Abstract
Forkhead box O (FOXO) transcription factors have a conserved function in regulating metazoan lifespan. A key function in this process involves the regulation of the cell cycle and stress responses including free radical scavenging. We employed yeast chronological and replicative lifespan assays, as well as oxidative stress assays, to explore the potential evolutionary conservation of function between the FOXOs and the yeast forkhead box transcription factors FKH1 and FKH2. We report that the deletion of both FKH genes impedes normal lifespan and stress resistance, particularly in stationary phase cells, which are non-responsive to caloric restriction. Conversely, increased expression of the FKHs leads to extended lifespan and improved stress response. Here we show the Anaphase-Promoting Complex (APC) genetically interacts with the Fkh pathway, likely working in a linear pathway under normal conditions, as fkh1Δ fkh2Δ post-mitotic survival is epistatic to that observed in apc5CA mutants. However, under stress conditions, post-mitotic survival is dramatically impaired in apc5CA fkh1Δ fkh2Δ, while increased expression of either FKH rescues APC mutant growth defects. This study establishes the FKHs role as evolutionarily conserved regulators of lifespan in yeast and identifies the APC as a novel component of this mechanism under certain conditions, likely through combined regulation of stress response, genomic stability, and cell cycle regulation.
Author Summary
Throughout human evolution, one question has remained constant: can we live forever? We are continuously bombarded with products, diets, and exercise regimens that supposedly add years to our life. Is there an alterable program, whether genetic or environmental, that can be tweaked to increase longevity? Medical advances have led to a dramatic increase in average lifespan over the last century. However, the maximum human lifespan has curiously remained constant. Recent research indicates that in many organisms a genetic program exists to control lifespan. The conservation of this genetic lifespan program extends into yeast where numerous longevity genes have been isolated and characterized. Interestingly, mutations that reduce genomic instability, glucose utilization, or oxidative damage extend lifespan in multiple organisms. Here we characterize one such set of genes, the FOXOs. In animals, these genes increase lifespan and suppress tumors, but have yet to be associated with longevity in yeast. By confirming that these genes play a similar role in yeast, we provide a tool to identify downstream factors triggered by the FOXOs, a feat which has not yet been accomplished in other systems. Considering the conservation of these factors, it is likely that our discoveries in yeast will be directly applicable to research into human cancer and aging.
Introduction
The evolutionarily conserved insulin-signaling pathway plays a critical role in multiple cellular processes [1]–[4]. Perhaps most important is the decisive role it plays in cellular, and organismal, survival. This pathway must be tightly regulated, as overactive insulin-signaling leads to increased survival of cells that would otherwise be shunted down the apoptotic pathway. This occurs by increased repression of stress response, pro-apoptotic, and DNA repair genes, thereby increasing the proliferative capacity, and oncogenic potential of these cells. Although the lifespan of damaged cells is increased under these conditions, this situation increases the probability that the organism will die prematurely due to disease onset. On the other hand, reduced insulin-signaling relieves repression of the stress response, cell cycle arrest and DNA repair pathways, increasing cell maintenance capacity and survival. It is now clear that there is a link between diabetes and cancer [5]–[7], diseases associated with the insulin-signaling pathways, highlighting the importance of understanding the precise activity of this pathway.
The insulin-signaling phosphorylation cascade activates AKT, which targets cellular factors that switch metabolism from catabolic to anabolic reactions, favoring growth and reproduction over maintenance and repair [8]. Major AKT targets include the forkhead box O family (FOXO) transcription factors (reviewed in [9]–[14]). The FOXOs are believed to serve diverse rolls in longevity determination and tumor suppression in metazoans from nematodes to humans. The FOXOs integrate signals from energy, growth factor and stress signaling cascades to regulate cell differentiation, cell-cycle progression, apoptosis, autophagy, DNA-damage repair, and scavenging reactive oxygen species. FOXO proteins have been shown to interact with multiple cofactors that mediate their activity through posttranslational modifications. Phosphorylation, ubiquitination, methylation, and acetylation regulate transcription factor efficiency and nuclear shuttling (reviewed in [9], [10]). Specifically, nutrient (insulin) and growth factor signals lead to cytosolic sequestering and ubiquitination of the FOXOs, targeting them for degradation; conversely, internal reactive oxygen species (ROS), DNA damage sensing and starvation signals can cause nuclear shuttling, and transcription factor activity. Thus, a dynamic and complex molecular network controls FOXO protein function, yet specific downstream targets remains speculative.
Saccharomyces cerevisiae is often utilized to elucidate regulation of fundamental eukaryotic mechanisms. However, the individual deletion of any of the four Forkhead box orthologs does not affect lifespan [15], suggesting a lack of functional conservation. However, two of the orthologs, FKH1 and FKH2, show genetic redundancy, as deletion of both genes is necessary to alter growth, cell morphology and gene transcription phenotypes [16]–[20]. Further evolutionary conservation for FKH1 and FKH2 is suggested by their requirement for ROS-induced cell cycle arrest [18], and cell cycle regulation through the regulation of both G1 and G2/M gene clusters [17], hallmarks of the human FOXO genes.
The Fkh1/2 regulated CLB2 gene cluster [17] encodes genes required for Anaphase-Promoting Complex (APC) activity (APC1, CDC5, CLB2, and CDC20), as well as APC targets (CLB2, CDC5, CDC20 and IQG1) [21]–[23]. The APC is a highly conserved multi-subunit ubiquitin-protein ligase (E3) that promotes mitotic progression and G1 maintenance by targeting cell cycle regulators, such as the securin Pds1 and the cyclin Clb2, for proteasome-dependent degradation [21], [23], [24]. The APC has been demonstrated to be critical for regulating genomic stability, and longevity in yeast and higher eukaryotic organisms [25]–[30]. In yeast, mutation to multiple APC subunits decreases replicative lifespan (RLS; measures mitotic longevity) and chronological lifespan (CLS; measures post-mitotic survival), while over-expression of APC10 increases RLS [29]. In mice, mutations to the APC regulator BubR1, a component of the spindle checkpoint, lead to premature aging defects [27], [31]. Consistent with this, we and others have provided evidence that the yeast APC plays a role in stress response by possibly targeting proteins that block proper stress response for degradation [28], [32], [33].
Our data supports a model where FKH1 and FKH2 function is evolutionarily conserved with higher eukaryotic FOXO proteins with regards to lifespan and oxidative stress resistance. We show that the FKHs are required for increased stress resistance and survival in response to severe caloric restriction (cultures maintained in water). Importantly, we identify the APC as a potential target of the FKHs under normal conditions, while functioning cooperatively under stress conditions.
Results
FKH1 and FKH2 encode redundant longevity determinants
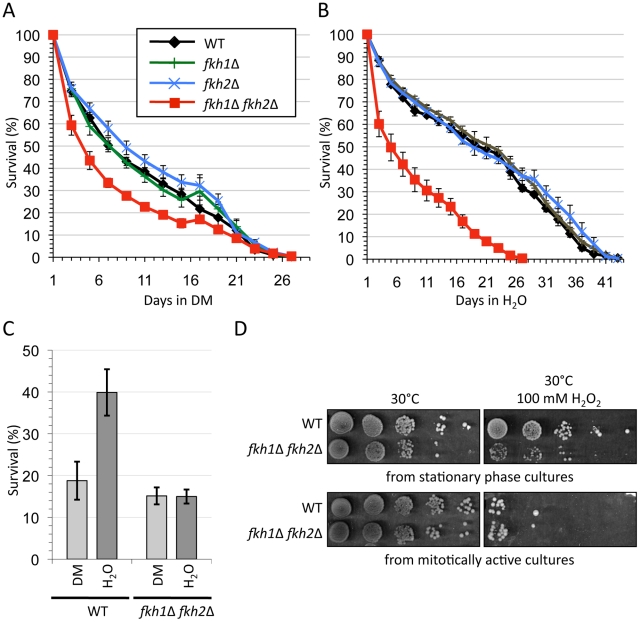
FOXO transcription factors regulate processes involved in the homeostasis of metazoan cells and tissues with the net outcome being lifespan extension and tumor suppression, yet many of the downstream targets remain unknown [9]–[14]. The budding yeast Saccharomyces cerevisiae is a powerful tool used to elucidate genetic and molecular mechanisms mediating many cellular processes, but independent deletion of the four yeast forkhead box protein encoding genes (FKH1, FKH2, HCM1, FHL1) does not alter CLS [15]. However, Fkh1 and Fkh2 have been shown to be phenotypically redundant, as they are required for M/G1 progression and cell cycle arrest in response to hydrogen peroxide [18]. These characteristics lead us to examine the role of both Fkh1 and Fkh2 in the regulation of yeast lifespan. We investigated the FKHs using the RLS assay, a measure of the mitotic lifespan of individual cells, finding that deletion of either individual FKH gene had no effect on RLS, as reported for CLS [15]. Double mutant cells could not be assayed using RLS due to their flocculent phenotype (data not shown). Therefore, we investigated the potential of the FKHs in regulating CLS, a measure of metabolic activity in post-mitotic stationary phase cells [34]. In cultures maintained in depleted complete media (DM), we also observed that single deletion of the FKH genes did not impair CLS. Deletion of both FKH1 and FKH2, on the other hand, reduced CLS (Figure 1A), with mean (50%) survival reached by day 8.5 for WT cultures, day 11 for fkh2Δ cultures, day 8 for fkh1Δ cultures, and only day 4 for fkh1Δ fkh2Δ cultures.
