Abstract
Objective
To assess the nine-month HIV-free survival of children with two strategies to prevent HIV mother-to-child transmission.
Design
Non-randomized interventional cohort study.
Setting
Four public health centers in Rwanda.
Participants
Between May 2005 and January 2007, all consenting HIV-infected pregnant women were included.
Intervention
Women could choose the mode of feeding for their infant: breastfeeding (BF) with maternal highly active antiretroviral therapy (HAART) for six months or formula feeding (FF). All received HAART from 28 weeks of gestation. Nine-month cumulative probabilities of HIV transmission and HIV-free survival were determined using the Kaplan-Meier method, and compared using the log-rank test. Determinants were analyzed using a Cox model analysis.
Results
Of the 532 first-live born infants, 227 (43%) were BF and 305 (57%) were FF. Overall, seven (1.3%) children were HIV-infected of whom six were infected in utero. Only one child in the BF group became infected between month 3 and month 7, corresponding to a 9-month cumulative risk of postnatal infection of 0.5% (95% CI 0.1–3.4%; P=0.24) with BF. Nine-month cumulative mortality was 3.3% (95% CI 1.6–6.9%) in the BF arm group and 5.7% (95% CI 3.6–9.2%) for the FF group (P=0.20). HIV-free survival by nine months was 95% (95% CI 91–97%) in the BF group and 94% (95% CI 91–96%) for the FF group (P=0.66), with no significant difference in the adjusted analysis (adjusted hazard ratio for BF: 1.2 (95% CI 0.5–2.9%).
Conclusions
Maternal HAART while BF could be a promising alternative strategy in resource-limited countries.
Keywords: Adult; Antiretroviral Therapy, Highly Active; methods; Breast Feeding; Cohort Studies; Female; HIV Infections; drug therapy; epidemiology; transmission; HIV-1; Humans; Infant; Infant Formula; administration & dosage; Infant, Newborn; Infectious Disease Transmission, Vertical; prevention & control; Post-Exposure Prophylaxis; methods; Pregnancy; Pregnancy Complications, Infectious; drug therapy; epidemiology; Rwanda; epidemiology; Survival Analysis
Keywords: Africa, PMTCT, HIV, antiretroviral, breastfeeding, formula feeding, postnatal transmission
INTRODUCTION
In developed countries, comprehensive prevention of mother-to-child transmission(PMTCT) programs that use antiretroviral treatment(ART) prophylaxis and alternatives to breastfeeding(BF), have been shown to be very effective, resulting in overall HIV perinatal transmission rates of less than 2%[1]. Similarly, low rates of perinatal transmission have been demonstrated in PMTCT programs in low-income countries[2–4]. However, when BF is employed, especially for extended periods and mixed with formula feeding(FF), subsequent transmission through BF clearly reduces the long-term efficacy of these prophylactic regimens[5–8]. Exclusive breastfeeding(EBF) with early cessation[9] and FF are two conceivable alternatives to prevent postnatal HIV transmission[4]. Several studies from low-income countries have reported postnatal transmission rates of less than 2% using alternatives to BF without a higher overall mortality rate[2, 10]. However, in operational settings, these alternatives have shown a worrying increase in overall mortality among FF children, probably due to the lack of access to clean water, incorrect dilution of formula, and inadequate access to formula or postnatal follow-up[11–12]. As a result, current World Health Organization(WHO) guidelines recommended EBF with early weaning if replacement feeding is acceptable, feasible, affordable, sustainable and safe [13]. Unfortunately, there are still concerns: EBF cannot completely avoid HIV transmission, but early weaning may lead to an increased morbidity in children[12]. With the increased access to ARV in Africa, two other approaches could be conceivable as well: giving prophylactic antiretroviral treatment to breastfed children born to HIV positive mothers[14–16] or to provide maternal highly active antiretroviral treatment(HAART) while BF[2, 17–18]. Although several studies provide indirect evidence for the efficacy of this latter intervention[19–21], well-designed studies evaluating the efficacy of this strategy in poor countries are virtually non-existent or on-going. No single study has formally compared maternal BF with HAART to FF within the same cohort in resource-limited countries.
Therefore, we implemented an interventional cohort study to assess these two interventions to prevent postnatal MTCT of HIV-1 in Rwanda: BF combined with maternal HAART for a maximum duration of six months versus exclusive FF. The purpose of this paper is to report the efficacy of these interventions based on HIV-1 transmission, mortality and HIV-free survival until nine months of age.
