drive embryonic stem cells to adopt the extraembryonic endoderm fate [54]. Thus, the repression of Gata6 may be important for maintaining a totipotent transcriptional state in germ cells. Similarly, Lef1 became sex-specifically depleted in XY supporting cells (Dataset S2A). Lef1 can interact with β-catenin in a transcriptional complex downstream of Wnt signaling [55], and this pathway antagonizes aspects of testis development [24], [56]. Thus, the depletion of Lef1 may be important for maintaining the male supporting cell fate. The sex-specifically depleted genes in XX supporting cells were enriched for cell cycle-related pathways and GO terms (Dataset S2C, S2D). Interestingly, both XX and XY supporting cells are arrested at E11.5 and only XY supporting cells re-enter the cell cycle; XX supporting cells remain non-proliferative [57], [58]. It was previously shown that XX cells express higher levels of cell cycle inhibitors [11] and that cell cycle genes are over-represented in XY supporting cells [15]. However, our data suggest a mechanism of cell cycle arrest involving the active repression of multiple genes important for cell cycle progression in XX supporting cells.
Additionally, we identified transcripts associated with a sexually undifferentiated progenitor cell for each lineage. All cell types had a large number of genes that were lineage-specific and sex-independent at E11.5 (Figure 3A, “♂+♀” category). The identification of shared expression in XX and XY cells demonstrates that there is a sexually undifferentiated progenitor for each lineage with a distinct transcriptional state. This was consistent with previous data suggesting that XX and XY supporting cells have a common origin [7] as well as the clustering results showing that supporting cells and interstitial/stromal cells are distinct lineages by E11.5 (Figure 2A).
Supporting cells exhibited the largest number of sexually dimorphic genes at E11.5 (Figure 3A, “♂” and “♀” categories), in accord with previous evidence that the supporting cell lineage adopts a sex-specific fate early in gonad development and instructs the other lineages as to which fate they should adopt [8], . Although it was clear that supporting cells began sex-specific differentiation by E11.5, XX and XY supporting cells still expressed sex-independent genes. Since the supporting cells are in the midst of their sex-specific differentiation at E11.5, the sex-independent genes likely represent remnants of the sexually-undifferentiated progenitor state. The XX and XY supporting cells adopt their distinct sex-specific states by E12.5.
The other three cell types exhibited few sex-specific genes at E11.5 (Figure 3A, categories “♂” and “♀”), showing that the differentiation of these lineages is delayed relative to that of the supporting cells. Germ cells and interstitial/stromal cells began to adopt lineage-specific, sex-specific transcriptional states by E12.5, a process that was further advanced by E13.5. This was again consistent with the dendrogram (Figure 2A). We identified few lineage-specific genes in the XX stroma at these stages. While some genes were over-expressed in XX stromal cells when compared only to XY interstitial cells, most were also dimorphic in another lineage (Figure S4). This may indicate that the XX stromal cells are delayed in their differentiation and/or closely related to the supporting cell lineage based on shared expression of female-associated genes (like Irx3 and Wnt4; Figure S4).
Predictions of the gonad differentiation models
These analyses defined the transcriptome shared between XX and XY progenitors for each lineage, and traced the timing of differentiation and acquisition of sex specific fate for each cell type. Informed by this analysis, we investigated whether the transcriptome shared by XX and XY progenitors in the germ cell and supporting cell lineages showed evidence of lineage priming toward the female or male fate. Progenitors that show lineage priming express markers of the various fates into which they can differentiate and subsequently silence genes associated with the fate not adopted as they differentiate [28]–[35].
Four predominant models have been proposed to account for gonad differentiation that have important historical antecedents in the sex determination literature, and make different predictions as to the “ground state” of gonadal progenitors (Figure 4A). It has been proposed that (1) the female state is a default pathway [19]–[21], (2) a female “Z” gene actively represses the male program [22], (3) both the female and male programs are actively initialized [23], and (4) the gonad is balanced between the male and female fates [24]–[27]. We logically extended and reframed these models in the context of lineage priming so that we could test their applicability to the differentiation of the individual cell types in the gonad at the level of the whole transcriptome.
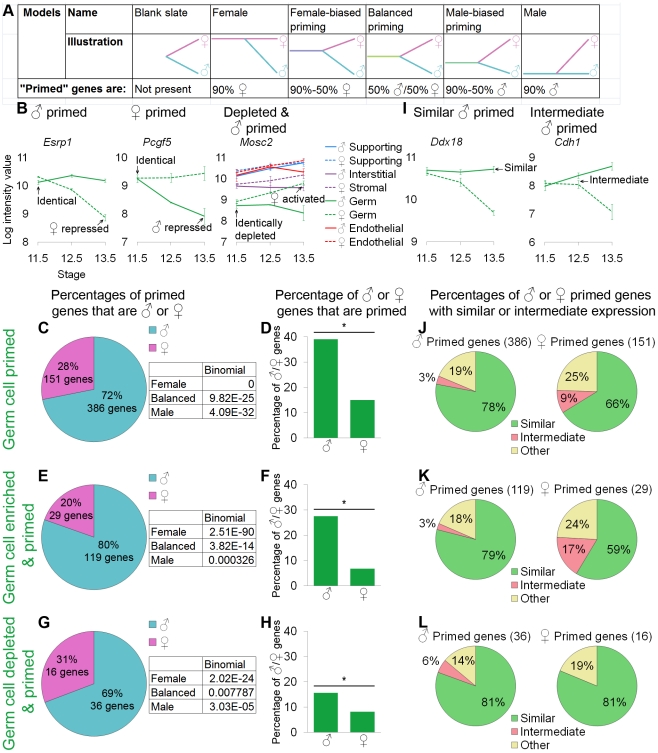
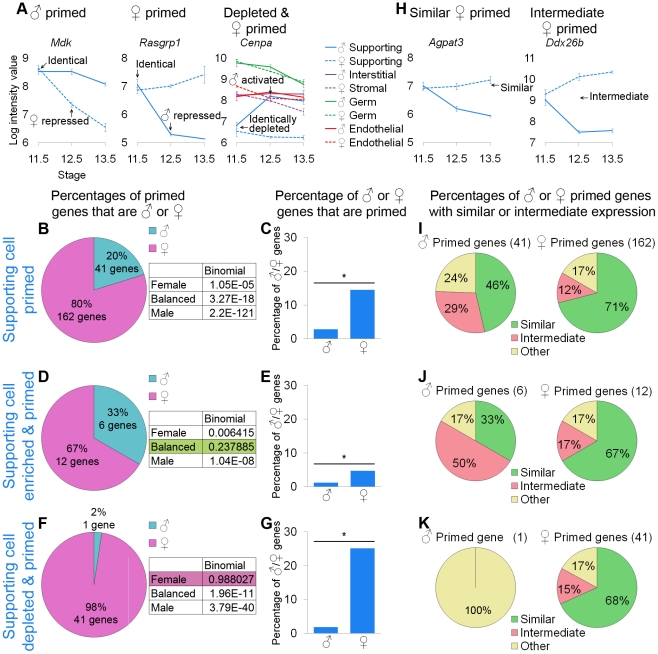
Figure 4. Germ cells showed lineage priming with a male bias.
(A) Models tested and their predictions for the relationship between the differentiated cells and their undifferentiated progenitors. (B, I) Graphs of the log-transformed, normalized intensity values. The error bars are standard error. Only the values for germ cells are shown, except in the depleted and primed example where all lineages are shown for comparison (B). (B) Esrp1 and Pcgf5 are examples of male- and female-primed genes, and Mosc2 is an example of a male-primed depleted gene. We used three different methods to identify primed genes: (C, D, and J) all primed genes were considered, (E, F, and K) only primed and lineage-specifically enriched genes were considered, and (G, H, and L) lineage-specifically depleted primed genes were analyzed. (C, E, and G) The percentages of primed genes that were male-primed and female-primed: all methods showed male-biased priming. The boxes contain the p-values from the binomial test with the expected percentages of the extreme models: 90% male genes (“Male”), 50% male and female genes (“Balanced”), and 90% female genes (“Female”) (see A). All of the extreme models were excluded because p-values were <0.05. (D, F, and H) The percentages of male or female genes that were primed. Significance (*) was determined with the hypergeometric test (p-value<0.05). (I) Graphs illustrating two primed genes whose expression in progenitors is “similar” to the differentiated cell in one sex or “intermediate” between the two sexes. (J–L) In all cases, for both sexes, the majority of primed genes were similarly expressed in germ cell progenitors and differentiated cells of one sex. Gene lists and permutation tests are provided in Dataset S4.
The “female” model predicts that the transcriptome shared by XY and XX progenitors should be predominately associated with the differentiated female fate (i.e., “female primed”). Conversely, the “male” model predicts that the transcriptome shared by XY and XX progenitors should be predominately associated with the differentiated male fate (i.e., “male primed”). If both the male and female programs are activated de novo as progenitors differentiate, those progenitors should be a “blank slate” in that they would not express transcripts associated with either the differentiated male or female cells. Alternatively, the progenitors could fit the “balanced priming” model and express a similar number of both male- and female-associated transcripts at the time when they are poised to adopt either fate. Finally, the progenitors could be primed to adopt either fate, but there could be more genes associated with the female (“female-biased priming”) or the male (“male-biased priming”) fate (Figure 4A).
Germ cell progenitors are primed with a bias toward the male fate
We analyzed the relationship between XX and XY germ cell progenitors and their final sexually differentiated fates in multiple ways to ensure that our methods did not skew the results. We defined a gene as “primed” if it showed identical levels of expression in XY and XX germ cells at E11.5 that were retained or elevated in one sex and not in the other at E13.5 (Figure 4B, Esrp1 and Pcgf5). This was an unbiased method of identifying genes characteristic of the female and male fates [60]. Similarly, we determined “expression” in the E11.5 sexually undifferentiated progenitors in an unbiased way. We inferred “expression” from the fact that these genes showed down-regulation in one of the sexes. In this approach, all primed genes were analyzed, regardless of whether they were specific to germ cells (Figure 4C, 4D).
In a second approach, we adopted more stringent, but still unbiased, definitions for the set of genes analyzed (Figure 4E, 4F). In this approach, we restricted our analysis to primed genes that were lineage-specifically enriched in E11.5 germ cells and in differentiated E13.5 germ cells of one sex (Figure 3A). Using an analogous method, we also explored depleted and primed genes that were specifically depleted in XY and XX E11.5 progenitors, were activated by one sex, and remained sex-specifically and lineage-specifically depleted in the other (Figure 4B, Mosc2, Figure 4G–4H). Gene lists and associated permutation tests are provided (Dataset S4A, S4C).
With all methods tested, germ cells showed a similar pattern indicating male-biased priming. When comparing the percentage of primed genes that were male or female primed, we observed a clear bias toward male genes (Figure 4C, 4E, 4G). We performed a binomial test using the expected values provided with the models (Figure 4A) to determine if we could exclude the extreme models. In all cases, the pattern of genes observed was significantly different from the extreme female and male models as well as the completely balanced priming model (Figure 4C, 4E, 4G). Thus, a male-biased priming model best described the transcriptome in undifferentiated XX and XY germ cell progenitors. This finding was consistent with the clustering dendrogram showing a closer relationship of the undifferentiated early germ cells to the late XY germ cells than to the late XX germ cells (Figure 2A).
We also wanted to ensure that this result was not a statistical artifact of the size of the underlying lists of male and female markers. For example, if the male program contained a larger number of genes than the female program, seeing a male bias in the number of primed genes could reflect the higher relative percentage of male pathway genes, rather than a real priming bias in the progenitor. Thus, we examined the percentage of male and female genes that showed priming (Fig 4D, 4F, and 4H). Again, in all cases, we saw that the same male bias was preserved. Given the large number and percentage (nearly 40% of male germ cell markers) of genes that showed priming (Figure 4C, 4D), the blank slate model could be discarded. Thus, germ cell progenitors showed male-biased priming, including the priming of depleted genes.
Lastly, we were interested in determining whether genes that showed priming were expressed at high or low levels. The expression levels of differentiation markers in the progenitor cells are low in the hematopoietic system [29], but high levels of expression of differentiation markers were observed in progenitors in the early embryo [36]. For our analysis, expression level was defined relative to the differentiated cell. A gene with “similar” expression was expressed in progenitors at a level similar to the level in sexually differentiated cells maintaining expression (Figure 4I, Ddx18), analogous to the high expression observed in the early embryo [36]. A gene with “intermediate” expression was expressed in progenitors at a level between the levels observed in the two sexes (Figure 4I, Cdh1), analogous to the low expression observed in hematopoietic cells [29]. We analyzed the expression level for genes identified as primed by each method (Figure 4J–4L).