Figure 1. FKH1 and FKH2 encode redundant determinants of lifespan and stress response.
(A) and (B) The cells shown were grown in CM (2% glucose) to stationary phase. The cells were either left in (A) depleted CM (DM), or (B) washed and resuspended in H20 for the remainder of the experiment. Colony counts were performed every other day throughout the experiment. The day when the colony count peaked was considered Day 1. Standard error is shown for at least 3 replicates. (C) Acute oxidative stress in stationary phase cells. WT and fkh1Δ fkh2Δ cells were grown to day 5 of stationary phase with maintenance in either DM or H2O. 100 mM H2O2 was then added to one half of each sample and incubated for 60 minutes at 30°C. Diluted cells were then plated on YPD media and the colony forming units were counted. Survival was determined by dividing the colony forming units following H2O2 treated by untreated samples. Standard error of at least 3 replicates is shown. (D) Chronic oxidative stress in mitotically active and stationary phase cells. WT and fkh1Δ fkh2Δ cells from overnight log phase cultures or day 5 stationary phase cultures were treated with 100 mM H2O2 at 30° for 1 hour, as above, then spot diluted onto YPD plates in the absence of stress. The plates were grown at 30°C for 3 days.
Controversy exists as to whether higher eukaryotic FOXOs, downstream targets of nutrient/insulin signaling, are contributing factors to caloric restriction-induced lifespan extension [35]–[39]. To examine whether the yeast FKHs play a role in caloric restriction, we examined the CLS of the FKH mutants by maintaining the post-mitotic cultures in distilled H2O. Water is believed to act as a form of severe caloric restriction (SCR) that simulates the low-nutrient environment that yeast in the wild would most likely encounter [40], [41]. Maintenance in H2O extended the mean survival of WT, fkh1Δ and fkh2Δ cultures to 19–21 days, while little change was observed in fkh1Δ fkh2Δ cultures with a mean survival of 5 days (Figure 1B). The lack of response in fkh1Δ fkh2Δ cultures maintained in H2O suggests that Fkh1 and Fkh2 play a redundant role in SCR-induced lifespan extension.
Although Fkh1 and Fkh2 have not previously been associated with longevity in yeast, they have been linked with stress response in mitotically active cells [18], which is associated with an evolutionarily conserved role in long lifespan [42]–[44]. To address whether the Fkhs' role in longevity is a manifestation of their involvement in stress resistance in post-mitotic cells, we treated WT and fkh1Δ fkh2Δ day 5 stationary phase cells maintained in either H2O or DM with 100 mM hydrogen peroxide (H2O2) for 1 hour (Figure 1C). WT day 5 stationary phase cultures exhibited increased resistance to H2O2 when maintained in water compared to DM. However, this effect was nullified in fkh1Δ fkh2Δ cultures, indicating that the Fkh proteins are required for stress resistance during stationary phase. A plate assay confirmed that stationary phase cells exhibit increased stress response compared to mitotically active cells, and that deletion of FKH1 and FKH2 diminishes this effect (Figure 1D).
The Fkh proteins are present during stationary phase and have a higher nuclear content in H2O
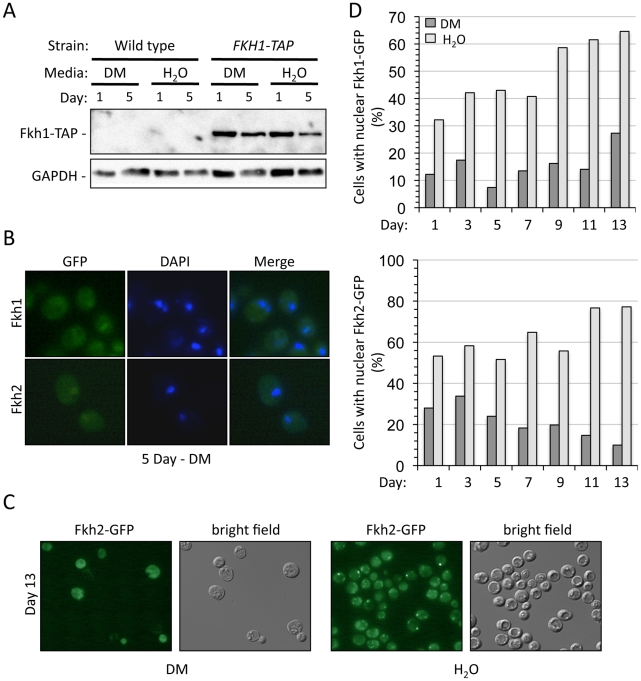
To further assess the role of the FKHs in normal and stressed post-mitotic lifespan, the endogenous genes encoding Fkh1 and Fkh2 were C-terminally TAP (tandem affinity purification)-tagged, with protein levels analyzed as cells aged in DM media and H2O (Figure 2A). Fkh1-TAP was indeed expressed as cells aged during stationary phase in DM and H2O. Fkh2-TAP was also observed in aging stationary phase cells, but at much lower levels (data not shown). Fkh1-TAP levels appear slightly lower in day 5 stationary phase cells compared to day 1, with very little difference between H2O and DM. Nonetheless, Fkh1 and Fkh2 proteins are expressed in post-mitotic cells.
Figure 2. The Fkh proteins are present in the nucleus of stationary phase cells.
(A) Cells expressing endogenously TAP-tagged FKH1 were grown to stationary phase and either left in DM or transferred to H2O for the remainder of the experiment. Samples were removed on days 1 and 5 for Western analysis using antibodies against the TAP epitope, or GAPDH as a load control. Fkh2-TAP was also observed in stationary phase cells (data not shown). (B) Cells expressing endogenously tagged FKH1- or FKH2-GFP were grown to day 5 stationary phase while maintained in DM. Cells were observed to harbor both Fkh1 and Fkh2 nuclear staining. (C) Day 13 stationary phase cells expressing Fkh2-GFP were imaged, showing reduced nuclear staining in cells maintained in DM. (D) The percentage of nuclear localized Fkh1-GFP or Fkh2-GFP was determined as cells aged in either DM or H2O.
Next, endogenous C-terminal GFP-tagged Fkh1 and Fkh2 were analyzed in aging cells to determine cellular localization. In day 5 stationary phase cultures maintained in DM, GFP fluorescence was observed to be nuclear in many cells (Figure 2B). When Fkh1-GFP and Fkh2-GFP were monitored in progressively aging cells in DM and in H2O, we observed that a larger proportion of the Fkh protein remained nuclear in H2O compared to DM. An example is shown in Figure 2C, where day 13 stationary phase cells appear healthier, with a larger proportion of nuclear Fkh2-GFP when maintained in H2O (Figure 2C). The percentage of cells harboring nuclear Fkh1-GFP or nuclear Fkh2-GPF was consistently observed to be greater when the cells were maintained in H2O compared to DM (Figure 2D). This suggests the presence and nuclear localization of the Fkhs may be necessary for normal CLS and stress resistance, especially in an SCR environment.