METHODS
Study Design and Setting
This was a non-randomized, interventional cohort study, named “AMATA”, which means “milk” in the local language (Kinyarwanda). It was conducted at four government-run health facilities: one rural health centre (70 km from Kigali, the capital city), one semi-rural health centre (15 km from Kigali) and two urban sites in Kigali. Each of these four sites had antenatal care services with PMTCT programs in place, providing access to treatment for HIV infection. Routine HIV testing and HAART are provided free of charge in Rwanda.
Study population
Between May 2005 and January 2007, all HIV-infected pregnant women entering the PMTCT programs in these four centres were invited to participate in the study from 28 weeks of gestation. The women were counselled about the risk of higher morbidity with FF and the risk of HIV through BF during two information prenatal sessions; those consenting to participate were given the choice of infant feeding mode at inclusion, prior to delivery.
Interventions
All enrolled women received HAART from 28 weeks of gestation irrespective of the study group. In line with the Rwandan national protocol, pregnant women with CD4 cell count <350 cells/μL and/or WHO clinical stage 4 were considered eligible for lifelong HAART, consisting (in 2005) of combined therapy with stavudine(D4T), lamivudine(3TC) and nevirapine(NVP). For the remainder (WHO clinical stage 1, 2, 3 and CD4 cell count >350/μL), a prophylactic HAART regimen containing zidovudine(AZT), 3TC and efavirenz(EFV) was started. EFV was chosen to avoid the hepatotoxicity due to NVP for women having high CD4 cell counts[22]. AZT was preferred over D4T in prophylactic HAART given its well documented efficacy in PMTCT.
After inclusion, those choosing FF had specific education sessions with the counsellors on safe preparation of formula, which was provided free of charge until six months of age. These women were also informed that they would be given an injection of 5 mg oestradiol just after delivery, to help suppressing the breast milk production. In the BF group, women were counselled to exclusively breastfeed until six months and then to perform rapid weaning. A supplement of “sosoma” (mixture of soya, sorghum and maize) was given during the weaning period for one month and was also given to the FF group. All food supplementation was stopped at seven months unless severe malnutrition (weight-for-age below the fifth percentile) was diagnosed, after excluding underlying medical problems. We used the Centers for Disease Control and Prevention curves[23], as the 2006 international WHO curves[24] were not available at the start of the study. After birth, prophylactic HAART was stopped for the non lifelong eligible women that had opted for FF unless they became eligible for HAART in between. For BF women, prophylactic HAART was given until seven months of age (until one month after weaning) to protect infants against the risk of postnatal transmission associated with mixed feeding if abrupt weaning was not done. In each case, a backbone of AZT and 3TC was given for seven days after stopping NVP/EFV, to reduce the risk of resistance[25].
Following WHO recommendations, all newborn infants exposed to HIV received NVP 2 mg/kg at birth and AZT 4 mg/kg twice-daily for seven days. At six weeks of age, cotrimoxazole was given to all infants until nine months of age and continued after for those infected[22].
Follow-up procedures
Follow-up of mother-infant pairs was done by the AMATA team, consisting of physicians, nurses and counsellors. Mother-infant pairs were examined clinically at birth (within 48 hours) and follow-up visits were scheduled at fifteen days, six weeks and three, six, seven and nine months post-partum. At each visit, adherence to HAART (pill count and questionnaires) and the feeding method were assessed by maternal interview and clinical examination and women were counselled accordingly. Breast health and feeding techniques were evaluated at each visit. If a breast-fed infant had received any liquids or solids even once (with the exception of drugs), they were then considered to have received “mixed feeding”, using the WHO definitions[26]. In case of problematic or insufficient BF, the need to add additional feeding was discussed and mixed feeding was an acceptable option. All women were counselled about family planning; those on an EFV-containing regimen were given three-monthly progesterone injections.
All care was provided for free, and transportation costs were reimbursed by the project. CD4 cell count and HIV-RNA plasma viral load(VL) of all women were measured at inclusion, at delivery (less than 48 hours after having given birth) and at six months postpartum.
CD4 cell count was done using a FACSCalibur instrument (BD Bioscience, Becton, Dickinson and company, USA). VL tests were done with the Real-time polymerase chain reaction(PCR) testing on COBAS TaqMan® 48 Analyzer (Roche, Switzerland).