A majority of both male and female primed genes were similarly expressed in the undifferentiated germ cell progenitors and the sexually differentiated cells, regardless of how the set of primed genes was defined or whether enriched or depleted genes were considered (Figure 4J–4L). Thus, not only did germ cells show male-biased priming, but the progenitors frequently expressed these primed genes at the same level as the sexually differentiated cells.
We analyzed the lists of genes that exhibited a primed expression pattern (including all primed genes), and were regulated in the same way (similar expression in the progenitor and sexually differentiated cells, Figure 4J) for enrichment of GO terms (Dataset S4D). The genes primed toward the male germ cell fate showed a strong enrichment for categories related to RNA biology, such as RNA binding (Dataset S4D). This is consistent with the previously reported importance of gene control at the RNA level during germ cell development, especially in the male [49], [61], [62].
All the data indicate that the germ cells are primed with a male-bias, and that these primed genes are generally expressed at a similar level in the progenitor and differentiated cell.
Supporting cell progenitors are primed with a bias toward the female fate
We also examined the relationship between supporting cell progenitors and their sexually differentiated states. We used the same method and tested the same models as with germ cells, but the end point of the analysis for supporting cells was E12.5 because their transcriptome changed little between E12.5 and E13.5 (Figure 3A, Figure S4, Dataset S2A, Dataset S3A). As in the germ cell analysis, we defined a gene as “primed” if it showed identical levels of expression in XY and XX supporting cells at E11.5, which were retained or elevated in one sex and not in the other by E12.5 (Figure 5A). The same variations of the analytical methods used for germ cells were also used to identify supporting cell-specifically enriched and depleted primed genes (Figure 5A). The gene lists, permutation test results, and GO terms are provided (Dataset S4B-S4D).
Figure 5. Supporting cells showed lineage priming with a female bias.
(A and H) Graphs of the log-transformed, normalized intensity values of genes. The error bars are standard error. Only the values for supporting cells are shown, except in the depleted and primed example where all cell types are shown. (A) Mdk and Rasgrp1 are examples of male- and female-primed genes and Cenpa is an example of a female-primed depleted gene. As in the germ cell analysis, we examined all primed genes (B, C, and I), primed and lineage-specifically enriched genes (D, E, and J), and primed and lineage-specifically depleted genes (F, G, and K). (B, D, and F) The percentages of primed genes that were male-primed and female-primed. The boxes contain the p-values from the binomial test with the expected percentages of the extreme models. (B) Using the first method, all of the extreme models could be excluded because they had a p-value<0.05. (D and F) However, using the second and third methods, the balanced and female models could not be excluded, respectively. (C, E, and G) Nevertheless, examining the percentage of male or female genes that were primed, all methods showed a significant (*) bias toward the female pathway, as determined by the hypergeometric test (p-value<0.05). Taken together, the data supported female-biased priming. (H) Graphs illustrating two primed genes, whose expression in the progenitor is “similar” to the differentiated cell of one sex, or “intermediate” between the two sexes. (I–K) The female-primed genes were predominantly similarly expressed, but the male-primed genes showed more variability. Gene lists and permutation tests are provided in Dataset S4.
The pattern in supporting cells was less consistent than the pattern observed for germ cells, but was indicative of female-biased priming. Examining all of the primed genes, there was a clear female bias both for the primed genes as a percentage of the priming program (Figure 5B) and the primed genes as a percentage of the XY and XX sexually dimorphic genes (Figure 5C). When restricting our analysis to the genes specifically enriched in supporting cells (Figure 5D, 5E), we observed a similar female bias in the percentage of XX and XY genes that were primed (Figure 5E), but the bias in the percentage of primed genes associated with the male and female pathways was not sufficient to exclude the balanced priming model (Figure 5D). This is likely a statistical artifact due to the small number of primed genes and the difference in size between the E12.5 XX and XY enriched supporting cell gene lists (Figure 3A). The genes specifically depleted in supporting cells showed a strong female bias (Figure 5F, 5G), such that the female model could not be excluded (Figure 5F). All of these analyses suggested female-biased priming, consistent with the clustering showing the closer relationship between the less differentiated early supporting cells and the late XX supporting cells compared to the late XY supporting cells (Figure 2A).
We also determined if the primed genes showed similar or intermediate expression in undifferentiated supporting cell progenitors (Figure 5H). For the female-primed genes, we consistently observed that most genes were similarly expressed (Figure 5I–5K). Expression levels of male-primed genes were more variable (Figure 5I–5K), which again could be due to the small number of genes analyzed. Nevertheless, supporting cell progenitors expressed at least some of the primed genes at levels similar to the sexually differentiated supporting cells.
However, the XX and XY supporting cells were already expressing sexually dimorphic genes by E11.5 (Figure 3A) and therefore were not fully undifferentiated at the start of our analysis. With the reporters available to us, collection of a pure population of progenitors from an earlier stage was not feasible. To determine whether our results were affected by the differentiation process already in progress, we reanalyzed a publically available microarray time course on sorted Sf1-EGFP cells from the urogenital ridge that included earlier time points than were collected here [11] (Figure 6, Figure S5, Dataset S5). While the Sf1-EGFP reporter allows for the collection of earlier time points, it also labels a mixed population that includes at least supporting cells and interstitial/stromal cells [11], [52]. This is concerning because different cell types can have different priming patterns (Figure 4, Figure 5). Therefore, we identified genes from the Sf1-EGFP data set that showed priming, and then restricted our analysis to only those genes whose expression patterns were associated with the supporting cell lineage as defined by our data set.
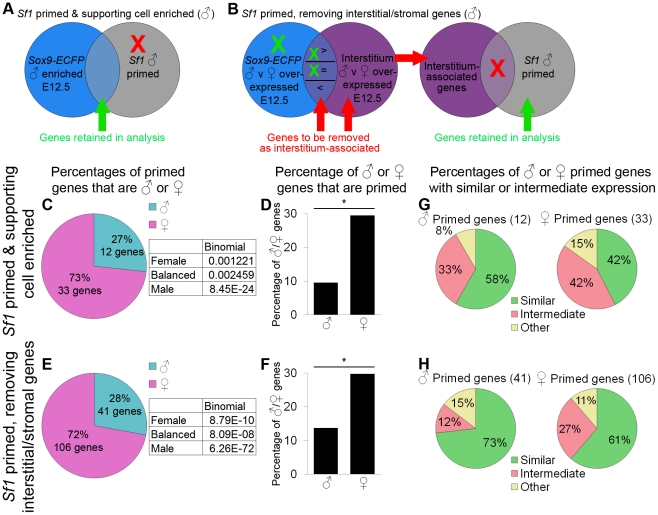
Figure 6. Data from sorted Sf1-EGFP cells also supported female-biased priming for supporting cells.
(A–B) Graphical illustrations of the genes included in our analysis of priming in the Sf1-EGFP data. Because the Sf1-positive population is a mixture of lineages, we used two methods to identify the primed genes associated with supporting cells. XY cells are illustrated in this example, but the same operations were also performed for XX cells. (A) “Sf1 primed and supporting cell enriched” genes were both male-primed in the Sf1-EGFP data (comparing E11.0 and E12.5) and lineage-specifically enriched in our XY Sry-EGFP/Sox9-ECFP purified supporting cells at E12.5. Red indicates genes being removed from the analysis, and green indicates genes being retained. (B) For the “Sf1 primed, removing interstitial/stromal genes”, we removed genes associated with the interstitial/stromal cells at E12.5 (i.e., sexually dimorphic in the interstitium/stroma) from the Sf1-EGFP primed genes. Genes that were expressed sexually dimorphically in both the interstitial/stromal cells and the supporting cells were removed only if expression was higher in the interstitial/stromal cells than in the Sry-EGFP/Sox9-ECFP supporting cells. The Sf1-EGFP primed genes that were enriched in the Sry-EGFP/Sox9-ECFP supporting cells (C, D, and G) and those that were identified by removing interstitial/stromal genes (E, F, and H) were analyzed separately. (C and E) The percentages of primed genes that were male-primed and female-primed. Both methods showed a female bias. The boxes contain the p-values from the binomial test with the expected percentages of the extreme models, and all extreme models could be rejected as having a p-value<0.05. (D and F) The percentage of male or female genes that were primed showed a significant (*) bias toward the female pathway, as determined by the hypergeometric test (p-value<0.05). (G and H) However, primed genes in both sexes were predominantly expressed at similar levels in progenitors and E12.5 supporting cells of one sex. While supporting cell progenitors have a female bias, they also express some markers of the male pathway at levels similar to male supporting cells at E12.5. Gene lists and permutation tests are provided in Dataset S5.
Using the same approach as in the analysis of the Sry-EGFP/Sox9-ECFP sorted supporting cell progenitors, we identified primed genes within the Sf1-EGFP undifferentiated progenitor population. To limit the contribution of genes from interstitial/stromal cells, we set the end point of the analysis at E12.5. E11.0 Sf1-EGFP cells were selected as the starting point for the analysis presented in Figure 6 because it was closest to the sexual divergence point of the primed genes, although the analysis from E10.5 is also provided (Figure S5, Dataset S5B).
While lists of all primed genes identified in the Sf1-EGFP cells are provided (Dataset S5A), we limited our analysis to only those genes associated with supporting cells. To define this gene set, we used two different methods utilizing our purified supporting cell (Sry-EGFP/Sox9-ECFP) and interstitial/stromal cell data as a reference. First, we used a rigorous threshold for inclusion and retained only those genes that were also found to be lineage-specifically and sex-specifically enriched in our E12.5 XX or XY Sry-EGFP/Sox9-ECFP supporting cells (Dataset S2A; Figure 6A, 6C, 6D, 6G). In a second approach, we used less rigorous criteria and removed genes sexually dimorphic in the interstitial/stromal cells (Figure 6B, 6E, 6F, 6H; Dataset S5A, S5C). Genes that were sexually dimorphic in both the Sry-EGFP/Sox9-ECFP supporting cells and the interstitial/stromal population were only removed if they were expressed at higher levels in interstitial/stromal cells than in supporting cells.
Regardless of the method used, these data also supported female-biased priming of the supporting cells. Most of the primed genes were female, although the progenitors expressed some male genes as well (Figure 6C–6F, Dataset S5A). Many of these genes were also similarly expressed (Figure 6G, 6H; Dataset S5A). This analysis is therefore consistent with the findings from the Sry-EGFP/Sox9-ECFP data. To investigate the overlap in these data sets, we determined whether similar transcripts were identified as primed in both the Sf1-EGFP and Sry-EGFP/Sox9-ECFP data sets (Figure S6). While a small percentage of the genes primed in the Sf1-EGFP cells were also identified as primed in the Sry-EGFP/Sox9-ECFP data, a larger proportion were already sexually dimorphic by E11.5, although indications of priming could be observed in some of the expression patterns (Figure S6). This analysis was also consistent with previous findings showing that individual genes we identified as primed were expressed in the supporting cell progenitors and then became sex-specific (Dax1/Nr0b1, Wnt4, Sox9, and Cbln4 [26], [63]–[66], Dataset S4B, Dataset S5A). Thus, the analysis of these two independent data sets produced consistent results for individual genes and reached the same overall conclusion that the supporting cells are primed with a female-bias.
Because nearly 30% of female genes showed priming in the Sf1-EGFP data (Figure 6D, 6F), the blank slate model can be rejected. The E11.5 Sry-EGFP/Sox9-ECFP progenitors showed a lower percentage of primed transcripts (Figure 5C, 5E) than the E11.0 Sf1-EGFP progenitors (Figure 6D, 6F), which may be explained by the fact that the Sry-EGFP/Sox9-ECFP supporting cell progenitors were already partially sexually differentiated by E11.5 (Figure 3A).
Discussion
A comprehensive understanding of organogenesis requires systems-level knowledge of transcriptional network dynamics underlying cell differentiation. By performing a microarray analysis on sorted cell populations in the fetal mouse gonad over the course of sex-specific differentiation, we quantified the transcription dynamics of diverse cell types as they build one of two different organs from similar pools of progenitors. This study provided an expression resource for the field of gonad development, but more importantly, it characterized the features of the biological system that could only be appreciated at the whole transcriptome level. By examining the system as a whole, we obtained new insights into patterns of cell fate determination and lineage commitment.