Increased expression of the FKHs enhances stress resistance, CLS, and RLS
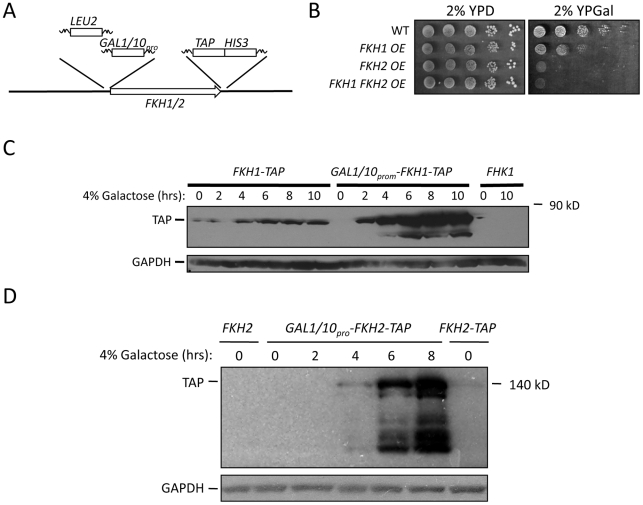
In higher eukaryotic systems increased expression of FOXO orthologues is associated with increased longevity and stress resistance [38], [45]. Thus, we predicted that an increase in survival would be expected with the overexpression of FKH1 and/or FKH2. Increased FKH expression was accomplished by integrating the GAL1/10 inducible promoter immediately upstream of the FKH1-TAP and FKH2-TAP start sites (Figure 3A). Cells overexpressing both FKH1 and FKH2 were created by crossing the appropriate strains. Growth of these cells on 2% glucose was comparable to WT, but growth was diminished when FKH overexpressing (OE) cells were grown on 2% galactose-supplemented media (Figure 3B). Fkh1-TAP and Fkh2-TAP were weakly expressed in 2% glucose, but massively expressed after 6 hrs in 2% galactose (Figure 3C, 3D). Lower concentrations of galactose (0.05–0.1%) did not influence vegetative growth (data not shown), but did improve stress resistance and longevity (Figure 4). First, we measured the ability of 5 day stationary phase cultures maintained in DM to survive a 1 hour treatment of 100 mM H2O2. For this experiment, once cells reached stationary phase the cells were split with one sample receiving a supplement of 0.05% galactose. After 5 days, samples were removed, treated with H2O2, and then diluted onto YPD plates to determine colony forming units. Controls were cells that did not receive H2O2. The FKH OE cultures exhibited increased survival in the absence of galactose, with improved resistance when supplemented with galactose (Figure 4A). These observations are consistent with a role for the Fkh proteins in stress resistance during stationary phase.
Figure 3. Increased FKH1 or FKH2 expression improves stress resistance and extends CLS and RLS.
(A) Schematic representation of scheme used to integrate the GAL1/10 promoter upstream of FKH1 and FKH2. The LEU2 PCR product, containing 300 basepairs of LEU2 promoter (incorporated for selection purposes), was flanked by 60 basepairs of homology to the FKH promoter and to the GAL1/10 promoter. The GAL1/10 promoter PCR fragment was flanked by 60 basepairs of homology to the 3′ end of LEU2 and to the 5′ end of the FKH gene. Cells were cotransformed with both products and selected on leu− plates. Cells harboring FKH1 and FKH2 under the control of the GAL1/10 promoter were generated by crossing the single integrated strains. (B) Cells overexpressing (OE) FKH1 and/or FKH2 from the GAL1/10 promoter were grown overnight in 2% YPD, then spot diluted onto the plates shown. The plates were incubated at 30°C for 2 to 5 days. (C) FKH1-TAP OE cells were grown overnight in 2% glucose. The next day, the cells were washed and resuspended in media containing 2% galactose. Samples were taken every two hours for 10 hours for Western analysis using antibodies against TAP and GAPDH. Cells expressing FKH1-TAP and FKH1 under their own promoter were used as controls. (D) FKH2-TAP OE cells were analyzed as described above for FKH1-TAP OE cells.
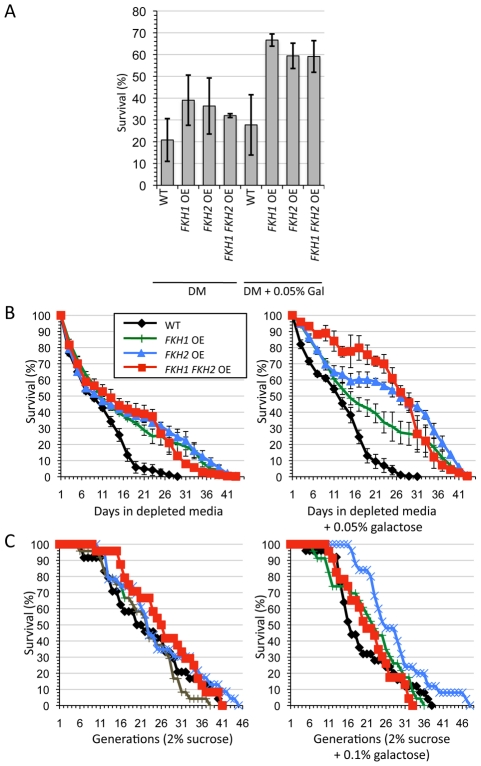
Figure 4. Increased expression of the FKH genes increases lifespan and stress response.
(A) The FKH OE cells were grown to stationary phase, then either maintained in DM, or 0.05% galactose was added. The cells were incubated for an additional 5 days, then split, with one half treated with 100 mM H2O2 for 1 hour. The other half served as the untreated control. Following the 1 hour incubation, the cells were diluted and plated onto YPD until colony forming units formed. Survival was determined by dividing the treated cells by the untreated cells. Standard error is shown for at least 3 replicates. (B) CLS was determined for the OE strains when maintained solely in DM (left panel) or in DM supplemented with 0.05% galactose (right panel). Standard error is shown for at least 3 replicates. (C) RLS was determined for the OE strains on 2% sucrose plates or sucrose plates supplemented with 0.1% galactose. Typical results are shown.
If the Fkh proteins do enhance stress resistance during stationary phase, then it is likely that increased FKH expression may prolong metabolic activity in these cells. CLS of WT and FKH OE cells was measured in DM in the presence and absence of 0.05% galactose (Figure 4B). Cells were grown in 2% glucose to stationary phase, then split, with one sample receiving a supplement of 0.05% galactose. Unaltered WT cells were used as a control. We observed that the addition of 0.05% galactose increased the CLS mean lifespan (50% survival) of the unaltered WT control from approximately 8.5 to 12 days. In the absence of galactose the OE strains exhibited mean lifespans of 9.5 to 12 days. However, in the presence of 0.05% galactose, the FKH1 OE strain experienced a 15 day mean lifespan, while FKH2 OE strains enjoyed mean lifespans of approximately 27 days. Yeast cell lifespan can be measured in post-mitotic stationary phase (CLS), or in rapidly dividing mitotic cells (RLS). Stress resistance plays a major role in determining both CLS and RLS, however not all genes that influence CLS also influence RLS. Sir2 in yeast is a good example of this [44]. To determine whether the Fkh proteins also influence RLS, we measured RLS in the cells employed in Figure 4B using 2% sucrose as a base carbon source in the presence and absence of 0.1% galactose (Figure 4C). In the absence of galactose, RLS of all strains was relatively unchanged. Upon 0.1% galactose supplementation, FKH2 OE cells had a markedly longer RLS. The mean lifespan of FKH1 OE cells was also increased, but not to the same extent as the FKH2 OE cells. We also observed increased RLS in FKH2 OE, but not FKH1 OE cells using 0.05% galactose (data not shown). The enhanced stress resistance and CLS observed in OE strains in the absence of galactose are not surprising as we previously documented the basal activity of the galactose promoter [33]. Our results are consistent with the Fkh proteins playing a role in responding to stress, which may indirectly lead to increased CLS and RLS. The effect appears to be greater during post-mitotic stationary phase cells, with Fkh2 perhaps playing a more pivotal role compared to Fkh1.