The HIV status of the infant was established with a HIV-1 DNA PCR assay using the Amplicor technique (version 1.5, Roche Molecular Systems, Pleasanton, CA).
Diagnosis of HIV infection in children
HIV DNA PCR tests were performed in children at birth, at six weeks, three months, seven and nine months of age. Infants were defined as having been infected in utero if the HIV-1 DNA PCR was positive within 48 hours of life and confirmed to be positive in the next test. Transmission was considered to have occurred peripartum if a negative result at birth was followed by a positive result at six weeks of age. The blood test done at day 15 was stored and performed only in case of different results between birth and six weeks PCR tests. Children with a positive HIV DNA PCR after day 15 were considered infected through breast milk if blood samples from birth and day 15 were negative. Every positive HIV test for children was confirmed by a second positive test before a final diagnosis of HIV infection was accepted.
Mortality and morbidity data were obtained through hospital inpatient and outpatient records or by interview of the family member at each scheduled visits.
Main Outcome Measures
Three main outcomes were measured at nine months of age among the liveborn children:
Cumulative incidence of mother-to-child HIV transmission (peri-natal and postnatal)
Cumulative infant mortality.
Cumulative incidence of HIV-free survival: HIV infection or death whichever came first.
Statistical Analysis
Every first-born liveborn child was included in the analysis in an intent-to-feed analysis, BF+HAART or FF. The allocation to a feeding group was based on the feeding option chosen before delivery. All newborns that died before 48 hours of life linked to anoxia and prematurity before any feeding were not included in the analysis, to exclude deaths unrelated to the feeding option. The Student t-test, Wilcoxon rank-sum test, χ2 test and Fisher’s exact test were used to determine differences in means, medians values and proportions between groups. Cumulative probability of events in the first nine months of life was calculated using Kaplan-Meier estimates with 95% Confidence Intervals(CI). This was appropriate since intervals between tests were less than three months[27]. Comparisons were made using the log-rank test. Infants who were lost to follow-up(LTFU) before reaching study end points were recorded at the date of their last HIV test available. LTFU was defined as having missed planned appointments after having been actively sought at home. The timing of acquisition of infection was estimated to have occurred midway between the dates of the last negative and the first positive test. Determinants for nine-month HIV-free survival were explored using a Cox model adjusted on the following variables : infant feeding modalities, and co-variants: maternal CD4 count at delivery, time on HAART prior to delivery, access to clean water, level of the mother’s education, mode of delivery and birth weight and sex of the infant. VL at delivery was not included, since data were missing for 18% of women. Data were entered and stored using Access TM software, data analysis was done with Epi Info TM(version 3.3.2), SPSS TM(version 16) and Stata TM(Version 9).
Sample size
The main judgement criterion was HIV-free survival, expected to be 95% overall at nine-months of age. To be able to detect a 7.5% difference in HIV-free survival (a constant HR of 0.38) with a power of 80%[28], 205 patients needed to be included in each group. Allowing for 10% LTFU, we decided to include at least 225 pregnant women in each group.
Ethics
The study was designed and conducted in collaboration with the Rwandan Ministry of Health. The National Ethical Committee of Rwanda, having an international registration, approved the study with annual evaluation of the study progress reports and re-evaluation of the protocol. An informed consent has been obtained for all patients included in this cohort.
RESULTS
Study population
From May 2005 to January 2007, 562 HIV-positive pregnant women were included in the study, of whom 240(42.7%) preferred BF under HAART and 322(57.3%) women chose FF. Eleven(2.0%) women were LTFU prior to delivery, and 551 infants were born during the study, of whom 11 were twins (second born excluded) and 14(2.5%) were stillborns. Overall, five(1.0%) newborns died within 48 h of life (four were premature, and one infant died on the second day of a neonatal infection that was clinically present at the time of birth; the mother had fever during expulsion). None of these five deaths were related to feeding exposure and were excluded from further analysis. Therefore 532 mothers-infant pairs were included in this analysis (Figure 1).
Figure 1.