Insights from whole transcriptome characterization of multiple gonadal lineages
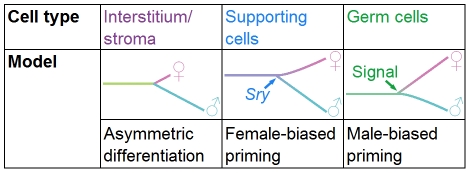
We characterized the transcriptomes of undifferentiated progenitors and analyzed their transition to sexually differentiated cells. All four lineages analyzed (including interstitial/stromal cells) have a sexually undifferentiated progenitor cell with a distinct transcriptome. Although we detected some overlapping sexually dimorphic expression patterns that may have biological significance between the supporting cells and interstitial/stromal cells (Figure S4), these lineages have transcriptionally distinct progenitors at E11.5 (Figure 3A). However, the differentiation of the interstitial and stromal cells is sexually asymmetric over this time period (Figure 7). Whereas the XY interstitium expressed lineage-specific transcripts, there were few lineage-specific transcripts in XX stromal cells even at E13.5 (Figure 3A). Thus, the XX stroma may not fully differentiate until after E13.5. The XX stroma also showed overlapping sexually dimorphic expression with XX supporting cells, in part due to pathways downstream of widespread Wnt signaling in the ovary (Figure S4).
Figure 7. Models of differentiation for the different gonadal lineages.
The interstitial/stromal cells differentiate asymmetrically over the time period examined, as we detected few genes specific to the XX stroma by E13.5, whereas, the XY interstitial population acquired a larger set of lineage-specific genes. Supporting cells are primed with a female bias. The natural progression of the primed state may be to adopt the female differentiated state, but in the presence of Sry the cells repress the female program and adopt the male fate. Conversely, germ cells are primed with a male bias. An extrinsic signal may be required from the mesonephros to induce the adoption of the female fate; otherwise, germ cells adopt the male fate.
We also provided global transcriptional evidence that the supporting cells are the first cell type in the gonad to adopt a sex-specific fate (Figure 3A), as predicted by previous experiments [10], [11], [46], [59]. While there are some gene expression differences between E12.5 and E13.5, the supporting cells appear to have adopted their sex-specific fates by E12.5 (Figure 3A, Figure S4, Dataset S2, Dataset S3). The sex-specific differentiation of interstitial/stromal cells and germ cells began at E12.5, when the supporting cells had essentially completed their differentiation process (Figure 3A).
Lineage- and sex-specific transcriptional depletion in the differentiating gonad
For supporting cells, germ cells, and endothelial cells, our methods were sensitive enough to detect lineage-specific transcript depletion that could be sex-specific or sex-independent (Figure 3A). Given that other studies have also reported specific gene depletion [16], [17], this likely represents a common regulatory logic in the transcription network of differentiating cells. For example, unique cell fate specification in the sea urchin involves the repression of widely-expressed genes to “lock-down” the selected fate [67]. We detected the lineage-specific repression of transcription factors likely involved in specifying alternative fates in both germ and supporting cells (i.e., Gata6 and Lef1, Figure 3, Dataset S2A). We also found evidence for lineage-specific repression of genes that regulate cell behavior. The transcriptome of XX supporting cells is characterized by the sex- and lineage-specific repression of cell cycle genes (Figure 3, Dataset S2), which is correlated with the failure of XX supporting cells to reenter the cell cycle [57], [58]. A similar phenomenon was reported in senescence and DNA damage arrest [68], [69], indicating this may be a widely-used mechanism of cell cycle arrest.
Depleted genes also showed evidence of lineage priming in progenitors with similar patterns as genes expressed and primed (Figure 4G, 4H; Figure 5F, 5G), particularly in germ cells. The association between components of the network that are expressed and repressed suggests that both are important for specifying cell fate and may be co-regulated as parts of the same transcriptional program [67].
Contrary to our findings in the other lineages, we did not detect lineage-specifically depleted genes in the XY interstitial or XX stromal cells. However, there may be heterogeneity within this population that masks repression characteristic of any one subfraction. Since the purity of the germ and supporting cell populations were likely important for detecting depletion, the ability to isolate distinct populations within the interstitium/stroma may be necessary to do the same for this population.
A role for priming in the bipotential supporting cell lineage
We determined that the supporting cell progenitors are primed with a female bias, indicating that both male and female genes are expressed in the progenitor, but that the female program is over-represented (Figure 7). This female-biased priming model bridges the more recent evidence for a balance in the gonad between the male and female fates [24]–[27] and the classic theory of the female “default” state [19]–[21]. Although we found genes characteristic of both the male and female programs in supporting cell progenitors, the over-representation of the female program in progenitors explains why the female fate is the “default” state in the absence of Sry.
The female fate may be the “default” state because the over-represented female program of supporting cell progenitors is self-sustaining without additional inputs and leads to silencing of the alternative testis pathway. The high level expression of primed genes may make the primed state in the progenitors unstable. Whereas, low-level expression of fate determinates has been associated with a stable primed state, the expression of determinates at high levels has been associated with instability of the primed state [70]. As priming with high level expression has also been noted in the early embryo [36], this may be a common developmental mechanism to ensure that development progresses and does not become stalled. Thus, this unstable primed state would naturally lead to female differentiation in the absence of an intervention from Sry. Under these circumstances, an ovary-determining gene, as proposed by Eicher and Washburn [23], would not be necessary.
Differences in the strength of the female bias may explain differences between humans and mice with respect to the effects of mutations in individual genes in the female pathway. For example, mutation of RSPO1 in humans resulted in female-to-male sex reversal [71]. However, deletion of Wnt4, Rspo1, or β-catenin in the mouse did not result in full female-to-male sex reversal [25], [66], [72], [73]. The female bias in mice may be sufficiently robust to rebound from the loss of any one of these genes. Conversely, a lower level of female-biased priming in humans could allow deletion of just one key factor in the female program to disrupt female development.
Bipotential supporting cells show priming toward the male and female fates (Figure 5, Figure 6). A previous study identified a female subnetwork in E11.5 XY gonads [27]. We identified many of these genes as female-primed (Wnt4, Fst, Rspo1, Dapk1, Pld1, Actr6, and Dock4, Dataset S5A). The expression of female genes in XX and XY supporting cell progenitors could be consistent with a female transcriptional state in the progenitors that is repressed by the activation of Sry in XY cells rather than by the concept of male and female priming.
However, the coexpression of male genes in both XX and XY supporting cell progenitors strongly supports the idea that these cells are primed to adopt either of their potential fates. These genes likely represent a male subnetwork operating in the early XX and XY cells independent of Sry, as Sry is not present in the XX cells. This work provides a molecular explanation for the concept of bipotentiality characterized by the coexpression of both male and female transcripts in the XX and XY supporting cell progenitors.
The mechanism of priming remains to be determined. It has been speculated that priming is a byproduct of open chromatin [29], [33], [70]. Bivalent chromatin has been reported in embryonic stem cells at loci expressed at low levels [74]. However, this is inconsistent with our findings in the gonad where primed genes in bipotential supporting cell precursors tended to be expressed at high levels. Other studies in the laboratory are aimed at investigating the state of chromatin at primed loci.
While priming may be important to establish a bipotential state, mounting evidence suggests that repression of the primed genes associated with the alternative sex is important for supporting or maintaining cell fate commitment. Genes important for testis (Sox9 and Dmrt1) [75]–[77] and ovary (Wnt4, Fst, and Rspo1) [25], [66], [78] development were identified as expressed in XX and XY supporting cell precursors and repressed in the opposite sex (Dataset S4B, Dataset S5A). Over-expression of Sox9 is known to result in female-to-male sex reversal [79]. Ectopic activation of the downstream target of WNT4, β-catenin, can reverse differentiation of XY supporting cells and trigger their differentiation as female cells [56]. Thus, the repression of genes associated with the opposite sex (which we identified in these experiments) may be as essential to the cell fate decision as the genes that are expressed.
Priming during germ cell development
The testicular-biased primed state of germ cell progenitors was surprising because germ cell fate is determined by the somatic environment [8]. Germ cells that enter an ovarian environment initiate meiosis (the female fate), while germ cells that enter a testis environment undergo mitotic arrest (the male fate) [80]. Historically, entry into meiosis was thought to be the default state for germ cell differentiation [8], [81]–[84]. However, the weight of current evidence indicates that meiosis (the female fate) is the result of an external inducing signal produced in the mesonephros and specifically degraded in the testis by Cyp26b1 [85]–[89]. While there is some evidence for a signal promoting the male fate [90]–[94], this signal may act by antagonizing the female-promoting signal [85], [91] and/or providing a permissive environment for male germ cell development [90].
The male-biased transcriptome of germ cell progenitors is consistent with a male developmental “default” state in the absence of the female-promoting signal (Figure 7). Interestingly, in both supporting and germ cells, the dominant fate-determining signal is associated with the fate under-represented in the progenitor's transcriptome (Figure 7). Sry expression in XY supporting cells is required to stabilize the male program and repress the female program. Similarly, the external female signal initiates the meiotic program in germ cells and represses the alternative male program. XY germ cells adopt the female fate if Cyp26b1 activity is eliminated (resulting in the presence of the meiosis-inducing signal), even in an otherwise male environment [85], [86]. Our priming model suggests that the over-represented male program requires only subtle reinforcement from the somatic environment. On the other hand, the under-represented female program cannot be stabilized without its instructive cue, but once that input is received, it is able to suppress the male program.
One reason why E13.5 XY germ cells share more transcriptional features with the progenitor than XX germ cells may be due to their maintenance in a more stem cell-like state (Dataset S4A) [95]. However, XY germ cells at E13.5 are not identical to the sexually undifferentiated germ cell progenitors at E11.5. Specifically, by E13.5, XY germ cells have repressed genes associated with the female germ cell program (Figure 4), which may explain why, even when put into a female environment after E11.5, XY germ cells can no longer adopt the female fate [8], [81].
Priming during differentiation
This study revealed previously unknown systems-level aspects of the differentiation of two critical cell types during gonad development, with implications for other developing cells. Supporting and germ cells arise from different embryonic origins and respond to different cues during their terminal differentiation, and yet both show priming. Priming may be a common feature of differentiation from multipotent progenitors at all levels, as it has now been identified in the early embryo [36], multipotent hematopoietic cells [29],bone marrow mesenchymal stem cells [28], germ cells, and somatic gonadal cells.
However, each priming program appears surprisingly lineage specific. Even the cells within the gonad do not share a common bias in their priming programs (Figure 7). Priming may limit the developmental potential of cells by preparing them to respond in a unique manner to the same signals used throughout development. Only certain avenues of differentiation are available while others are closed [28], [30], [60]. For example, male supporting cells and male germ cells exposed to similar Fgf9 signals adopt different fates [24], [91], [92], [96] because they have different underlying transcriptional networks that prepare the cell to respond differently. In vivo, a supporting cell progenitor cannot become a germ cell because the required transcriptional avenues are not available. The ability to induce pluripotent cells from differentiated cells in vitro may be related to the ability to return the cell to a primitive primed state, where many avenues of differentiation are open.
We identified priming patterns using simple, yet flexible, statistical methods that can be applied to any microarray time course on a single purified cell population isolated immediately preceding and following differentiation. While we were able to analyze the priming of lineage-specific enriched and depleted transcripts to validate our results (Figure 4, Figure 5), the results were similar regardless of the method used. Because having other cell types for comparison is not required, this method can be broadly applied to other systems exploring differentiation.
Systems biology entails the use of both whole genome analysis and molecular genetic approaches to inform each other [97]. While studies disrupting the function of individual genes have clearly identified critical components of the system, they are unlikely to be sufficient on their own to fully elucidate the combinatorial interactions within the complex transcriptional network governing organ development. Recent studies show that developmental transcriptional networks are highly buffered and contain redundant factors, suggesting that many important network players may not have a developmental phenotype when disrupted [27], [98]. While some transcriptional profiles uncovered in this study may have no functional relevance, others likely contribute to robustness of the system and allow it to rebound from perturbation.
In conjunction with the traditional functional studies examining individual genes, our understanding of gonad organogenesis (and development in general) is facilitated by a whole system view of the process as this approach reveals novel phenomena that cannot be identified by studying single genes. This analysis leads to many new and exciting hypotheses related to the role of priming in the differentiation of gonadal cells and provides new insight into the processes of cellular differentiation and lineage commitment.