The Fkh proteins and the Anaphase-Promoting Complex (APC) work together to mediate post-mitotic survival
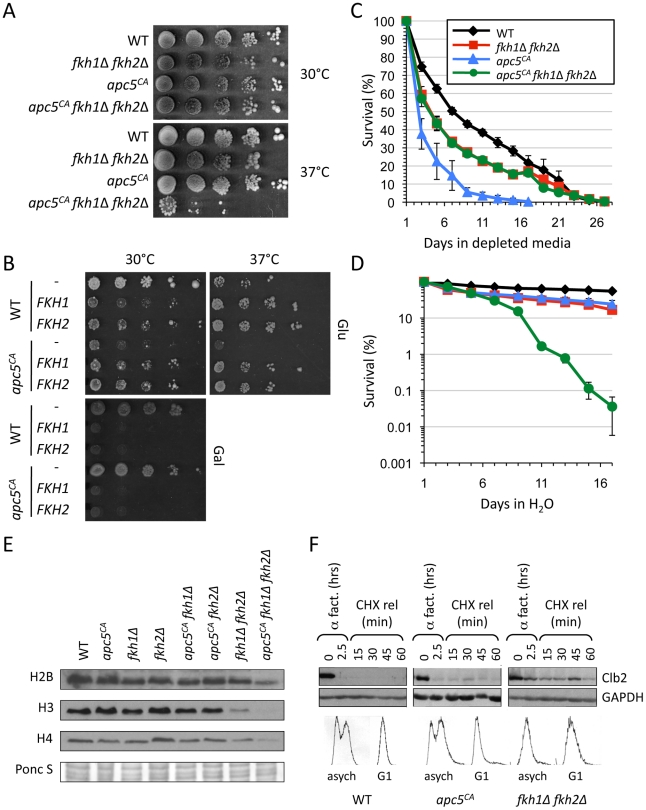
The advantage of using yeast for genetic studies is the relative ease of identifying interacting partners for proteins and genes of interest. Thus, we sought possible downstream Fkh targets that may be involved in stress response and longevity. One possible target of the Fkh transcription factors is the Anaphase-Promoting Complex (APC). The APC is an evolutionarily conserved ubiquitin-protein ligase (E3) that targets proteins that inhibit mitotic progression and exit, as well as G1 maintenance, for ubiquitin- and proteasome-dependent degradation [21], [23]. We previously observed APC mutants to exhibit reduced CLS and RLS, while increased APC10 expression extended RLS [29], [46]. Furthermore, APC mutants are sensitive to DNA damaging agents, and exhibit both chromatin assembly and histone modification defects [28], [33], [46]–[49]. Consistent with the APC's involvement in histone biogenesis and lifespan, we recently demonstrated that yeast cells harboring histone modification defects are subject to reduced RLS [50]. The APC appears to play an evolutionarily conserved role in lifespan, as mutations to mouse BubR1, a component of the spindle checkpoint that inhibits APC function, resulted in inappropriate APC activity and premature aging phenotypes [27]. A possible link between the APC and the Fkhs was revealed by a previous microarray analysis of fkh1Δ fkh2Δ cells, where APC1 (APC subunit), CDC5 (APC activator/target), CLB2 (APC activator/target), CDC20 (APC activator/target), and IQG1 (APC target), were identified as responsive to the Fkh transcription factors [17], [21], [22]. A subsequent analysis of CLB2 mRNA expression during the cell cycle (CLB2 mRNA synthesis is cell cycle regulated) showed that it was defective in fkh1Δ fkh2Δ mutants [16]. Clb2, a mitotic cyclin that is an important activator of the APC, later becomes targeted by the APC for degradation to allow exit from mitosis [21]. To test our hypothesis that APC activity may be targeted and activated by the Fkh proteins, we created fkh1Δ fkh2Δ cells harboring a mutation in the APC subunit Apc5 to enable genetic analyses. APC5 encodes an essential APC subunit (the apc5CA allele used in our studies contains a two basepair deletion at the 5′ end of the coding region, most likely creating an N-terminally truncated protein [28]; unpublished data). Cells with the apc5CA mutation grow slowly at temperatures above 36°C, which can be recovered or exacerbated by genetic alteration of negative or positive regulators, making this an excellent allele to identify APC5 interacting partners [29], [33], [47], [49], [51].
First, we examined the growth characteristics of apc5CA fkh1Δ fkh2Δ mutants. Deletion of both FKH genes severely impaired apc5CA growth at the restrictive temperature, but not at the permissive temperature (Figures 5A). Deletion of both FKH genes also impaired the growth of apc10Δ cells and was lethal in the apc11-13 background (unpublished data). This preliminary investigation provides evidence that the APC and the Fkhs may share a common function.
Figure 5. The Fkh proteins and the APC work together to promote extended CLS and stress response.
(A) The FKH genes were deleted in apc5CA cells by multiple rounds of genetic crosses. The strains shown were grown overnight at 30°C in YPD, then spot diluted onto YPD plates and incubated at 30 or 37°C. (B) WT and apc5CA cells were transformed with plasmids expressing FKH1 or FKH2 from a galactose inducible promoter, or an empty vector control plasmid. Individual transformed colonies were grown overnight and then spot diluted onto SD ura- plates supplemented with either 2% glucose or 2% galactose. The plates were incubated at 30 or 37°C. (C) The mutants used above were grown to stationary phase and maintained in depleted media (DM) for the remainder of the experiment. Colony forming units were determined every other day and a survival curve was plotted. Standard error is shown for at least 3 repeats. (D) CLS was determined for the strains used above. Rather than maintenance in DM, the cells were washed once they reached stationary phase and maintained in H2O for the remainder of the experiment. Standard error is shown for at least 3 repeats. The experiments in (C) and (D) were started from the same cultures. (E) The panel of strains shown were grown overnight at 30°C to early log phase growth. Proteins were extracted and analyzed by Westerns to assess total histone H2B, H3 and H4 protein levels. A Ponceau S stained gel is included to shown equivalence of protein load. (F) WT, apc5CA and fkh1Δ fkh2Δ cells encoding BAR1 were grown to early log phase, then arrested with 2 µg/ml α factor for 1.5 hours in pH 3.5 YPD media. Another 2 µg/ml α factor was added followed by another hour of incubation. Cycloheximide was then added with continued incubation. Samples were removed at the times shown and Clb2 protein levels were determined by Western blotting. Antibodies against GAPDH were included as a load control. The same blot was divided and used for the Clb2 and GAPDH Westerns. Samples were taken before and after G1 arrest for FACS analysis.
If the Fkhs and the APC do share a common function, then increased expression of the FKHs may restore the apc5CA temperature sensitive (ts) growth phenotype. Thus, we expressed plasmid borne FKH1 and FKH2 under the control of the GAL1/10 promoter in WT and apc5CA cell. The cells were grown in 2% glucose supplemented media, then spot diluted onto plates containing either 2% galactose or 2% glucose (Figure 5B). At 30°C, expression of the FKHs on glucose-supplemented media was slightly detrimental to WT cells, but beneficial to apc5CA cells. Galactose-driven genes do have basal activity in the presence of glucose [33]. On galactose plates, overexpression of either FKH was toxic, as observed above (Figure 3B). At 37°C, expression of either FKH gene on glucose plates improved growth of both WT and apc5CA cells. We have also observed that increased FKH1 expression suppressed the ts defect in additional APC mutants, including apc10Δ, apc11-13, cdc16-1, cdc23-1, apc5CA apc10Δ and apc5CA cdc26Δ cells (data not shown). These observations suggest that under conditions of stress, whether temperature or impaired APC activity, moderately increased FKH expression improves the capacity of the cell to cope.
Next, we asked whether deletion of both FKHs influenced apc5CA CLS. Cells expressing the different combinations of mutations were grown to stationary phase, then split, with one half resuspended in H2O, and the other half left in DM. Equal volumes were then plated on the days shown to generate survival curves (Figure 5C, 5D). In DM, apc5CA cells rapidly lost viability compared to WT and fkh1Δ fkh2Δ cells (Figure 5C). Interestingly, the triple mutant survived as long as fkh1Δ fkh2Δ cells. This suggests that deletion of both FKH1 and FKH2 is epistatic to the apc5CA allele under the conditions tested. In other words, under normal media conditions using DM, the Fkhs appear to act upstream of the APC. When the experiment was conducted by maintaining the cells in H2O for the duration of the experiment, a different survival profile was observed (Figure 5D). WT (20 days vs 7 days) and apc5CA (5 days vs 2.5 days) cells both responded to H2O conditions by exhibiting a longer mean CLS. However, fkh1Δ fkh2Δ cells had the same CLS in H2O as in DM, which was similar to apc5CA cells in H2O, whereas the triple mutant had a greatly reduced CLS in H2O. As stated above, the failure of fkh1Δ fkh2Δ cells to survive longer under SCR conditions, such as H2O, suggests that the Fkhs are required for long life under SCR conditions. The similar H2O CLS observed in fkh1Δ fkh2Δ and apc5CA cells, and the dramatically reduced H2O CLS in the triple mutant indicates that the Fkhs and the APC may have redundant functions under stress conditions. This contrasts with the Fkh/APC epistatic interaction that appears to occur under DM conditions. This could reflect dual roles for the Fkh proteins; as cell cycle regulators under normal conditions, and as stress response proteins under stress conditions [17], [18], [52], [53]. Lastly, these observations clearly identify another non-mitotic function for the APC. The APC has been shown to function in other non-mitotic activities, such as meiosis, quiescence, differentiation, metabolism, maintenance of post-mitotic neurons, and interestingly, memory in mice [30], [54]–[58].
In addition to controlling CLS, the APC and the Fkhs are also involved in histone metabolism, but likely through very different mechanisms. The Fkhs are redundant activators of cell cycle dependent histone expression [17]. On the other hand, histones and histone modifications are reduced when genes encoding different APC subunits, such as APC5, APC9, APC10, APC11, CDC16, CDC23 and CDC26, are mutated [33]. The mechanism involved remains elusive, but it is likely post-transcriptional, as histone mRNAs are unaltered in APC mutants [33]. Our analysis of total histone levels in the different mutant combinations indicates that histone control is indeed through different redundant mechanisms, as histone levels are greatly reduced in the triple mutant compared to the single and double mutants (Figure 5E). However, it remains possible that the Fkhs drive histone synthesis through direct transcriptional control and indirectly via the APC.