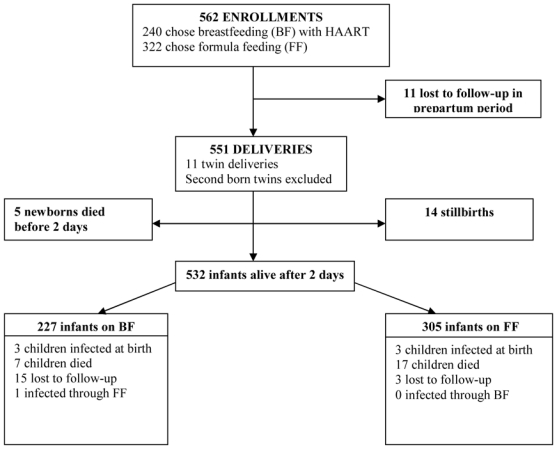
Amata cohort profile. Rwanda, 2005–2007
Delivery characteristics of these women and infants are summarized in Table 1. Women choosing BF were slightly younger, had higher CD4 cell count and were less likely to be eligible for lifelong HAART than women choosing FF. They had also less access to clean water. VL at delivery was similar in both groups but 95(17.8%) VL were missing at delivery due to laboratory technical problems. Women in the FF were also more likely to have started life-long ART prior to inclusion in the study. Overall, 256(48.1%) women were on HAART for life after inclusion.
Table 1.
Baseline and delivery characteristics of HIV infected mothers and their liveborn infants by infant-feeding group: formula feeding(FF) or breastfeeding(BF) with maternal HAART; AMATA cohort, Rwanda, 2005–2007
| Overall N= 532 |
F F N= 305 |
BF + HAART N= 227 |
P value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Maternal age | 532 | 305 | 227 | ||||
| Median (years) | 29 | 29 | 28 | 0.000 | |||
| Interquartile range | 25–33 | 26–34 | 24–32 | ||||
| WHO clinical stage (N) | 532 | 305 | 227 | ||||
| 1 & 2 | 470 | 88.3% | 266 | 87.2% | 204 | 89.9% | 0.345 |
| 3 & 4 | 62 | 11.7% | 39 | 12.8% | 23 | 10.1% | |
| Maternal CD4 cell count (N) | 471 | 274 | 197 | ||||
| Mean (/mm3) | 461 | 434 | 498 | 0.005 | |||
| Interquartile range (/mm3) | 279–592 | 241–545 | 326–659 | ||||
| Delivery maternal VL copies | 431 | 247 | 184 | ||||
| <40 copies/ml | 221 | 51.3% | 126 | 51.0% | 94 | 51.6% | 0.898 |
| <1000 copies/ml | 385 | 89.3% | 225 | 91.1% | 160 | 86.9% | 0.168 |
| Duration of HAART (N) | 523 | 300 | 223 | ||||
| Mean (weeks) | 19.5 | 21.8 | 16.4 | 0.766 | |||
| Interquartile range (weeks) | 7–14 | 6–15 | 8–13 | ||||
| Eligible for HAART (N) | 256 | 48.1% | 164 | 53.8% | 92 | ||
| Started at enrolment | 78 | 14.7% | 42 | 13.8% | 36 | 15.9% | 0.007 |
| Started before enrolment | 178 | 33.5% | 122 | 40.0% | 56 | 24.7% | |
| Educational level (N) | 518 | 301 | 217 | ||||
| None/Primary | 461 | 89.0% | 267 | 88.7% | 194 | 89.4% | 0.802 |
| Secondary/University | 57 | 11.0% | 34 | 11.3% | 23 | 10.6% | |
| Water type/source (N) | 518 | 301 | 217 | ||||
| Piped indoor | 61 | 11.8% | 39 | 13.0% | 22 | 10.1% | 0.043 |
| Public tap | 363 | 70.1% | 219 | 72.8% | 144 | 66.4% | |
| Source water | 85 | 16.4% | 40 | 13.3% | 45 | 20.7% | |
| River/Stagnant water | 9 | 1.7% | 3 | 1.0% | 6 | 2.8.% | |
| Electricity at home (N) | 520 | 302 | 218 | ||||
| Yes | 117 | 22.5% | 74 | 24.5% | 43 | 19.7% | 0.197 |
| Place of delivery (N) | 532 | 305 | 227 | ||||
| Health facilities | 485 | 91.2% | 275 | 90.2% | 210 | 92.5% | 0.345 |
| Home | 47 | 8.8% | 30 | 9.8% | 17 | 7.5% | |
| Mode of delivery (N) | 532 | 305 | 227 | ||||
| Caesarian section | 86 | 16.2% | 47 | 15.4% | 39 | 17.0% | 0.583 |
| Gestational age ( N) | 532 | 305 | 227 | ||||
| Mean (weeks) | 39.7 | 39.5 | 39.8 | 0.032 | |||
| Interquartile range (Weeks) | 39–41 | 39–41 | 39–41 | ||||
| Infant Birth Weight (N) | 532 | 305 | 227 | ||||
| Mean (kg) | 3.1 | 3.1 | 3.1 | 0.281 | |||
| Interquartile range (kg) | 2.8–3.4 | 2.8–3.4 | 2.8–3.4 | ||||
| <2.5 kg | 2.6 | 2.0 | 6 | 0.038 | |||
| Infant sex (N) | 532 | 305 | 227 | ||||
| Female | 262 | 49.2% | 152 | 49.8% | 111 | 48.9% | 0.830 |