Materials and Methods
Mice
All animals were maintained and experiments were conducted according to DUMC-IACUC and NIH guidelines, based on existing protocols. We used six different transgenic mouse lines with fluorescent reporters: Sry-EGFP [Tg(Sry-EGFP)92Ei] (sex determining region of Chr Y) [7], Sox9-ECFP (SRY-box containing gene 9) [39], Mafb-EGFP [Mafbtm1Jeng] (a gift from S. Takahashi; v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B) [40], αSma-EYFP (a gift from J. Lessard; the official gene name of αSma is Acta2; actin, alpha 2, smooth muscle) [45], Flk1-mCherry [Tg(Kdr-mCherry)1Medi] (a gift from M. Dickinson; the official gene name of Flk1 is Kdr; kinase insert domain protein receptor) [43], [44], and Oct4-EGFP [Tg(Pou5f1-EGFP)2Mnn] (the official gene name of Oct4 is Pou5f1; POU domain, class 5, transcription factor 1) [42]. In most cases, males from these lines were crossed to CD-1 females (an outbreed line from Charles River), and gonads from multiple embryos were generally pooled to reduce the impact of strain variation. All of the males were homozygous for the marker, with the exception of Mafb-EGFP males. This line is a targeted insertion of GFP into the Mafb locus, which results in a Mafb mutant when homozygous. Mafb-EGFP embryos collected in this study were therefore heterozygous for Mafb; however, we know of no defects in gonad development in Mafb heterozygotes (data not shown). To increase the fluorescence intensity for both the αSma-EYFP (maintained on an FVB/CD-1 mixed line) and Flk1-mCherry (maintained on the outbreed CD-1 line) reporters, homozygous males were crossed to homozygous females.
Sry-EGFP and Sox9-ECFP reporters were used to collect supporting cells. In the Sry-EGFP line, the Sry promoter drives expression of GFP in cells competent to activate the Sry promoter in both XX and XY gonads. This labels the supporting cell lineage in both sexes, but because the transgene lacks the SRY open reading frame, transgenic XX gonads express no SRY protein and develop as normal ovaries [7]. While Sry-EGFP expression persists in XX supporting cell precursors through E13.5, expression of the transgene is reduced in XY supporting cell precursors after E11.5 in our hands (data not shown), similar to endogenous Sry expression [99]. Therefore, Sry-EGFP was used to isolate supporting cells from XX and XY samples at E11.5, and XX E12.5 and E13.5 samples. Sox9-ECFP was used to sort XY supporting cells at E12.5 and E13.5. All cells that later express Sox9 can be lineage traced from Sry-positive cells [46]. Sox9 is also the direct downstream target of SRY [100]. This Sox9-ECFP reporter specifically labels XY supporting cells (Figure S1). Thus, we did not expect that the use of Sox9-ECFP would affect our analysis of the supporting cells. We validated that this was the case by ensuring the genes we identified in the Sox9-ECFP population substantially overlapped those identified in a previous study using the Sry-EGFP reporter [10], and that some genes continued to be similarly expressed in the XX Sry-EGFP and XY Sox9-ECFP supporting cells (data not shown).
Collection of gonadal lineages
Timed matings were performed, with the day the vaginal plug was detected considered E0.5. Embryos were collected at E11.5, E12.5, and E13.5. For the Mafb-EGFP line, only GFP-positive embryos were used for sorting. The sex of the gonad is obvious by eye at E12.5 and E13.5. The E11.5 embryos were genotyped to determine the sex as previously described using primers to detect Kdm5c (X chromosome) and Kdm5d (Y chromosome) [27], [101].
To collect gonadal cells, the urogenital ridge and dorsal aorta were removed, and the gonad/mesonephric complex was isolated. In most cases, the gonad was separated from the mesonephros. However, for the Oct4-EGFP sorts, the gonad was left attached because Oct4 expression is highly specific to germ cells (data not shown). For the E11.5 Flk1-mCherry sorts, only the anterior and posterior portions of the mesonephros were removed by cutting at a 45° angle from the end of the gonad. The gonad vasculature arises from a plexus in the mesonephros [5]; thus, the gonadal and a portion of the mesonephric endothelial cells represent one population. This procedure retained the mesonephric plexus while removing most of the vasculature associated with the mesonephric ducts. At E12.5 and E13.5, the mesonephros was removed completely for the Flk1-mCherry sorts.
XY and XX gonads were separately pooled from one or more litters and incubated in 250 µl 0.25% Trypsin EDTA (Gibco #25200) at 37°C for 5–10 minutes. The trypsin was removed and replaced with 400 µl PBS with or without 4 µl RNase-free DNase (Promega #M6101). The tissue was dissociated, and the cells were passed through a strainer (BD Falcon #352235). FACS was performed by the Duke Comprehensive Cancer Center Flow Cytometry Shared Resource. The positive fraction was pelleted, the liquid supernatant was removed, and the cells were immediately frozen at −80°C.
Preparation of samples and arrays
Generally, cells from multiple embryos were pooled. RNA was extracted from over 100,000 cells to as few as 10,000 cells using the RNeasy Micro kit (Qiagen #74004) following the manufacturer's instructions for “Cells.” However, the protocol was started at step 2 (disruption with RLT), and β-ME was not added. The cells from multiple sorts were pooled during disruption with RLT (if necessary), step 3 (homogenization) was skipped, and in step 10 three RPE washes were performed.
Samples were prepared for the Affymetrix Genechip Mouse Gene 1.0 ST Arrays (#901168) using the Nugen WT-Ovation Pico RNA Amplification System (#3300), WT-Ovation Exon Module (#2000), and the Encore Biotin Module (#4200), following the manufacturer's instructions. For purification following the Pico and Exon kits, the Qiagen QIAquick PCR Purification Kit (#28104) was used following the instructions provided by Nugen. Fragmented and labeled product was submitted to the Duke Institute for Genome Sciences and Policy Microarray Facility for hybridization and reading.
We ran a total of 91 arrays. This included the arrays on our five sorted cell types at the three time points with separate XX and XY samples in biological triplicate as well as one array on a whole P1 mouse RNA sample(a gift from S. Potter) for normalization across GUDMAP.
Immunofluorescence
Samples were fixed, stained, and imaged as whole gonads with the mesonephros attached as previously described [102]. Some samples were first processed through a methanol series and stored at −80°C prior to rehydration and staining [103]. Primary antibodies used were: anti-3β-HSD (Santa Cruz sc-30820, 1∶100 in samples processed through methanol; TransGenic Inc. KO607, 1∶500; K. Morohashi generously provided a non-commercial antibody before the TransGenic Inc. antibody was available), anti-PECAM1 (BD Pharmingen 553370, 1∶250), anti-SRY (a gift from P. Koopman and D. Wilhelm), and anti-SOX9 (a gift from P. Koopman and D. Wilhelm). Secondary antibodies used included Alexa 647- and 488- conjugated secondary antibodies (Molecular Probes, 1∶500) and Cy3- and Cy5-conjugated secondary antibodies (Jackson ImmunoResearch, 1∶500). DAPI (Sigma-Aldrich) was used to label nuclei.
Initial array processing and analysis
The .cel files were processed with Partek Genomics Suite version 6.5 (6.11.0207) by RMA with quantile normalization and median polish summarization at the transcript cluster (gene) level. Probes were adjusted for GC content and probe sequence. The data were transformed into log base 2. We removed all transcript clusters that did not have a cross hybridization category of 1 (perfect match) in the Affymetrix annotation, that did not have a gene symbol, or that did not have a log base 2 normalized expression value >6 in at least two out of three replicates of at least one sample. The genes that passed this filtering step, and only these genes, were used in subsequent analyses.
In the case of the analysis of the αSma-EYFP cells (Figure S2), this initial processing of the arrays included all 91. However, for all other analyses, we used data generated by processing only 72 of our arrays because the αSma-EYFP and P1 whole mouse data were not included. These processed data on the 72 arrays were used in all portions of the analysis and are provided as a resource for the community (Dataset S1).
Partek Genomics Suite was used to generate the hierarchical clustering dendrograms and perform the ANOVA sources of variation analysis (Figure 2, Figure S3). The clustering methods used are described in the figure legends. One of the E11.5 XY endothelial samples was somewhat of an outlier in the clustering. This may be due in part to its processing, which resulted in unusually low (but still adequate) yield after the amplification with the Pico kit. However, we do not believe this compromised the sample as it still clustered with endothelial cells.
Pairwise comparisons used to identify genes of interest
This analysis was performed at the level of the transcript cluster (gene), but some genes have multiple transcript clusters. Thus, the lists of transcript clusters may include multiple entries for the same gene. For graphical display of the numbers of genes identified (Figure 3, Figure 4, Figure 5, Figure 6, Figure S4, Figure S5, Figure S6), each gene was counted only once (i.e., duplicates were removed). However, in tables, all transcript clusters are shown (i.e., duplicates are not removed) (Dataset S2, Dataset S3, Dataset S4, Dataset S5).
To identify genes of interest, we adopted a simple and flexible method using multiple or single pairwise comparisons between samples (analogous to methods used previously [53]). The same p-value and fold change cutoffs were used throughout. In all cases where we identified a difference between samples, we used a p-value cutoff of 0.05, and a fold change cutoff of 1.5 for each comparison. A gene was deemed to be identically expressed in two samples if the p-value was>0.05 and the fold change was between −1.5 and 1.5. When multiple pairwise comparisons were done, the intersection of the multiple lists generated was taken as the genes of interest.
To identify sex-specifically and lineage-specifically enriched genes (using E12.5 XY supporting cells as an example), we used the following pairwise comparisons with the above cutoffs:
The gene was more highly expressed in XY supporting cells than XX supporting cells at E12.5 (i.e., sex-specific expression).
The gene was more highly expressed in XY supporting cells than the XY interstitium, germ cells, and endothelial cells at E12.5 (XY supporting cells versus XY interstitium, XY supporting cells versus XY germ cells, etc.) (i.e., lineage-specific expression).
The intersection of these multiple lists were the genes considered enriched in XY supporting cells at E12.5 (Figure 3A). Because the XY supporting cells were not compared to other XX lineages (e.g., XX germ cells), genes could be identified as enriched in both the XY supporting cells and a different XX lineage. Similar comparisons were also used to identify sex- and lineage-specifically depleted genes, but a gene was deemed “depleted” when its expression was higher in all other cell lineages than the E12.5 XY supporting cells in the example.
To identify sex-independent and lineage-specifically enriched genes indicative of sexually undifferentiated progenitor expression (Figure 3A), we used the following pairwise comparisons (using the E11.5 supporting cells as an example):
The gene was more highly expressed in XY supporting cells than the XY interstitium, germ cells, and endothelial cells at E11.5 (i.e., lineage-specific expression among XY cells).
The gene was more highly expressed in XX supporting cells than the XX stroma, germ cells, and endothelial cells at E11.5 (i.e., lineage-specific expression among XX cells).
The gene was identically expressed in XX and XY supporting cells at E11.5 (i.e., identically expressed in progenitors).
Again, similar comparisons were used to identify the lineage-specifically depleted genes, but a gene was deemed “depleted” when its expression was higher in all other cell lineages than the E11.5 XY and XX supporting cells in the example. Occasionally, multiple transcript clusters for a gene may behave differently. In this case that gene may be identified in multiple lists. For example, different Myo9a transcript clusters were identified as sex-specifically enriched in E11.5 XX supporting cells and sex-independently enriched in E11.5 XX and XY supporting cells (Dataset S2A). The different behavior of the different transcript clusters in the gene could be caused by off-target probe binding or alterative splicing.
Leydig cell genes were a special case because both our sorted interstitial and “endothelial” cells contained Leydig cells (Figure 1C–1E). To identify Leydig cell genes (Figure 3A), using E13.5 as an example, we used the following pairwise comparisons:
The gene was more highly expressed in the XY interstitium than XY supporting cells and germ cells at E13.5 (i.e., lineage-specific expression).
The gene was more highly expressed in XY endothelial cells than XY supporting cells and germ cells at E13.5.
The gene was more highly expressed in the XY interstitium than the XX stroma at E13.5 (i.e., sex-specific expression).
The gene was more highly expressed in XY endothelial cells than XX endothelial cells at E13.5.
Depleted genes were identified similarly, but a gene was deemed “depleted” when its expression was higher in all other cell lineages than the E13.5 XY interstitium and endothelial cells in the example. The genes identified as E13.5 Leydig cell genes were removed from the E12.5 and E13.5 XY endothelial cell gene lists (Dataset S2A). In all cases, we used the E13.5 Leydig cell lists to remove the maximum number of genes associated with Leydig cells. Some of these genes were also identified in the XY interstitial lists, and they appear in both lists of identified genes (Dataset S2A), but the overlapping genes were removed from the Leydig cell bar for the graphical depiction (Figure 3A).