As a direct assessment of whether the Fkhs control an APC function, we measured Clb2 stability in apc5CA and fkh1Δ fkh2Δ mutants. Clb2 is a B-type cyclin that is targeted by the APC for degradation in order to exit mitosis [21]. CLB2 transcripts are also controlled by the Fkhs [16], [17]. WT, apc5CA and fkh1Δ fkh2Δ cells were grown to early log phase at 30°C, then arrested in G1 using α factor. The cells were determined to be arrested in G1 using microscopy (data not shown) and FACS analysis (Figure 5F). The cells were then released into cycloheximide, with samples removed every 15 minutes for protein analysis using antibodies against endogenous Clb2. In both WT and apc5CA cells Clb2 levels were decreased in G1 arrested cells when compared to asynchronous cells (Figure 5F). In fkh1Δ fkh2Δ cells however, the degradation of Clb2 was reduced in G1 arrested cells. However, it should be noted that fkh1Δ fkh2Δ cells accumulated in G1 in asynchronously grown cells, as indicated by FACS, perhaps reflecting the inability to degrade mitotic cyclins. These observations are consistent with a model where the Fkh transcription factors act in a positive manner upstream of the APC, perhaps through transcriptional activation of APC subunits, activators and targets.
The APC and the Fkhs function in the stress response pathway
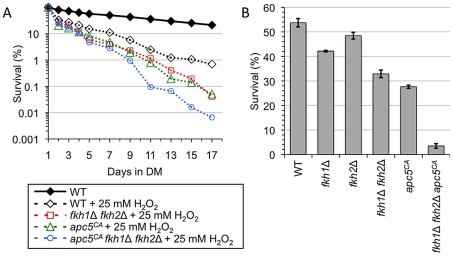
Since the APC is associated with maintaining lifespan in multiple systems [27], [29], [30], [31], we tested whether the APC may also be involved in oxidative stress resistance. To test this hypothesis, we conducted CLS in the presence of oxidative stress in WT, apc5CA, fkh1Δ fkh2Δ and apc5CA fkh1Δ fkh2Δ cells. The cells were grown to stationary phase followed by the addition of 25 mM H2O2 (Figure 6A), with cell counts determined every other day. The results show WT cells had a reduced lifespan in response to 25 mM H2O2, while the mutants were further impaired. The lifespan of the triple mutant was dramatically reduced compared to apc5CA and fkh1Δ fkh2Δ cells in H2O2, as it was in water (Figure 5D). These observations provide additional evidence that the triple mutant is extremely sensitive to stress and likely perceives water as a severe stress, rather than a form of caloric restriction. To our knowledge, few mutations have been described that act in a negative manner to SCR.
Figure 6. The APC and the Fkh proteins provide overlapping function to respond to oxidative stress.
(A) CLS was performed using the strains shown in the presence of 25 mM H2O2. The cells were grown to stationary phase, and then H2O2 was added to the cultures. Colony forming units were determined every other day for the remainder of the experiment. Survival curves are shown. (B) The strains described above were used to determine resistance to oxidative stress. Day 5 stationary phase cells were exposed to 100 mM H2O2 for 1 hour at 30°C. Controls were not treated with H2O2. Diluted cells were then plated onto YPD plates until colonies formed. The percent survival was determined, with standard error shown for at least 3 replicates.
To investigate the roles of the APC and the Fkh proteins in stress resistance further, stationary phase cultures were treated with 100 mM H2O2 for 30 minutes at 30°C, and then plated to determine cell viability. While 53.2% of WT cells survived 100 mM H2O2, only 21.3% of apc5CA cells and 31.3% of the fkh1Δ fkh2Δ survived the treatment (Figure 6B). However, the apc5CA fkh1Δ fkh2Δ mutant was dramatically impaired, with only 3.5% surviving this treatment. Taken together, our data indicates that the APC and the Fkh proteins have overlapping functions in response to oxidative stress in post-mitotic cells, opposed to the epistatic interaction observed in unstressed cells (Figure 5C, Figure 7). Consistent with an APC involvement in post-mitotic CLS, the APC is also required for stress response in post-mitotic cells.
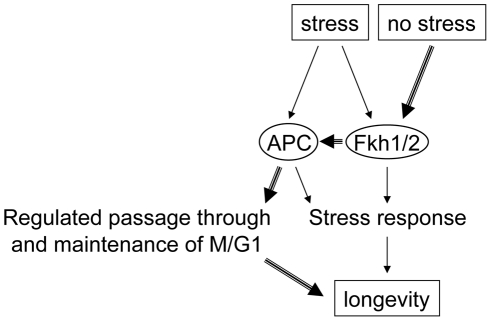
Figure 7. Model depicting how the APC and the Fkh proteins may interact under normal and stress conditions.
Under normal conditions, the Fkh proteins play a role in activating the APC. This in turn mediates progression through mitosis and maintenance of G1. This interaction is depicted by bold arrows. Under stress conditions, both the APC and the Fkh proteins respond via different mechanisms. The Fkh proteins likely promote the expression of stress response proteins. The APC acts according to a mechanism that remains uncharacterized, but likely requires ubiquitin-dependent processes.
Discussion
This report provides evidence to support an evolutionarily conserved role for the yeast forkhead transcription factors Fkh1 and Fkh2 in lifespan determination and stress response. FKH1 and FKH2 act redundantly in controlling CLS and post-mitotic stress response, as deletion of both genes is required to observe CLS and stress response defects (Figure 1), as previously described for other phenotypes [16], [17]. Importantly, we show that a modest increase in FKH1 or FKH2 expression results in increased CLS, RLS and stress resistance (Figure 4). The Fkhs may have a dual function in cell cycle progression and in stress response. A microarray analysis initially identified such a role, as arrest of fkh1Δ fkh2Δ cells in G1 identified a series of genes involved in cell cycle progression, whereas the transcript profile identified in asynchronous fkh1Δ fkh2Δ cells was composed of stress response genes [17]. Importantly, we identify an evolutionarily conserved cell cycle regulator, the Anaphase-Promoting Complex (APC), as a potential downstream target of the Fkh transcription factors (Figure 5, Figure 6). Genetic interaction studies between APC and Fkh mutants revealed a possible division of Fkh labor. For example, the CLS of apc5CA and fkh1Δ fkh2Δ cells showed an epistatic interaction under normal conditions (Figure 5C), suggesting the Fkhs may be upstream of the APC. However, under stress conditions, such as maintenance in H2O (Figure 5D) and in the presence of 25 mM H2O2 (Figure 6A), the CLS of the triple mutant was dramatically impaired beyond any of the single and double mutants, defining a synergistic interaction. This most likely reflects a role for the Fkhs in activating the transcription of genes involved in stress response (Figure 7). Thus, under non-stress conditions, the Fkhs may play a role in driving APC activity that leads to controlled progression through mitosis and into G1. Since the APC is required for genomic stability, activating the APC would be predicted to increase the fidelity of chromosome segregation, reducing nondysjunction events, thereby increasing the potential for a healthier and longer cellular lifespan. The APC also plays a role in stress response (Figure 6B). Previously, it was shown cells lacking the Cdh1 APC activator were sensitive to multiple stresses, such as ethanol, caffeine and salt [32], and we have shown that apc5CA and apc10Δ cells are sensitive to UV and methylmethanesulfonate (MMS) [28], [33]. Stabilization of the APC targets Clb2 and Hsl1 also increased stress sensitivity [32], indicating the APC may activate the stress response by alleviating inhibitory signals. The Fkhs' contribution to longevity is most likely an indirect reaction to stress, occurring via at least two pathways, one involving transcription of stress genes [17], [18], and the other through driving APC activity (Figure 7). It has long been established that cells better equipped to repair damage and respond to stress stand a better chance to live a healthier and potentially longer lifespan [34], [42], [43], [59]. These observations demonstrate the evolutionary conservation of the FOXO transcription factors in yeast, which respond to stress and extend cellular lifespan.
Fkh1, Fkh2, and severe caloric restriction (SCR)
The initial focus of this work was to determine whether the conserved yeast Fkh proteins were involved in longevity, as shown with metazoan FOXOs. Our work clearly demonstrates a need for the Fkh proteins for extended CLS and RLS. However, our work also demonstrates that the Fkh proteins are necessary for extended lifespan in response to SCR. Recently, the Rim15 stress responsive transcription factor was identified as a major mediator of SCR lifespan extension [15]. Deletion of RIM15 blocked extended lifespan in ras2Δ, sch9Δ and tor1Δ strains, indicating that the phenomenon of SCR funnels through Rim15. Interestingly, although deletion of RIM15 in the extremely long-lived ras2Δ sch9Δ mutant reduced lifespan under normal conditions, this strain could still respond to SCR, suggesting other factors can respond to SCR in the absence of Rim15. Our data provides the possibility that Fkh1/Fkh2 may fulfill this role. Future work will require an analysis of strains lacking FKH1, FKH2 and RIM15.