Tolerance of and adherence to the interventions
No mother interrupted her HAART treatment, although 11(2.1%) required drug substitution due to toxicity, one during pregnancy and ten after delivery. Of all these side effects, seven were due to AZT-related anaemia, and two were due to D4T-related lipodystrophy. In addition, one woman interrupted EFV treatment due to a severe depression and one case of rash due to NVP was observed. No HIV-related deaths were reported. Adherence to exclusive BF was very good (94.2%). None of the infants of the 13 women who practiced mixed feeding were infected.
Outcomes
Overall, seven children were infected with HIV-1 of which six in utero (three in each infant feeding group). Only one child in the BF group became infected between month 3 and month 7 and no child acquired HIV infection between birth and nine months in the FF group. In the BF group, the cumulative probability of HIV-1 transmission at six weeks and nine months was 1.3% (95%CI 0.4–4.1%) and 1.8% (95%CI 0.7–4.8%), respectively. In the FF group, these cumulative probabilities were similar at six weeks and nine months estimated to be 1% (95%CI: 0.3–3.0%). Over the first nine months, the probability of HIV-1 transmission was not statistically different between both groups (log-rank test, P=0.43). The one infant who acquired HIV infection in the BF group represented a cumulative risk of postnatal infection of 0.5% (95%CI 0.1–3.4%; P=0.24) at nine months of life. Although the infecting BF mother was receiving HAART, she had been suffering from gastritis with severe vomiting from four months post-partum. This, in combination with prolonged fasting for religious reasons, made her adherence and drug absorption questionable. Finally, while hospitalized for severe vomiting, she suddenly ceased BF at five months. Her plasma VL result at six months postpartum showed a VL of 1600 copies/ml.
Overall, by nine months of age, seven(3.1%) children had died in the BF group and seventeen(5.6%) in the FF group. For the 22 infants who died before 9 months of age and were HIV-negative at birth, a negative PCR result was available for all of them by 3 months prior to death. The nine-month cumulative probability of death for the BF group was 3.3% (95%CI 1.6–6.9%) and 5.7% (95%CI 3.6–9.2%) for the FF group, with no statistically significant difference (log-rank test, P=0.20).
Among the 532 exposed liveborn children, 29 were HIV-1 infected or dead at nine month of age. As shown in figure 2, the nine-month cumulative HIV-free survival was 95% (95%CI 91–97%) in the BF group and 94% (95%CI 91–96%) in the FF group (log-rank test, P=0.66).
Figure 2.
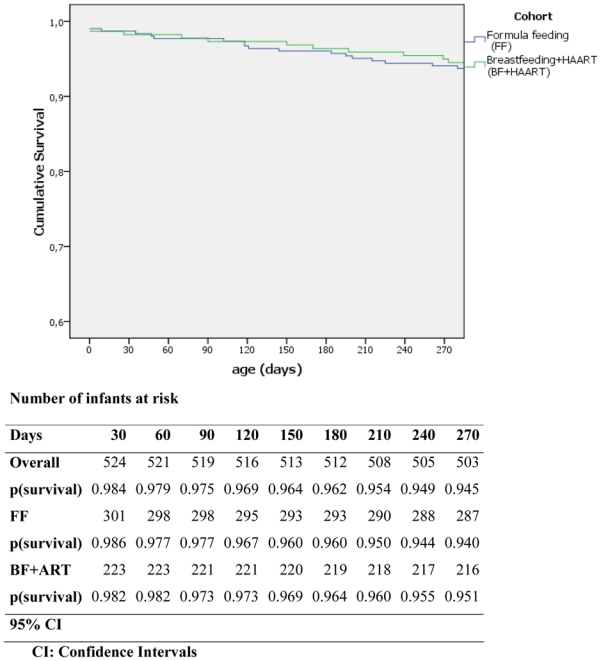
Kaplan Meier HIV-free survival in infants in the formula and breastfed with maternal HAART group. AMATA cohort, Rwanda, 2005–2007
After adjustment for potential confounders in the adjusted analysis, no significant difference in HIV-free survival was seen between the two intervention groups, with an adjusted HR of 1.2 (95%CI 0.5–2.9%) for BF versus FF (Table 2). The only statistically significant factors retained were maternal CD4 cell count below 350/μL and birth weight below 2.5 kg. If LTFU was considered as dead/infected in a sensitivity analysis, an adjusted HR of 1.9 (95%CI 0.9–4.0%) for BF compared to FF was found (data not shown).