Permutation testing was done to estimate the false discovery rate in the gene lists. The array data from the samples being used in the generation of a gene list (in the first example above: XY supporting cells, XX supporting cells, XY interstitium, XY germ cells and XY endothelial cells; all at E12.5) were permuted. The series of operations was run on the permuted columns, and the number of genes generated from each permutation was stored. The permutations were performed 200 times, and the mean number of genes was used to compute the false discovery rate. In all cases, we considered a false discovery rate of 20% or less as acceptable (Dataset S2B). Most lists actually had a much lower false discovery rate. In any case where some of the genes were removed from the list, such as the removal of Leydig genes from endothelial cell lists, this operation was ignored in the permutation tests.
The lists of transcript clusters for the lists considered significant were inputted into DAVID (http://david.abcc.ncifcrf.gov/) [104], [105] to identify pathway and GO term enrichment. The full list of transcript clusters used in the analysis (Dataset S1A) was inputted as the background. We included all KEGG and BioCarta pathways as well as GO_FAT molecular function (MF) and Biological Process (BP) terms with a p-value>0.05 (Dataset S2C, S2D).
Primed genes were identified by multiple methods (Figure 4, Figure 5). In general, we defined a gene as primed when it was expressed in the progenitor, then repressed by one sex and maintained or activated by the other sex. To identify all male-primed genes, using germ cells as an example, we used the following pairwise comparisons:
The gene was identically expressed in XX and XY germ cells at E11.5 (i.e., identically expressed in progenitors).
The gene was more highly expressed in XY than XX germ cells at E13.5 (i.e., specific to XY cells).
The gene was more highly expressed in XX germ cells at E11.5 than at E13.5 (i.e., this gene is repressed in XX cells).
If the gene was also more highly expressed in XY germ cells at E11.5 than at E13.5, it was removed (i.e., genes showing differential repression were eliminated).
In the second analysis, we used more stringent criteria to define genes characteristic of the progenitor cells at E11.5 and differentiated cells at E13.5 by incorporating information on lineage-specific expression. To identify the enriched and primed genes, we used the above comparisons in addition to requiring that:
The gene was more highly expressed in XY germ cells at E11.5 than XY supporting cells, interstitium, and endothelial cells at E11.5.
The gene was more highly expressed in XX germ cells at E11.5 than XX supporting cells, stroma, and endothelial cells at E11.5.
The gene was more highly expressed in XY germ cells at E13.5 than XY supporting cells, interstitium, and endothelial cells at E13.5.
Finally, we also wanted to explore the possibility of depleted gene priming. Continuing with the example of XY germ cells, we used the following pairwise comparisons to identify depleted and primed genes:
The gene was identically expressed in XX and XY germ cells at E11.5 (i.e., identically expressed in progenitors).
The gene was more highly expressed in XY supporting cells, interstitium, and endothelial cells at E11.5 than XY germ cells at E11.5 (i.e., lineage specific repression).
The gene was more highly expressed in XX supporting cells, stroma, and endothelial cells at E11.5 than XX germ cells at E11.5.
The gene was more highly expressed in XX than XY germ cells at E13.5 (i.e., remains repressed in XY cells).
The gene was more highly expressed in XY supporting cells, interstitium, and endothelial cells at E13.5 than XY germ cells at E13.5 (i.e., lineage-specific repression).
The gene was more highly expressed in XX germ cells at E13.5 than at E11.5 (i.e., this gene is activated in XX cells).
If the gene was also more highly expressed in XY germ cells at E13.5 than at E11.5, it was removed (i.e., genes showing differential activation were eliminated).
The same methods were used to analyze priming in the supporting cells, but E12.5 was used as the end point of the analysis. Permutation tests were run on all of these lists of primed genes.
We then examined these primed genes in two ways. First, we compiled all genes identified as male or female primed and determined the percentage associated with each sex (Figure 4C, 4E, 4G; Figure 5B, 5D, and 5F). We used a binomial test for the different extreme models to determine whether priming showed a sex-specific bias. A one-tailed test was used for the “female” and “male” models, which were defined as predicting that 90% of the genes were female-primed or male-primed, respectively. A two-tailed binomial test was used for the balanced model, which predicts 50% male and 50% female genes. Any model resulting in a p-value<0.05 was excluded. If all models were excluded, an intermediate model was selected.
To ensure that this result was not a statistical artifact of the size of the underlying lists of male and female markers, we also displayed primed genes as a percentage of the total “male” or “female” genes (Figure 4D, 4F, 4H; Figure 5C, 5E, 5G). These male and female genes were determined in different ways to account for differences in the method of defining primed genes. When identifying all primed genes, the list of all testis genes included everything identified in step 2 alone of the process for generating the primed genes. For the enriched and primed genes, testis genes were required to meet the requirements of step 2 and 7. For the depleted and primed genes, testis genes were required to meet steps 4 and 5. A 2×2 contingency table (with the actual numbers of genes, not percentages) and a two-tailed hypergeometric test were used to determine if there were a significant difference in the percentage of primed genes in the male and female programs. A p-value<0.05 was considered significant. A significant p-value meant that sex and priming were not independent variables and that there was a bias in the representation of the two programs in the progenitor cells.
We further characterized the primed genes identified by each method based on their expression level. We divided these into two categories: similar and intermediate expression relative to the expression in the differentiated cells (Figures 4J–4L, Figure 5I–5K). For similarly expressed genes, using XY germ cells as an example, the gene was required to be primed and identically expressed in XY germ cells at E11.5 and E13.5. For intermediate expression genes, the gene was required to be primed and more highly expressed in XY germ cells at E13.5 than E11.5 (or more highly expressed at E11.5 for depleted and primed genes). Any gene that did not fall into one of these categories, or had transcript clusters that fell into both similar and intermediate categories, was counted as “other.” If a gene had transcript clusters that fell into the defined similar or intermediate categories and others that did not fall into either, the gene was still counted as similar or intermediate. GO term enrichment for all of the primed genes with similar expression for both supporting and germ cells was determined using DAVID as described above.
Sf1-EGFP array analysis
To examine earlier expression in supporting cells, we reanalyzed previously generated microarray data from Sf1-EGFP sorted cells [11] (raw data available at http://www.ebi.ac.uk/arrayexpress/browse.html?keywords=Nef&expandefo=on). Because these data used a different array format (Affymetrix Mouse Genome 430 2.0 arrays), they were analyzed separately. The .cel files were processed with Partek Genomics Suite in the same manner as our own, and the same criteria were used to remove probe sets. The only difference was that rather than using the cross-hybridization category, the annotation grade was obtained from NetAffx (http://www.affymetrix.com) [106], and only probes with unambiguous A and B grade annotation were retained. Control probes were also removed.
We used the same method to identify and analyze primed genes as described above. This analysis used the Sf1-EGFP data at E11.0 (or E10.5) and E12.5 (Figure 6, Figure S5, Dataset S5). The priming analysis was limited to probes associated with the supporting cells by two methods, although the list of all primed genes is provided (Dataset S5A, S5B). First, we examined only genes that were lineage-specifically enriched in our XX or XY E12.5 Sry-EGFP/Sox9-ECFP data (Figure 3A, Dataset S2A) and Sf1-EGFP primed (Figure 6A, 6C, 6D, 6G; Figure S5A, S5B, S5E). Because different arrays were used, we compared between the two datasets using the gene symbol. If the gene symbol for an Sf1-EGFP primed gene was also found in the lineage-specific Sry-EGFP/Sox9-ECFP lists, the gene was retained in the analysis. Second, we removed the genes associated with the interstitial/stromal cells at E12.5 from the Sf1-EGFP primed genes (Figure 6B, 6E, 6F, 6H; Figure S5C, S5D, S5F). Using XY cells as an example, we identified the genes to remove using the following pairwise comparisons and removal steps:
We identified genes more highly expressed in XY interstitial cells than XY supporting cells at E12.5.
We identified genes more highly expressed in XY supporting cells than XX supporting cells and also XY interstitial cells than XX stromal cells, then removed the genes identified in step 1.
We identified all genes more highly expressed in XY interstitial cells than the XX stromal cells, and removed all of the genes remaining in step 2. This list contains genes sexually dimorphic in the interstitial cells and not the supporting cells, and genes sexually dimorphic in both the interstitial/stromal cells and the supporting cells if expression was higher in the interstitial/stromal cells.
The lists of the genes identified for removal is provided in Dataset S5C. Again, the comparison between the two data sets was based on the gene symbol: gene symbols found in the interstitial/stromal-associated lists were removed from the Sf1-EGFP primed list.
To calculate the percentage of male and female genes showing priming (Figure 6D, 6F; Figure S5B, S5D), we used the same method used in analyzing all primed genes in our own data. However, in addition to being more highly expressed in one sex at E12.5, the gene was also required to pass the appropriate filter used for identifying supporting cell-associated genes.
Supporting Information
RMA normalized values and intensity value graphing. We provided an excel file with (A) the log-transformed RMA normalized intensity values used in our analysis and (B) the graphic format used to display gene expression in each cell type over time. The user can copy any row from (A) with values into row 2 of (B) (yellow) to generate the graph for that gene. Only transcript clusters included in the analysis are provided, and the grounds for removing transcript clusters are provided in the Materials and Methods.
(XLSX)
Lineage-specific gene lists, permutation tests, pathway enrichment, and GO term enrichment. (A) Gene lists generated by multiple pairwise comparisons, graphically illustrated in Figure 3A. The lists are arranged in order of lineage, stage, enrichment or depletion, and sex. Genes are in alphabetical order. (B) Results of permutation tests for all the gene lists displayed in Figure 3A. Separate entries are listed for the median and mean number of transcript clusters and individual genes found in the permutation tests. The number of genes in the actual list and the percentage of false positives based on the mean number of genes are also provided. (C) The lists of KEGG and BioCarta pathways enriched with a p-value<0.05 in each list with a false discovery rate <20%. (D) The list of biological process (“BP”) and molecular function (“MF”) GO terms enriched with a p-value<0.05 in each significant list. In both cases, the lists are arranged in order of lineage, stage, sex, and enrichment or depletion. The terms in the list are ranked from lowest to highest p-value.
(XLSX)
Lists of sexually dimorphic genes, overlapping expression, and permutation tests. (A) Gene lists generated by a single pairwise comparison between XY and XX cells of a lineage graphically illustrated in Figure S4A. Lists of all sexually dimorphic genes in each lineage at each stage are provided first, followed by lists of genes sexually dimorphic in multiple lineages. Genes are in alphabetical order. The gene lists are arranged by stage, sex, and lineage. When transcript clusters for a single gene behaved differently, in all cases the gene was counted in the highest overlapping category. For example, if a gene had one transcript cluster sexually dimorphic in only one lineage and another that was dimorphic in two lineages, it was counted as being dimorphic in both lineages. “§” next to a gene name indicates the gene was counted as “♀ E13.5 over-expressed in all cell types” (the triple overlap in the Venn diagram in Figure S4A) even though it appeared in other categories (Syngap1 and Myo9a). (B) Results of permutation tests for the single pairwise comparisons. (C) Lists of genes that were sexually dimorphic in only two lineages, expressed identically in both lineages, and enriched in these two lineages compared to the remaining two lineages (Figure S4E). Genes are in alphabetical order. The gene lists are arranged by lineage, sex, and stage. The gene considered in the triple overlap (Syngap1) is again marked by the § and removed from the analysis.
(XLSX)
Germ cell and supporting cell primed gene lists, permutation tests, and GO term enrichment. (A and B) Gene lists generated by multiple pairwise comparisons to identify germ cell (A) and supporting cell (B) primed genes, graphically illustrated in Figure 4 and Figure 5. Genes are in alphabetical order. The gene lists are arranged by method of identifying primed genes, and sex. The genes with similar and intermediate expression are provided after each list of primed genes. “§” next to a gene name indicates its transcript clusters appeared in both the similar and intermediate expression lists, and so was counted in Figure 4 and Figure 5 as “Other”. (C) Results of permutation tests for the lists of primed genes. (D) The lists of biological process (“BP”) and molecular function (“MF”) GO terms enriched with a p-value<0.05 in all of the primed genes with similar expression (Figure 4J, Figure 5I). The lists are arranged in order of lineage and sex. The terms in the lists are ranked from lowest to highest p-value.