The APC is likely a downstream target of the stress responsive Fkh proteins
We tested the hypothesis that the Fkh proteins play a role in lifespan by contributing to APC activation. Our results support this hypothesis, as (i) low-level expression of FKH1 or FKH2 suppressed APC mutant growth phenotypes; (ii) deletion of both FKH1 and FKH2 exacerbated APC mutant histone and growth phenotypes in mitotically active cells; and (iii) deletion of FKH1 and FKH2 stabilized the APC substrate Clb2. Furthermore, stress resistance and lifespan in the presence of stress were markedly worse in the triple mutant compared to the single and double mutants. These results demonstrate that the APC and the Fkhs function together in both mitotic and post-mitotic cells. While fkh1Δ fkh2Δ cells do not respond to SCR, apc5CA fkh1Δ fkh2Δ exhibit decreased CLS under these conditions. This suggests that H2O is perceived as much more than a low level stress in the triple mutant, which is consistent with the Hormesis hypothesis of aging, a theory that postulates low level stresses turn on stress defense mechanisms, leading to potenially longer life [60]. Nonetheless, these observations implicate the APC as a player in caloric restriction and stress response.
We observed that APC and FKH mutants interacted differently depending on the growth conditions. Figure 7 presents a model describing how this may occur. Under non-stress conditions, such as growth on YPD or maintenance of stationary phase cells in the depleted media (DM), there was little interaction (growth on YPD at 30°) or an epistatic interaction (CLS in DM). The interaction could be interpreted to define a pathway where the Fkhs act upstream of the APC. It could be as simple as the Fkhs driving the transcription of the APC subunit Apc1, and the APC activators Clb2, Cdc5 and Cdc20 [16]. This interaction is likely more complicated than direct classical epistasis, perhaps involving stoichiometric alteration of APC activators, subunits and/or substrates. The deletion of the FKHs in APC mutant strains may bring about a homeostatic balance between APC activity and substrate levels. On the other hand, under stress conditions, such as high temperature or the addition of H2O2, apc5CA fkh1Δ fkh2Δ cells exhibited a synergistic interaction. This type of interaction occurs when multiple proteins drive a similar activity in a redundant manner, thus requiring that more than one mutation must occur to expose a phenotype. Both the Fkhs and the APC are required for response to stress, but likely act in very different manners. The Fkhs drive expression of many stress response genes [16], whereas the molecular mechanisms employed by the APC to combat stress may involve APCCdh1, which has been found to regulate different stress responses through the degradation of substrates such as Clb2 and Hsl1 as a part of the APC's role in G1 maintenance [32]. An additional role the APC likely plays in stress revolves around histone metabolism. We have shown that APC mutants exhibit defects in histone maintenance, chromatin assembly and histone modifications [28], [33], [47]–[49], [51]. It is well established that histone modifications, such as phosphorylation of H2A/H2AX, methylation of H3 Lys79, and acetylation of H3 Lys56 and H4 Lys16, are required for recruitment of DNA repair enzymes to sites of DNA damage [61]–[65]. Therefore, under stress conditions, the Fkhs and the APC likely play separate, yet overlapping roles in ensuring the cell responds to stressful damaging agents in a positive manner.
Our studies provide a potential link between the Fkh transcription factors and the APC that ties the APC together with stress response. This provides insight into a molecular mechanism whereby the APC facilitates longevity. The evolutionarily conserved nature of our results, and the established role the APC plays in tumor development and progression, suggests that the APC may be a potential downstream target of the insulin signaling pathway. A recent report supports this scenario, as the insulin driven AKT1 kinase phosphorylates the APCCdh1 substrate Skp2, an SCF component, which inhibits Skp2 degradation [66]. This enables SCF activity and promotes cell cycle progression. These studies offer the basis for further studies in understanding APC-dependent longevity.
Conclusions
This study identifies the yeast forkhead box containing proteins Fkh1 and Fkh2 as regulators of lifespan, allowing for the characterization of upstream and downstream regulation by these factors. This may provide insight into how the highly related FOXO proteins in mammals regulate both lifespan extension and tumor suppression. Our data supports a model where FKH1 and FKH2 are functionally orthologous with the metazoan FOXOs, which opens the door to genetic manipulation in yeast for further exploration into the function and mechanisms controling these important metabolic and stress regulators.
Materials and Methods
Yeast strains and plasmids
The yeast strains used in this study are shown in Table 1. The fkh1Δ::kanMX6 and fkh2Δ::kanMX6 strains, obtained from the ResGen library (provided by W. Xiao, U. of Saskatchewan), were repeatedly backcrossed to our S288c background strains to generate apc5CA, apc10Δ and apc11-13 congenic partners. apc11-13 cells were a kind gift from T. Hunter (Salk Institute). Cells harboring APC9, APC10 and CDC26 deletions were acquired from W. Xiao and backcrossed repeatedly to our S288c background. cdc16-1, cdc23-1 and the isogenic wild type were generously provided by D. Stuart (U. of Alberta). The C-terminal FKH1-TAP and FKH2-TAP strains were generously provided by A. Ghavidel (U. of Toronto). PCR based methods were used to TAP-tag FKH1 and FKH2 in various mutants. Cells expressing endogenously GFP-tagged FKH1 and FKH2 were obtained from Open BioSytems. The galactose inducible FKH1-HA and FKH2-HA encoding plasmids were obtained from the Research Genetics library of tandem affinity tagged plasmids purchased by W. Xiao (U. of Saskatchewan). The GAL1/10 promoter was integrated upstream of the FKH1 and FKH2 genes by PCR-based homologous integration approach. Two sets of primers were designed for this approach. First, primers were designed to amplify the LEU2 gene (plus 300 basepairs of the promoter) flanked on the 5′ side by 60 nucleotides of sequence homologous to the immediate promoter regions of FKH1 or FKH2, and the 3′ side by 60 nucleotides homologous to the GAL1/10 promoter. The second primer set was designed to amplify GAL1/10 promoter flanked on the 5′ side by 60 nucleotides homologous to the 3′ end of the LEU2 gene and on the 3′ end by 60 nucleotides homologous to the first 60 nucleotide of FKH1 or FKH2. Primer sequences are available upon request. The two PCR fragments were transformed together into WT cells. Leu+ transformants were selected for further analysis.
Table 1. Saccharomyces cerevisiae strains used in this study.
| Strain | Genotype | Source |
| YTH5 | MATα ade2 his3Δ200 lys2Δ201 ura3-52 | Harkness et al. 2002 |
| YTH6 | MATα ade2 his3Δ200 lys2Δ201 ura3-52 | Harkness et al. 2002 |
| YTH457 | MATα ade2 his3Δ200 leu2-3,112 ura3-52 apc5CA | Harkness et al. 2002 |
| YTH1235 | MATa ade2 his3Δ200 leu2-3,112 lys2Δ201 ura3-52 | Harkness et al. 2002 |
| YTH1636 | MATa ade2 his3Δ200 leu2-3,112 ura3-52 | Harkness et al. 2004 |
| YTH1637 | MATα ade2 his3Δ200 leu2-3,112 ura3-52 apc5CA-PA::His5 | Harkness et al. 2004 |
| YTH1693 | MAT(?)his3 leu2 met15 ura3 apc10Δ::kanMX6 | Harkness et al. 2005 |
| YTH2290 | MATa his3Δ1 Δleu2 Δmet15 Δura3 fkh1Δ::kanMX6 | W. Xiao |
| YTH2291 | MATa his3Δ1 Δleu2 Δmet15 Δura3 fkh2Δ::kanMX6 | W. Xiao |
| YTH2427 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 fkh1Δ::kanMX6 | This study |
| YTH2431 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 fkh1Δ::kanMX6 apc5CA-PA::His5 | This study |
| YTH2444 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 fkh2Δ::kanMX6 | This study |
| YTH2449 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 fkh2Δ::kanMX6 apc5CA-PA::His5 | This study |
| YTH2578 | MATa ade2 his3 lys2(?) Δmet15(?) ura3 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH2579 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH2581 | MAT(?) ade2 his3 lys2(?) Δmet15(?) ura3 apc5CA-PA::His5 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH2582 | MATa ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc5CA-PA::His5 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH3124 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc10Δ::kanMX6 fkh1Δ::kanMX6 | This study |
| YTH3143 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc11-13 fkh2Δ::kanMX6 | This study |
| YTH3346 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc10Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH3405 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc11-13 fkh1Δ::kanMX6 | This study |
| YTH3408 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc10Δ::kanMX6 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH3409 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc10Δ::kanMX6 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH3926 | as YTH1235, but FKH1-TAP::HIS3 | This study |
| YTH3929 | as YTH1235, but FKH2-TAP::HIS3 | This study |
| YTH3930 | as YTH457, but FKH1-TAP::HIS3 | This study |
| YTH3933 | apc10Δ::kanMX6 FKH1-TAP::HIS3 (YTH1693×3926) | This study |
| YTH3999 | MAT(?) apc10Δ::kanMX6 FKH2-TAP::HIS3 (YTH1693×3929) | This study |
| YTH4110 | as YTH457, but FKH2-TAP::HIS3 | YTH3409 |
| YTH3409 | MAT(?) ade2 his3 leu2 lys2(?) Δmet15(?) ura3 apc10Δ::kanMX6 fkh1Δ::kanMX6 fkh2Δ::kanMX6 | This study |
| YTH4265 | as YTH3929, but LEU2::GAL1/10prom-FKH2-TAP::HIS3 | This study |
| YTH4269 | MATα ade2 his3Δ200 leu2-3,112 lys2Δ201 ura3-52 | This study |
| YTH4315 | MATa his3Δ1 Δleu2 Δmet15 Δura3 FKH1-GFP::HIS3 | Open Biosystems |
| YTH4316 | MATa his3Δ1 Δleu2 Δmet15 Δura3 FKH2-GFP::HIS3 | Open Biosystems |
| YTH4515 | MATα LEU2::GAL1/10prom-FKH1-TAP::HIS3 | This study (YTH4265×4269) |
| YTH4517 | LEU2::GAL1/10prom-FKH1-TAP::HIS3 LEU2::GAL1/10prom-FKH2-TAP::HIS3 | This study (YTH4515×4516) |
Media and methods
Media were prepared as previously described [28], [33]. Segregation of the kanMX6 cassette was determined by patching spores onto 0.2 mg/ml geneticin-supplemented YPD plates. Mutants containing two or more kanMX6 marked alleles were generated by selecting tetrads in which G418 resistance segregated in a 2∶2 fashion. Escherichia coli strains JM109 and DH10B were used to propagate DNA plasmids. DNA manipulations such as DNA minipreps, and yeast and E. coli transformations were carried out according to standard protocols [67]. Spot dilution assays were conducted by pipeting 3 µl of cells from samples generated from a 10-fold dilution series onto the various media shown and grown at the temperatures indicated. The starting spot generally contained 5×104 cells. To assess resistance to oxidative stress, cultures were grown in 2% YPD at 30°C for 5 days and then diluted to an OD600 of 1 in depleted media (DM). Each of these cultures was divided into two samples and 100 mM H2O2 (EMD Chemicals) was added to one sample. Both samples were then incubated at 30°C for 1 hour. Viability was determined by plating diluted cells onto 2% YPD and comparing the growth of the H2O2 treated culture to that of the non-treated control culture. Clb2 stability was determined by growing the indicated cells to early log phase at 30°C, then adding 2 µg/ml α factor, when using BAR1 strains, in media at pH 3.5 to arrest cells in G1. After 1.5 hours, another 2 µg/ml was added, with continued incubation for another 1 hour. After this treatment, cells were arrested in G1. Cell cycle arrest was confirmed by microscopic visualization of the cells and by flow cytometry. The α factor was then washed away and fresh media containing 10 µg/ml cycloheximide was added. The incubation was continued at 30°C, with samples removed every 15 minutes for Western analysis using antibodies against endogenous Clb2 and GAPDH. FACS was performed as described previously [28]. To characterize fkh1Δ fkh2Δ FACS profiles the following changes were made to overcome the persistent cell/cell contacts inherent to this mutant. 1 ml of culture (OD600 0.4) was resuspended in 50 µl followed by the addition 10 µl of 12.5 U/µl lyticase. This solution was incubated for 15 minutes at room temperature, followed by the addition of 500 µl 50 mM Tris. The cells were then sonicated for 6 seconds at output 4. The cells were centrifuged, resuspended in 1 ml 70% EtOH and processed as usual past this step.
Lifespan assays
Chronological lifespan was performed as previously described [29], [40], [41]. Briefly, overnight CM (2% glucose) cultures were diluted to OD600 0.5 in fresh CM with a flask to culture volume ratio of 5∶1. The incubation continued (200 RPM) at 30°C. Each day the same volume of culture was diluted and plated to evaluate colony forming units (CFU) as a measure of viability. When the CFU counts peaked, this was deemed stationary phase and denoted as Day 1. Every two days CFU were determined and compared to Day 1. For severe caloric restriction (SCR) experiments, once stationary phase was reached in CM (Day1), cultures were washed and resuspended in sterile distilled H2O, with washes of equal volume of water every two to four days to remove metabolites produced by the cells. Galactose and hydrogen peroxide were added to appropriate cultures upon reaching Day 1 to final concentrations of 0.05% and 25 mM respectively.
For fluorescent localization, samples were obtained from CLS cultures, washed and mounted in PBS or Ultracruz mounting medium (Santa Cruz Biotechnology sc-24941) and imaged using 100× oil immersion with an Olympus BX51 fluorescent microscope. Images were captured using an INFINITY 3-1UM camera, and analyzed with Infinity Analyse software version 5.0.3 (Lumenera).
Replicative lifespan experiments were performed as previously described [29]. The plates were stored at 4°C each night. The experiments were performed blind; the genotypes of the strains and conditions used were not revealed to the experimenter until the final mother stopped producing daughters.
Western analysis
Yeast whole cell protein extraction was performed as previously described [28]. Samples were resolved on a 15% acrylamide SDS-PAGE gel, which was stained with Coomassie brilliant blue R-250 (OmniPUR) or transferred directly to nitrocellulose membrane (PALL) at 400 mAmps for 1 hour. Protein loading was analyzed using ImageJ 1.37v software (NIH). Equalized samples were then transferred to nitrocellulose membrane. For Western analysis, the membranes were blocked in 5% non-fat milk (Biorad) and PBST overnight at 4°C. The membranes were then incubated with primary antibody in 5% non-fat milk and PBST for 1.5 hours at room temperature or overnight at 4°C. Rabbit polyclonal anti-H2B (Abcam), polyclonal anti-H3 (Abcam), and polyclonal anti-H4 (Abcam) were used at a 1∶4000 dilution. Rabbit polyclonal anti-Clb2 (Santa Cruz; Y-180) was used at 1∶2000. The TAP antibody (Open Biosystems) was used at a dilution of 1∶1000. Mouse monoclonal anti-GAPDH (Sigma) was used at a dilution of 1∶20,000. The membranes were then washed 3 times in PBST for 15 minutes, and incubated in 1∶10,000 dilution of secondary antibody in 5% non-fat milk and PBST for 1 hour at room temperature. After another 3 washes in PBST for 15 minutes, the membranes were processed with Enhanced Chemiluminescence reagent (PerkinElmer) and exposed to Kodak film.