(XLSX)
Sf1-EGFP primed gene lists and permutation tests. (A and B) Gene lists generated by multiple pairwise comparisons, graphically illustrated in Figure 6 and Figure S5. Genes are in alphabetical order. The gene lists are arranged by the method of selecting supporting cell primed genes, and sex. The list of all primed genes identified in the Sf1-EGFP data without limiting the list to genes associated with supporting cells, “all”, are also provided. Lists of similar and intermediate expression primed genes follow each list of primed genes (except for the first). Lists are provided starting the priming analysis at both E11.0 (A) and E10.5 (B). (C) Gene lists of interstitial/stromal associated genes that were used to remove interstitial/stromal genes from the priming analysis as illustrated in Figure 6B. (D) Lists of genes illustrated in Figure S5G that were primed at E10.5 or E11.0 and became sexually dimorphic 12 hours later. Genes are in alphabetical order. (E) Lists of genes illustrated in Figure S5H. Of the genes that were primed at E10.5 or E11.0 and remained similarly expressed in XX and XY cells 12 hours later, we identified genes that showed a significant and similar change in expression in both XX and XY cells. Genes are in alphabetical order. (F) Lists of genes illustrated in Figure S6A that were primed in the Sf1-EGFP and Sry-EGFP/Sox9-ECFP data, were primed in the Sf1-EGFP data and dimorphic in the Sry-EGFP/Sox9-ECFP data, and a subset of these E11.5 dimorphic genes that showed indications of previous priming in the Sry-EGFP/Sox9-ECFP cells. Genes are in alphabetical order.
(XLSX)
Lineage-specific fluorescent tags used for FACS. Images of E13.5 XY and XX gonads with DAPI (blue) and each fluorescent marker used: Sox9-ECFP and Sry-EGFP (cyan) labeling supporting cells, Mafb-EGFP (purple) labeling the interstitial/stromal cells, Oct4-EGFP (green) labeling germ cells, and Flk1-mCherry (red) labeling endothelial cells. Scale bar = 100 µm.
(TIF)
αSma-EYFP labeled a heterogeneous population containing supporting cell precursors. (A) Images of E13.5 XY and XX gonads with DAPI (blue) and αSma-EYFP (pink) labeling the interstitial/stromal cells. (B) Graphs of the log-transformed, normalized intensity values. The error bars are standard error. The Sry transcript is expressed at similarly high levels in both XY supporting cells and αSma-EYFP cells at E11.5, and declines rapidly in both cell types. Expression of Sry is lower in the Mafb-EGFP cells. However, the pattern seen with Sry did not hold true for most supporting cell markers: Sox9 is expressed at a lower level in both αSma-EYFP and Mafb-EGFP cells than in supporting cells. (C) SRY and SOX9 proteins are also present in αSma-EYFP cells. Antibodies against SRY (red) and SOX9 (blue) co-label αSma-EYFP (green) cells. Cells with αSma-EYFP and SRY alone are indicated with arrows, whereas cells with αSma-EYFP, SRY, and SOX9 are indicated with arrowheads. Scale bar = 25 µm. This suggests that αSma-EYFP is expressed in a heterogeneous population of early gonadal cells containing supporting cell progenitors.
(TIF)
Alternative methods showed generally similar patterns indicating the importance of lineage, sex, and stage. Clustering dendrograms of the individual arrays generated using (A) Average linkage with Euclidean distance as a distance metric and (B) Complete linkage with Pearson's dissimilarity as a distance metric. Consistent with Figure 2A, the arrays cluster primarily by lineage, and secondarily by sex and stage. The largest differences were in the relationship of the somatic populations to each other, although the same clusters could always be identified. (C) Examining the sources of variation with the median F ratio shows a similar pattern to the mean F ratio (Figure 2B) with the primary source of variation being lineage.
(TIF)
Overlap of genes that are sexually dimorphic in at least one lineage. (A) The number of genes over-expressed (“sexually dimorphic”) in XY or XX cells of each lineage at each stage. Genes were identified by a single pairwise comparison between XX and XY cells for each lineage at each stage (Dataset S3A). Many more genes are identified here than in Figure 3A because additional pairwise comparisons were performed to restrict the analysis to genes that showed lineage-specificity in Figure 3A. The area proportional Venn diagrams were generated using Venn Diagram Plotter v1.4.3740 from PNNL and OMICS.PNL.GOV (http://omics.pnl.gov/software/VennDiagramPlotter.php). The sizing of the Venn diagrams relative to each other is approximate. Endothelial cells were not analyzed. The numbers shown indicate the number of genes exclusively in each portion of the diagram (except for E11.5 for which the total number of genes dimorphic in the supporting cells is shown). Lists marked “ns” had a false positive rate >20% (Dataset S3B). The overlapping areas on the Venn diagrams indicate genes sexually dimorphic in multiple lineages. Most genes dimorphic in the XX stroma were also dimorphic in another lineage. (B–D) Graphs of the log-transformed, normalized intensity values. The error bars are standard error. Endothelial cell values are not shown. (B) Many genes sexually dimorphic in multiple lineages were over-expressed in one of the lineages, as was the case for Sox9. However, this could be explained by the low and variable contamination expected after FACS. To address this issue, we used antibody stains of sorted cells to estimate that the XY E13.5 germ cells had <1% contamination with supporting cells, but the XY E13.5 interstitium was more variable and had between 1% and 15% supporting cell contamination (data not shown). Therefore, patterns similar to Sox9 were not further analyzed. (C–D) However, not all genes sexually dimorphic in multiple lineages had a pattern consistent with low level contamination. (C) Axin2, a Wnt/β-catenin transcriptional target gene [107], was highly expressed in the three XX cell types examined, indicative of widespread Wnt signaling in the ovary. (D) Similarly, Irx3 (Iroquois related homeobox 3), a gene known to be ovary-specific [108], showed convergent expression in the XX stroma and supporting cells. We identified more genes with a pattern similar to Irx3 as sexually dimorphic in only two lineages, expressed identically in both lineages, and enriched in these two lineages compared to the remaining two (Dataset S3C). Genes sexually dimorphic in all three lineages were not analyzed. There were few genes meeting these criteria in the overlap between the germ cells and other lineages (Dataset S3C), but (E) more genes met these criteria in the overlap between interstitial/stromal cells and supporting cells (Dataset S3C). The XX stroma in particular expressed several transcripts at similar levels to the XX supporting cells, although the populations are not identical as the XX supporting cells have many more lineage-specific transcripts (Figure 3A). Some of these shared transcripts are downstream of Wnt4 signaling in the ovary (Calb1, Fgfr2, Irx3, Sema3a, and Tkt) [109], and Wnt4 itself showed this pattern. Thus, at least some of these similarities may be attributable to widespread Wnt signaling in the ovary, as reported by Axin2 expression in all 3 lineages (C).
(TIF)
E10.5 Sf1-EGFP primed genes generally supported female-biased priming, but the E11.0 analysis was more informative. The analysis of the Sf1-EGFP primed genes (beginning the analysis at E10.5 and comparing to Sf1-EGFP cells at E12.5) was also limited to genes enriched in the Sry-EGFP/Sox9-ECFP supporting cells at E12.5 (A, B, and E) or to those genes identified by removing interstitial/stromal genes (C, D, and F). (A and C) The percentages of primed genes that were male-primed and female-primed. The first, but not the second, method showed a female bias. The boxes contain the p-values from the binomial test with the expected percentages of the extreme models. The balanced model can be rejected with the first (A), but not the second (C), method. (B and D) Examining the percentage of male or female genes that were primed similarly showed a significant (*) bias toward the female pathway, as determined by the hypergeometric test (p-value<0.05), for the first (B), but not the second (D), method (ns). (E and F) The primed genes for both sexes are predominantly similarly expressed in progenitors and E12.5 differentiated cells. However, E10.5 may not be the appropriate starting point for the priming analysis. (G) No primed genes (identified by removing interstitial/stromal genes) became dimorphic between E10.5 and E11.0 (“0”). Thus, starting the analysis at E11.0 does not result in the loss of any information. Starting at E11.0 is also preferable because it is closer to the divergence point. (H) Between E10.5 and E11.0, 98% of the primed genes were identically expressed in XX and XY samples at both E10.5 and E11.0, and 76% of the primed genes were identically expressed in XX and XY samples at both E11.0 and E11.5 (data not shown). Of these genes, 16% were changing expression level in the same way (see I) in both XX and XY cells between E10.5 and E11.0, whereas only 4% fell in this category between E11.0 and E11.5. This difference was significant (*), as determined by the hypergeometric test with a p-value<0.05, and affects how primed genes are called. This problem is illustrated in (I) by the graphs of the log-transformed, normalized intensity values from Sf1-EGFP cells (black) of two genes identified as primed at E10.5, but not at E11.0. Tpx2 showed significant identical changes in expression in XX and XY cells between E10.5 and E11.0. A similar pattern was observed in many other genes that did not reach significance, such as Ppil5, and so this pattern may be more pervasive than indicated by the number meeting the significance thresholds shown in H. This type of expression pattern confounds the analysis when we compare expression at E10.5 to E12.5 to identify primed genes. This may be caused by changes in the number of supporting cell progenitors, or alternatively, the continued transcriptional development of the progenitors between E10.5 and E11.0 may establish the priming program immediately preceding sexual divergence. For these reasons, we chose to start the analysis at E11.0. The gene lists for these analyses are provided in Dataset S5.
(TIF)
Overlap of primed genes between the Sf1-EGFP and Sry-EGFP/Sox9-ECFP data sets. (A) To cross-validate the analysis, we determined whether genes primed in the Sf1-EGFP data (removing interstitial/stromal genes, Figure 6E) were also identified as primed in the Sry-EGFP/Sox9-ECFP data (all genes with a priming pattern, Figure 5B). Some of the same primed genes were identified in both data sets (blue). Many more of the Sf1 primed genes were already sexually dimorphic in the Sry-EGFP/Sox9-ECFP cells by E11.5 (light and dark green). This is not surprising since primed genes were already becoming dimorphic at E11.5 based on the Sf1-EGFP data (Figure S5G). Some of the genes that were already sexually dimorphic in the E11.5 Sry-EGFP/Sox9-ECFP cells showed some indication of previous priming in the Sry-EGFP/Sox9-ECFP data (light green). These genes met the same requirements for defining a primed pattern outlined in the Materials and Methods, but rather than being identical at E11.5, the sex for which the gene was primed had higher expression at E11.5. This pattern is illustrated in (B) by the graphs of the log-transformed, normalized intensity values for the Sf1-EGFP (black) and Sry-EGFP/Sox9-ECFP (blue) cells for the gene Dock4. The error bars are standard error. Together, these primed or E11.5 dimorphic categories account for over half of the Sf1-EGFP primed genes, indicating both arrays showed consistent results for many genes. A number of Sf1-EGFP primed genes (48%) were not identified as primed or sexually dimorphic in Sry-EGFP/Sox9-ECFP cells at E11.5 (yellow). This was expected as probe sets for the two arrays, as well as the cell types collected, were different. The identification of similar patterns for the same genes in these two different data sets despite their differences gives us confidence in the results. The gene lists for these analyses are provided in Dataset S5F.
(TIF)
Acknowledgments
We gratefully acknowledge Bruce Aronow's helpful advice in selecting appropriate methods for processing our arrays. We thank Serge Nef for the outstanding publically available Sf1-EGFP data set that he produced. We would also like to thank Nugen for their excellent customer service and technical support at all stages of the sample processing, the Duke Institute for Genome Sciences and Policy Microarray Facility for their assistance collecting these data, and Duke Comprehensive Cancer Center Flow Cytometry Shared Resource for their exceptional efforts to assist us in collecting the cells we required to make this study possible. Finally, we appreciate the supportive environment of the Duke Center for Systems Biology.
Footnotes
The authors have declared that no competing interests exist.