Acknowledgments
We kindly thank Ata Ghavidel, Tony Hunter, Dave Stuart, and Wei Xiao for strains and plasmids. Emma Turner is acknowledged and thanked for providing comments on the manuscript. We thank Donna Lindsay for her role in troubleshooting the over-expression replicative lifespan assay.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by an operating grant from the Canadian Institutes for Health Research (CIHR) and an infrastructural grant from the Canadian Foundation for Innovation (CFI) New Investigators award to TAAH. SDLP is supported by a Natural Sciences and Engineering Research Council (NSERC) Doctorate award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28:4985–4995. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366:28–34. doi: 10.1098/rstb.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw LM. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle. 2011;10:1750–1756. doi: 10.4161/cc.10.11.15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 6.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wysocki PJ, Wierusz-Wysocka B. Obesity, hyperinsulinemia and breast cancer: novel targets and a novel role for metformin. Expert Rev Mol Diagn. 2010;10:509–519. doi: 10.1586/erm.10.22. [DOI] [PubMed] [Google Scholar]
- 8.Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22:573–577. doi: 10.1016/j.cellsig.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Berdichevsky A, Guarente L. A stress response pathway involving sirtuins, forkheads and 14-3-3 proteins. Cell Cycle. 2006;5:2588–2591. doi: 10.4161/cc.5.22.3513. [DOI] [PubMed] [Google Scholar]
- 10.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 11.Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- 12.Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol. 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Wang Y, Zhu WG. Applications of post-translational modifications of FoxO family proteins in biological functions. J Mol Cell Biol. 2011;3:276–282. doi: 10.1093/jmcb/mjr013. [DOI] [PubMed] [Google Scholar]
- 14.Monsalve M, Olmos Y. The Complex biology of FOXO. Curr Drug Targets. 2011;12:1322–1350. doi: 10.2174/138945011796150307. [DOI] [PubMed] [Google Scholar]
- 15.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollenhorst PC, Bose ME, Mielke MR, Muller U, Fox CA. Forkhead genes in transcriptional silencing, cell morphology and the cell cycle: overlapping and distinct functions for FKH1 and FKH2 in Saccharomyces cerevisiae. Genetics. 2000;154:1533–1548. doi: 10.1093/genetics/154.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu G, Spellman PT, Volpe T, Brown PO, Botstein D, et al. Two yeast forkhead genes regulate the cell cycle and pseudohyphal growth. Nature. 2000;406:90–94. doi: 10.1038/35017581. [DOI] [PubMed] [Google Scholar]
- 18.Shapira M, Segal E, Botstein D. Disruption of yeast forkhead-associated cell cycle transcription by oxidative stress. Mol Biol Cell. 2004;15:5659–5669. doi: 10.1091/mbc.E04-04-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voth WP, Yu Y, Takahata S, Kretschmann KL, Lieb JD, et al. Forkhead proteins control the outcome of transcription factor binding by antiactivation. EMBO J. 2007;26:4324–4334. doi: 10.1038/sj.emboj.7601859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherriff JA, Kent NA, Mellor J. The Isw2 chromatin-remodeling ATPase cooperates with the Fkh2 transcription factor to repress transcription of the B-type cyclin gene CLB2. Mol Cell Biol. 2007;27:2848–2860. doi: 10.1128/MCB.01798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- 22.Ko N, Nishihama R, Tully GH, Ostapenko D, Solomon MJ, et al. Identification of yeast IQGAP (Iqg1p) as an anaphase-promoting-complex substrate and its role in actomyosin-ring-independent cytokinesis. Mol Biol Cell. 2007;18:5139–5153. doi: 10.1091/mbc.E07-05-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C). Q Rev Biophys. 2011;44:153–190. doi: 10.1017/S0033583510000259. [DOI] [PubMed] [Google Scholar]
- 24.Passmore LA. The anaphase-promoting complex (APC): the sum of its parts? Biochem. Soc Trans. 2004;32:724–727. doi: 10.1042/BST0320724. [DOI] [PubMed] [Google Scholar]
- 25.Hartwell LH, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer RE, Hogan E, Koshland D. Mitotic transmission of artificial chromosomes in cdc mutants of the yeast, Saccharomyces cerevisiae. Genetics. 1990;125:763–774. doi: 10.1093/genetics/125.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 28.Harkness TAA, Davies GF, Ramaswamy V, Arnason TG. The ubiquitin-dependent targeting pathway in Saccharomyces cerevisiae plays a critical role in multiple chromatin assembly regulatory steps. Genetics. 2002;162:615–632. doi: 10.1093/genetics/162.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harkness TAA, Shea KA, Legrand C, Brahmania M, Davies GF. A functional analysis reveals dependence on the anaphase-promoting complex for prolonged life span in yeast. Genetics. 2004;168:759–774. doi: 10.1534/genetics.104.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Shin YH, Hou L, Huang X, Wei Z, et al. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat Cell Biol. 2008;10:1083–1089. doi: 10.1038/ncb1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr Opin Cell Biol. 2005;17:583–589. doi: 10.1016/j.ceb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Simpson-Lavy KJ, Sajman J, Zenvirth D, Brandeis M. APC/CCdh1 specific degradation of Hsl1 and Clb2 is required for proper stress responses of S. cerevisiae. Cell Cycle. 2009;8:3003–3009. [PubMed] [Google Scholar]
- 33.Turner EL, Malo ME, Pisclevich MG, Dash MD, Davies GF, et al. The Saccharomyces cerevisiae Anaphase Promoting Complex interacts with multiple histone-modifying enzymes to regulate cell cycle progression. Eukaryot Cell. 2010;9:1418–1431. doi: 10.1128/EC.00097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuin A, Castellano-Esteve D, Ayté J, Hidalgo E. Living on the edge: stress and activation of stress responses promote lifespan extension. Aging. 2010;2:231–237. doi: 10.18632/aging.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 36.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008;7:187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- 39.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 41.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Methods Mol Biol. 2007;371:89–95. doi: 10.1007/978-1-59745-361-5_8. [DOI] [PubMed] [Google Scholar]
- 42.Pijl H. Longevity. The allostatic load of dietary restriction. Physiol Behav. 2011;Jun 2 doi: 10.1016/j.physbeh.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 43.Tang BL. Sirt1's systemic protective roles and its promise as a target in antiaging medicine. Transl Res. 2011;157:276–284. doi: 10.1016/j.trsl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, et al. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 2007;6:429–438. doi: 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 46.Harkness TAA. The Anaphase Promoting Complex and aging: the APCs of longevity. Curr Genomics. 2006;7:263–272. [Google Scholar]
- 47.Harkness TAA, Arnason TG, Legrand C, Pisclevich MG, Davies GF, Turner EL. Contribution of CAF-I to anaphase-promoting-complex-mediated mitotic chromatin assembly in Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:673–684. doi: 10.1128/EC.4.4.673-684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harkness TAA. Chromatin assembly from yeast to man: Conserved factors and conserved molecular mechanisms. Current Genomics. 2005;6:227–240. [Google Scholar]
- 49.Islam A, Turner EL, Menzel J, Malo ME, Harkness TA. Antagonistic Gcn5-Hda1 interactions revealed by mutations to the Anaphase Promoting Complex in yeast. Cell Div. 2011;6:13. doi: 10.1186/1747-1028-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feser J, Truong D, Das C, Carson JJ, Kieft J, et al. Elevated histone expression promotes life span extension. Mol Cell. 2010;39:724–735. doi: 10.1016/j.molcel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnason TG, Pisclevich MG, Dash MD, Davies GF, Harkness TAA. Novel interaction between Apc5p and Rsp5p in an intracellular signaling pathway in Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:134–146. doi: 10.1128/EC.4.1.134-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar R, Reynolds DM, Shevchenko A, Shevchenko A, Goldstone SD, Dalton S. Forkhead transcription factors, Fkh1p and Fkh2p, collaborate with Mcm1p to control transcription required for M-phase. Curr Biol. 2000;10:896–906. doi: 10.1016/s0960-9822(00)00618-7. [DOI] [PubMed] [Google Scholar]
- 53.Pic A, Lim FL, Ross SJ, Veal EA, Johnson AL, et al. The forkhead protein Fkh2 is a component of the yeast cell cycle transcription factor SFF. EMBO J. 2000;19:3750–3761. doi: 10.1093/emboj/19.14.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manchado E, Eguren M, Malumbres M. The anaphase-promoting complex/cyclosome (APC/C): cell-cycle-dependent and -independent functions. Biochem Soc Trans. 2010;38:65–71. doi: 10.1042/BST0380065. [DOI] [PubMed] [Google Scholar]
- 56.Kuczera T, Stilling RM, Hsia HE, Bahari-Javan S, Irniger S, et al. The anaphase promoting complex is required for memory function in mice. Learn Mem. 2010;18:49–57. doi: 10.1101/lm.1998411. [DOI] [PubMed] [Google Scholar]
- 57.Homer H. New insights into the genetic regulation of homologue disjunction in mammalian oocytes. Cytogenet Genome Res. 2011;133:209–222. doi: 10.1159/000324118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eguren M, Manchado E, Malumbres M. Non-mitotic functions of the Anaphase-Promoting Complex. Semin Cell Dev Biol. 2011;22:572–578. doi: 10.1016/j.semcdb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 60.Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol. 1998;33:61–66. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 61.Chambers AL, Downs JA. The contribution of the budding yeast histone H2A C-terminal tail to DNA-damage responses. Biochem Soc Trans. 2007;35:1519–1524. doi: 10.1042/BST0351519. [DOI] [PubMed] [Google Scholar]
- 62.Conde F, Refolio E, Cordón-Preciado V, Cortés-Ledesma F, Aragón L, et al. The Dot1 histone methyltransferase and the Rad9 checkpoint adaptor contribute to cohesin-dependent double-strand break repair by sister chromatid recombination in Saccharomyces cerevisiae. Genetics. 2009;182:437–446. doi: 10.1534/genetics.109.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burgess RJ, Zhang Z. Roles for Gcn5 in promoting nucleosome assembly and maintaining genome integrity. Cell Cycle. 2010;9:2979–2985. doi: 10.4161/cc.9.15.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lévesque N, Leung GP, Fok AK, Schmidt TI, Kobor MS. Loss of H3 K79 trimethylation leads to suppression of Rtt107-dependent DNA damage sensitivity through the translesion synthesis pathway. J Biol Chem. 2010;285:35113–35122. doi: 10.1074/jbc.M110.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vempati RK. DNA damage in the presence of chemical genotoxic agents induce acetylation of H3K56 and H4K16 but not H3K9 in mammalian cells. Mol Biol Rep. 2011;39:303–308. doi: 10.1007/s11033-011-0739-9. [DOI] [PubMed] [Google Scholar]
- 66.Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, et al. Current protocols in molecular biology. 1995. Wiley, NY.