NIH/GUDMAP (http://www.gudmap.org/) provided funding to the laboratory (as a subcontract to GUDMAP U01 DK070219) and influenced the design of the data collection as part of the GUDMAP initiative to establish a public resource for gene expression in the urogenital tract. GUDMAP did not influence the analysis of the data, the decision to publish, or the preparation of this manuscript. BC and SAJ were funded by this GUDMAP grant. SCM, AN, and DMM were funded by NIH-P50GM081883 (http://www.nih.gov/). BC, SAJ, DMM, and LM were funded by NIH-HD039963. TD was funded by an NIH-NRSA post-doctoral fellowship (F32 HD058433-01). JC was supported by a March of Dimes (http://www.marchofdimes.com/) research grant to BC (MOD 1-FY10-355) and an American Heart Association (http://www.heart.org/HEARTORG/) predoctoral fellowship. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 2.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 3.Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- 4.Wylie C. Germ cells. Cell. 1999;96:165–174. doi: 10.1016/s0092-8674(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 5.Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- 8.Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 9.Swain A, Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13:755–767. doi: 10.1101/gad.13.7.755. [DOI] [PubMed] [Google Scholar]
- 10.Bouma GJ, Hudson QJ, Washburn LL, Eicher EM. New candidate genes identified for controlling mouse gonadal sex determination and the early stages of granulosa and Sertoli cell differentiation. Biol Reprod. 2010;82:380–389. doi: 10.1095/biolreprod.109.079822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Mise N, Fuchikami T, Sugimoto M, Kobayakawa S, Ike F, et al. Differences and similarities in the developmental status of embryo-derived stem cells and primordial germ cells revealed by global expression profiling. Genes Cells. 2008;13:863–877. doi: 10.1111/j.1365-2443.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 13.Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:417–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- 14.Rolland AD, Lehmann KP, Johnson KJ, Gaido KW, Koopman P. Uncovering gene regulatory networks during mouse fetal germ cell development. Biol Reprod. 2011;84:790–800. doi: 10.1095/biolreprod.110.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouma GJ, Affourtit JP, Bult CJ, Eicher EM. Transcriptional profile of mouse pre-granulosa and Sertoli cells isolated from early-differentiated fetal gonads. Gene Expr Patterns. 2007;7:113–123. doi: 10.1016/j.modgep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–806. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 17.Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- 18.Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, et al. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 2008;15:781–791. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jost A. Recherches sur la diff'erenciation sexuelle de l'embryon de lapin. Arch Anat Microsc Morph Exp. 1947:271–315. [Google Scholar]
- 20.McLaren A. Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays. 1991;13:151–156. doi: 10.1002/bies.950130402. [DOI] [PubMed] [Google Scholar]
- 21.Capel B. Sex in the 90s: SRY and the switch to the male pathway. Annu Rev Physiol. 1998;60:497–523. doi: 10.1146/annurev.physiol.60.1.497. [DOI] [PubMed] [Google Scholar]
- 22.McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci U S A. 1993;90:3368–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eicher EM, Washburn LL. Genetic control of primary sex determination in mice. Annu Rev Genet. 1986;20:327–360. doi: 10.1146/annurev.ge.20.120186.001551. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, et al. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, et al. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 26.Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–767. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- 27.Munger SC, Aylor DL, Syed HA, Magwene PM, Threadgill DW, et al. Elucidation of the transcription network governing mammalian sex determination by exploiting strain-specific susceptibility to sex reversal. Genes Dev. 2009;23:2521–2536. doi: 10.1101/gad.1835809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delorme B, Ringe J, Pontikoglou C, Gaillard J, Langonne A, et al. Specific lineage-priming of bone marrow mesenchymal stem cells provides the molecular framework for their plasticity. Stem Cells. 2009;27:1142–1151. doi: 10.1002/stem.34. [DOI] [PubMed] [Google Scholar]
- 29.Hu M, Krause D, Greaves M, Sharkis S, Dexter M, et al. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- 30.Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity. 2009;30:493–507. doi: 10.1016/j.immuni.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zipori D. The nature of stem cells: state rather than entity. Nat Rev Genet. 2004;5:873–878. doi: 10.1038/nrg1475. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, et al. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 33.Enver T, Greaves M. Loops, lineage, and leukemia. Cell. 1998;94:9–12. doi: 10.1016/s0092-8674(00)81215-5. [DOI] [PubMed] [Google Scholar]
- 34.Hipp JA, Hipp JD, Atala A, Soker S. Functional genomics: new insights into the ‘function’ of low levels of gene expression in stem cells. Curr Genomics. 2010;11:354–358. doi: 10.2174/138920210791616680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golan-Mashiach M, Dazard JE, Gerecht-Nir S, Amariglio N, Fisher T, et al. Design principle of gene expression used by human stem cells: implication for pluripotency. FASEB J. 2005;19:147–149. doi: 10.1096/fj.04-2417fje. [DOI] [PubMed] [Google Scholar]
- 36.Guo G, Huss M, Tong GQ, Wang C, Li Sun L, et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 38.Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, et al. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26:5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeFalco T, Takahashi S, Capel B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol. 2011;352:14–26. doi: 10.1016/j.ydbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo PE, Hubner K, Scholer H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev. 2002;115:157–160. doi: 10.1016/s0925-4773(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 43.Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, et al. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec (Hoboken) 2009;292:333–341. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poche RA, Larina IV, Scott ML, Saik JE, West JL, et al. The Flk1-myr::mCherry mouse as a useful reporter to characterize multiple aspects of ocular blood vessel development and disease. Dev Dyn. 2009;238:2318–2326. doi: 10.1002/dvdy.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cool J, Carmona FD, Szucsik JC, Capel B. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2008;2:128–133. doi: 10.1159/000143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004;274:271–279. doi: 10.1016/j.ydbio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Yao HH, Aardema J, Holthusen K. Sexually dimorphic regulation of inhibin beta B in establishing gonadal vasculature in mice. Biol Reprod. 2006;74:978–983. doi: 10.1095/biolreprod.105.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeanes A, Wilhelm D, Wilson MJ, Bowles J, McClive PJ, et al. Evaluation of candidate markers for the peritubular myoid cell lineage in the developing mouse testis. Reproduction. 2005;130:509–516. doi: 10.1530/rep.1.00718. [DOI] [PubMed] [Google Scholar]
- 49.Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, et al. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 50.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 51.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 53.Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, et al. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, et al. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 56.Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, et al. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- 58.Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, et al. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX----XY chimaeric mouse testes. Development. 1988;102:443–450. doi: 10.1242/dev.102.2.443. [DOI] [PubMed] [Google Scholar]
- 60.Mansson R, Hultquist A, Luc S, Yang L, Anderson K, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Raz E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000;1:REVIEWS1017. doi: 10.1186/gb-2000-1-3-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cook MS, Munger SC, Nadeau JH, Capel B. Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development. 2011;138:23–32. doi: 10.1242/dev.057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 64.Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 65.Bradford ST, Hiramatsu R, Maddugoda MP, Bernard P, Chaboissier MC, et al. The cerebellin 4 precursor gene is a direct target of SRY and SOX9 in mice. Biol Reprod. 2009;80:1178–1188. doi: 10.1095/biolreprod.108.071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 67.Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- 68.Larsson O, Scheele C, Liang Z, Moll J, Karlsson C, et al. Kinetics of senescence-associated changes of gene expression in an epithelial, temperature-sensitive SV40 large T antigen model. Cancer Res. 2004;64:482–489. doi: 10.1158/0008-5472.can-03-1872. [DOI] [PubMed] [Google Scholar]
- 69.Badie C, Itzhaki JE, Sullivan MJ, Carpenter AJ, Porter AC. Repression of CDK1 and other genes with CDE and CHR promoter elements during DNA damage-induced G(2)/M arrest in human cells. Mol Cell Biol. 2000;20:2358–2366. doi: 10.1128/mcb.20.7.2358-2366.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 71.Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 72.Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, et al. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- 74.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 75.Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, et al. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- 78.Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, et al. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 80.Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323:76–93. doi: 10.1016/j.mce.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 81.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 82.Upadhyay S, Zamboni L. Ectopic germ cells: natural model for the study of germ cell sexual differentiation. Proc Natl Acad Sci U S A. 1982;79:6584–6588. doi: 10.1073/pnas.79.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- 84.Farini D, Scaldaferri ML, Iona S, La Sala G, De Felici M. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev Biol. 2005;285:49–56. doi: 10.1016/j.ydbio.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 85.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 86.Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao X, et al. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun. 2011;2:151. doi: 10.1038/ncomms1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Byskov AG. Does the rete ovarii act as a trigger for the onset of meiosis? Nature. 1974;252:396–397. doi: 10.1038/252396a0. [DOI] [PubMed] [Google Scholar]
- 89.O W, Baker TG. Initiation and control of meiosis in hamster gonads in vitro. J Reprod Fertil. 1976;48:399–401. doi: 10.1530/jrf.0.0480399. [DOI] [PubMed] [Google Scholar]
- 90.DiNapoli L, Batchvarov J, Capel B. FGF9 promotes survival of germ cells in the fetal testis. Development. 2006;133:1519–1527. doi: 10.1242/dev.02303. [DOI] [PubMed] [Google Scholar]
- 91.Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, et al. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell. 2010;19:440–449. doi: 10.1016/j.devcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, et al. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–880. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- 93.Francavilla S, Zamboni L. Differentiation of mouse ectopic germinal cells in intra- and perigonadal locations. J Exp Zool. 1985;233:101–109. doi: 10.1002/jez.1402330114. [DOI] [PubMed] [Google Scholar]
- 94.Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development. 2008;135:1415–1425. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 97.Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annu Rev Genomics Hum Genet. 2001;2:343–372. doi: 10.1146/annurev.genom.2.1.343. [DOI] [PubMed] [Google Scholar]
- 98.Brady SM, Zhang L, Megraw M, Martinez NJ, Jiang E, et al. A stele-enriched gene regulatory network in the Arabidopsis root. Mol Syst Biol. 2011;7:459. doi: 10.1038/msb.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bullejos M, Koopman P. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn. 2001;221:201–205. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- 100.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 101.Mroz K, Carrel L, Hunt PA. Germ cell development in the XXY mouse: evidence that X chromosome reactivation is independent of sexual differentiation. Dev Biol. 1999;207:229–238. doi: 10.1006/dbio.1998.9160. [DOI] [PubMed] [Google Scholar]
- 102.Cook MS, Coveney D, Batchvarov I, Nadeau JH, Capel B. BAX-mediated cell death affects early germ cell loss and incidence of testicular teratomas in Dnd1(Ter/Ter) mice. Dev Biol. 2009;328:377–383. doi: 10.1016/j.ydbio.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barske LA, Capel B. Estrogen represses SOX9 during sex determination in the red-eared slider turtle Trachemys scripta. Dev Biol. 2010;341:305–314. doi: 10.1016/j.ydbio.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 104.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 105.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu G, Loraine AE, Shigeta R, Cline M, Cheng J, et al. NetAffx: Affymetrix probesets and annotations. Nucleic Acids Res. 2003;31:82–86. doi: 10.1093/nar/gkg121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns. 2005;5:756–762. doi: 10.1016/j.modgep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 109.Coveney D, Ross AJ, Slone JD, Capel B. A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation. Gene Expr Patterns. 2008;8:529–537. doi: 10.1016/j.gep.2008.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RMA normalized values and intensity value graphing. We provided an excel file with (A) the log-transformed RMA normalized intensity values used in our analysis and (B) the graphic format used to display gene expression in each cell type over time. The user can copy any row from (A) with values into row 2 of (B) (yellow) to generate the graph for that gene. Only transcript clusters included in the analysis are provided, and the grounds for removing transcript clusters are provided in the Materials and Methods.
(XLSX)
Lineage-specific gene lists, permutation tests, pathway enrichment, and GO term enrichment. (A) Gene lists generated by multiple pairwise comparisons, graphically illustrated in Figure 3A. The lists are arranged in order of lineage, stage, enrichment or depletion, and sex. Genes are in alphabetical order. (B) Results of permutation tests for all the gene lists displayed in Figure 3A. Separate entries are listed for the median and mean number of transcript clusters and individual genes found in the permutation tests. The number of genes in the actual list and the percentage of false positives based on the mean number of genes are also provided. (C) The lists of KEGG and BioCarta pathways enriched with a p-value<0.05 in each list with a false discovery rate <20%. (D) The list of biological process (“BP”) and molecular function (“MF”) GO terms enriched with a p-value<0.05 in each significant list. In both cases, the lists are arranged in order of lineage, stage, sex, and enrichment or depletion. The terms in the list are ranked from lowest to highest p-value.
(XLSX)
Lists of sexually dimorphic genes, overlapping expression, and permutation tests. (A) Gene lists generated by a single pairwise comparison between XY and XX cells of a lineage graphically illustrated in Figure S4A. Lists of all sexually dimorphic genes in each lineage at each stage are provided first, followed by lists of genes sexually dimorphic in multiple lineages. Genes are in alphabetical order. The gene lists are arranged by stage, sex, and lineage. When transcript clusters for a single gene behaved differently, in all cases the gene was counted in the highest overlapping category. For example, if a gene had one transcript cluster sexually dimorphic in only one lineage and another that was dimorphic in two lineages, it was counted as being dimorphic in both lineages. “§” next to a gene name indicates the gene was counted as “♀ E13.5 over-expressed in all cell types” (the triple overlap in the Venn diagram in Figure S4A) even though it appeared in other categories (Syngap1 and Myo9a). (B) Results of permutation tests for the single pairwise comparisons. (C) Lists of genes that were sexually dimorphic in only two lineages, expressed identically in both lineages, and enriched in these two lineages compared to the remaining two lineages (Figure S4E). Genes are in alphabetical order. The gene lists are arranged by lineage, sex, and stage. The gene considered in the triple overlap (Syngap1) is again marked by the § and removed from the analysis.
(XLSX)
Germ cell and supporting cell primed gene lists, permutation tests, and GO term enrichment. (A and B) Gene lists generated by multiple pairwise comparisons to identify germ cell (A) and supporting cell (B) primed genes, graphically illustrated in Figure 4 and Figure 5. Genes are in alphabetical order. The gene lists are arranged by method of identifying primed genes, and sex. The genes with similar and intermediate expression are provided after each list of primed genes. “§” next to a gene name indicates its transcript clusters appeared in both the similar and intermediate expression lists, and so was counted in Figure 4 and Figure 5 as “Other”. (C) Results of permutation tests for the lists of primed genes. (D) The lists of biological process (“BP”) and molecular function (“MF”) GO terms enriched with a p-value<0.05 in all of the primed genes with similar expression (Figure 4J, Figure 5I). The lists are arranged in order of lineage and sex. The terms in the lists are ranked from lowest to highest p-value.
(XLSX)
Sf1-EGFP primed gene lists and permutation tests. (A and B) Gene lists generated by multiple pairwise comparisons, graphically illustrated in Figure 6 and Figure S5. Genes are in alphabetical order. The gene lists are arranged by the method of selecting supporting cell primed genes, and sex. The list of all primed genes identified in the Sf1-EGFP data without limiting the list to genes associated with supporting cells, “all”, are also provided. Lists of similar and intermediate expression primed genes follow each list of primed genes (except for the first). Lists are provided starting the priming analysis at both E11.0 (A) and E10.5 (B). (C) Gene lists of interstitial/stromal associated genes that were used to remove interstitial/stromal genes from the priming analysis as illustrated in Figure 6B. (D) Lists of genes illustrated in Figure S5G that were primed at E10.5 or E11.0 and became sexually dimorphic 12 hours later. Genes are in alphabetical order. (E) Lists of genes illustrated in Figure S5H. Of the genes that were primed at E10.5 or E11.0 and remained similarly expressed in XX and XY cells 12 hours later, we identified genes that showed a significant and similar change in expression in both XX and XY cells. Genes are in alphabetical order. (F) Lists of genes illustrated in Figure S6A that were primed in the Sf1-EGFP and Sry-EGFP/Sox9-ECFP data, were primed in the Sf1-EGFP data and dimorphic in the Sry-EGFP/Sox9-ECFP data, and a subset of these E11.5 dimorphic genes that showed indications of previous priming in the Sry-EGFP/Sox9-ECFP cells. Genes are in alphabetical order.
(XLSX)
Lineage-specific fluorescent tags used for FACS. Images of E13.5 XY and XX gonads with DAPI (blue) and each fluorescent marker used: Sox9-ECFP and Sry-EGFP (cyan) labeling supporting cells, Mafb-EGFP (purple) labeling the interstitial/stromal cells, Oct4-EGFP (green) labeling germ cells, and Flk1-mCherry (red) labeling endothelial cells. Scale bar = 100 µm.
(TIF)
αSma-EYFP labeled a heterogeneous population containing supporting cell precursors. (A) Images of E13.5 XY and XX gonads with DAPI (blue) and αSma-EYFP (pink) labeling the interstitial/stromal cells. (B) Graphs of the log-transformed, normalized intensity values. The error bars are standard error. The Sry transcript is expressed at similarly high levels in both XY supporting cells and αSma-EYFP cells at E11.5, and declines rapidly in both cell types. Expression of Sry is lower in the Mafb-EGFP cells. However, the pattern seen with Sry did not hold true for most supporting cell markers: Sox9 is expressed at a lower level in both αSma-EYFP and Mafb-EGFP cells than in supporting cells. (C) SRY and SOX9 proteins are also present in αSma-EYFP cells. Antibodies against SRY (red) and SOX9 (blue) co-label αSma-EYFP (green) cells. Cells with αSma-EYFP and SRY alone are indicated with arrows, whereas cells with αSma-EYFP, SRY, and SOX9 are indicated with arrowheads. Scale bar = 25 µm. This suggests that αSma-EYFP is expressed in a heterogeneous population of early gonadal cells containing supporting cell progenitors.
(TIF)
Alternative methods showed generally similar patterns indicating the importance of lineage, sex, and stage. Clustering dendrograms of the individual arrays generated using (A) Average linkage with Euclidean distance as a distance metric and (B) Complete linkage with Pearson's dissimilarity as a distance metric. Consistent with Figure 2A, the arrays cluster primarily by lineage, and secondarily by sex and stage. The largest differences were in the relationship of the somatic populations to each other, although the same clusters could always be identified. (C) Examining the sources of variation with the median F ratio shows a similar pattern to the mean F ratio (Figure 2B) with the primary source of variation being lineage.
(TIF)
Overlap of genes that are sexually dimorphic in at least one lineage. (A) The number of genes over-expressed (“sexually dimorphic”) in XY or XX cells of each lineage at each stage. Genes were identified by a single pairwise comparison between XX and XY cells for each lineage at each stage (Dataset S3A). Many more genes are identified here than in Figure 3A because additional pairwise comparisons were performed to restrict the analysis to genes that showed lineage-specificity in Figure 3A. The area proportional Venn diagrams were generated using Venn Diagram Plotter v1.4.3740 from PNNL and OMICS.PNL.GOV (http://omics.pnl.gov/software/VennDiagramPlotter.php). The sizing of the Venn diagrams relative to each other is approximate. Endothelial cells were not analyzed. The numbers shown indicate the number of genes exclusively in each portion of the diagram (except for E11.5 for which the total number of genes dimorphic in the supporting cells is shown). Lists marked “ns” had a false positive rate >20% (Dataset S3B). The overlapping areas on the Venn diagrams indicate genes sexually dimorphic in multiple lineages. Most genes dimorphic in the XX stroma were also dimorphic in another lineage. (B–D) Graphs of the log-transformed, normalized intensity values. The error bars are standard error. Endothelial cell values are not shown. (B) Many genes sexually dimorphic in multiple lineages were over-expressed in one of the lineages, as was the case for Sox9. However, this could be explained by the low and variable contamination expected after FACS. To address this issue, we used antibody stains of sorted cells to estimate that the XY E13.5 germ cells had <1% contamination with supporting cells, but the XY E13.5 interstitium was more variable and had between 1% and 15% supporting cell contamination (data not shown). Therefore, patterns similar to Sox9 were not further analyzed. (C–D) However, not all genes sexually dimorphic in multiple lineages had a pattern consistent with low level contamination. (C) Axin2, a Wnt/β-catenin transcriptional target gene [107], was highly expressed in the three XX cell types examined, indicative of widespread Wnt signaling in the ovary. (D) Similarly, Irx3 (Iroquois related homeobox 3), a gene known to be ovary-specific [108], showed convergent expression in the XX stroma and supporting cells. We identified more genes with a pattern similar to Irx3 as sexually dimorphic in only two lineages, expressed identically in both lineages, and enriched in these two lineages compared to the remaining two (Dataset S3C). Genes sexually dimorphic in all three lineages were not analyzed. There were few genes meeting these criteria in the overlap between the germ cells and other lineages (Dataset S3C), but (E) more genes met these criteria in the overlap between interstitial/stromal cells and supporting cells (Dataset S3C). The XX stroma in particular expressed several transcripts at similar levels to the XX supporting cells, although the populations are not identical as the XX supporting cells have many more lineage-specific transcripts (Figure 3A). Some of these shared transcripts are downstream of Wnt4 signaling in the ovary (Calb1, Fgfr2, Irx3, Sema3a, and Tkt) [109], and Wnt4 itself showed this pattern. Thus, at least some of these similarities may be attributable to widespread Wnt signaling in the ovary, as reported by Axin2 expression in all 3 lineages (C).
(TIF)
E10.5 Sf1-EGFP primed genes generally supported female-biased priming, but the E11.0 analysis was more informative. The analysis of the Sf1-EGFP primed genes (beginning the analysis at E10.5 and comparing to Sf1-EGFP cells at E12.5) was also limited to genes enriched in the Sry-EGFP/Sox9-ECFP supporting cells at E12.5 (A, B, and E) or to those genes identified by removing interstitial/stromal genes (C, D, and F). (A and C) The percentages of primed genes that were male-primed and female-primed. The first, but not the second, method showed a female bias. The boxes contain the p-values from the binomial test with the expected percentages of the extreme models. The balanced model can be rejected with the first (A), but not the second (C), method. (B and D) Examining the percentage of male or female genes that were primed similarly showed a significant (*) bias toward the female pathway, as determined by the hypergeometric test (p-value<0.05), for the first (B), but not the second (D), method (ns). (E and F) The primed genes for both sexes are predominantly similarly expressed in progenitors and E12.5 differentiated cells. However, E10.5 may not be the appropriate starting point for the priming analysis. (G) No primed genes (identified by removing interstitial/stromal genes) became dimorphic between E10.5 and E11.0 (“0”). Thus, starting the analysis at E11.0 does not result in the loss of any information. Starting at E11.0 is also preferable because it is closer to the divergence point. (H) Between E10.5 and E11.0, 98% of the primed genes were identically expressed in XX and XY samples at both E10.5 and E11.0, and 76% of the primed genes were identically expressed in XX and XY samples at both E11.0 and E11.5 (data not shown). Of these genes, 16% were changing expression level in the same way (see I) in both XX and XY cells between E10.5 and E11.0, whereas only 4% fell in this category between E11.0 and E11.5. This difference was significant (*), as determined by the hypergeometric test with a p-value<0.05, and affects how primed genes are called. This problem is illustrated in (I) by the graphs of the log-transformed, normalized intensity values from Sf1-EGFP cells (black) of two genes identified as primed at E10.5, but not at E11.0. Tpx2 showed significant identical changes in expression in XX and XY cells between E10.5 and E11.0. A similar pattern was observed in many other genes that did not reach significance, such as Ppil5, and so this pattern may be more pervasive than indicated by the number meeting the significance thresholds shown in H. This type of expression pattern confounds the analysis when we compare expression at E10.5 to E12.5 to identify primed genes. This may be caused by changes in the number of supporting cell progenitors, or alternatively, the continued transcriptional development of the progenitors between E10.5 and E11.0 may establish the priming program immediately preceding sexual divergence. For these reasons, we chose to start the analysis at E11.0. The gene lists for these analyses are provided in Dataset S5.
(TIF)
Overlap of primed genes between the Sf1-EGFP and Sry-EGFP/Sox9-ECFP data sets. (A) To cross-validate the analysis, we determined whether genes primed in the Sf1-EGFP data (removing interstitial/stromal genes, Figure 6E) were also identified as primed in the Sry-EGFP/Sox9-ECFP data (all genes with a priming pattern, Figure 5B). Some of the same primed genes were identified in both data sets (blue). Many more of the Sf1 primed genes were already sexually dimorphic in the Sry-EGFP/Sox9-ECFP cells by E11.5 (light and dark green). This is not surprising since primed genes were already becoming dimorphic at E11.5 based on the Sf1-EGFP data (Figure S5G). Some of the genes that were already sexually dimorphic in the E11.5 Sry-EGFP/Sox9-ECFP cells showed some indication of previous priming in the Sry-EGFP/Sox9-ECFP data (light green). These genes met the same requirements for defining a primed pattern outlined in the Materials and Methods, but rather than being identical at E11.5, the sex for which the gene was primed had higher expression at E11.5. This pattern is illustrated in (B) by the graphs of the log-transformed, normalized intensity values for the Sf1-EGFP (black) and Sry-EGFP/Sox9-ECFP (blue) cells for the gene Dock4. The error bars are standard error. Together, these primed or E11.5 dimorphic categories account for over half of the Sf1-EGFP primed genes, indicating both arrays showed consistent results for many genes. A number of Sf1-EGFP primed genes (48%) were not identified as primed or sexually dimorphic in Sry-EGFP/Sox9-ECFP cells at E11.5 (yellow). This was expected as probe sets for the two arrays, as well as the cell types collected, were different. The identification of similar patterns for the same genes in these two different data sets despite their differences gives us confidence in the results. The gene lists for these analyses are provided in Dataset S5F.
(TIF)