Table 2.
Multivariate Cox model analysis to assess the correlates of HIV infection or death within the first nine months of life in, Rwanda, 2005–2007 (N=465)a
| HRb | P | Adjusted HRb | P | |
|---|---|---|---|---|
| Infant feeding group | ||||
| Formula feeding (FF) | 1 | 1 | ||
| Breastfeeding (BF) | 1.0 (0.4–2.3) | 0.99 | 1.2 (0.5–1.9) | 0.68 |
| Baseline CD4 count at enrolment | ||||
| > 350 cells/μL | 1 | 1 | ||
| ≤ 350 cells/μL | 2.6 (1.1.6.0) | 0.03 | 2.7 (1.1–6.4) | 0.02 |
| Time on HAART prior to delivery | ||||
| > 2 months | 1 | 1 | ||
| ≤ 2 months | 1.4 (0.6–3.2) | 0.45 | 1.3 (0.5–3.0) | 0.55 |
| Access to running water | ||||
| Yes | 1 | 1 | ||
| No | 2.9 (0.4–21.7) | 0.30 | 2.4 (0.3–18.8) | 0.39 |
| Education | ||||
| Post primary | 1 | 1 | ||
| None/Primary | 2.4 (0.7–8.1) | 0.16 | 2.2 (0.6–7.6) | 0.22 |
| Caesarian section | ||||
| Yes | 1 | 1 | ||
| No | 4.0 (0.5–30.0) | 0.17 | 3.8 (0.5–28.6) | 0.19 |
| Infant sex | ||||
| Female | 1 | 1 | ||
| Male | 2.0 (0.8–5.0) | 0.12 | 2.3 (0.9–5.7) | 0.08 |
| Infant birth weight | ||||
| > 2.5 kg | 1 | 1 | ||
| ≤ 2.5 kg | 3.2 (1.2–8.6) | 0.02 | 3.3 (1.1–9.4) | 0.03 |
Excluding 67 mother-infant pairs with missing data
HR: hazard ratio (95% confidence interval)
DISCUSSION
This is the first study reporting the field efficacy of two concomitant PMTCT interventions to reduce postnatal transmission of HIV. Whereas FF has been the recommended option in developed countries, this intervention is not feasible for many African women and carries a higher risk of morbidity and mortality in resource-limited settings that needs to be balanced against its benefits in preventing postnatal transmission. With the antiretroviral era in Africa since 2004, providing HAART to women while BF is another conceivable option, offering a culturally appropriate alternative. In our study, following a backbone of maternal HAART according to 2006 WHO criteria[25], both postnatal approaches were found to be safe and effective, with similar nine-month HIV-free survival of about 95%. The overall nine-month transmission rate was about 1.3%, one of the lowest MTCT rate ever reported from low-income countries[29] and similar to those reported from industrialized countries. We attribute this strong effect to the combination of starting HAART prior to delivery, antiretroviral prophylaxis to the newborn and continued HAART for six months while BF.
In a meta-analysis, the risk of HIV transmission by EBF was estimated around 0.7% per child-month of BF follow-up[6]. In other recent African studies, the lowest reported rates of transmission with EBF by 3–6 months have varied between 1.3 % and 5.6%[2–3, 30–31]. Although these studies are difficult to compare directly, our postnatal transmission rate for women BF under HAART would suggest a significant reduction in postnatal transmission. We found only one other published report (DREAM Program), assessing the efficacy of BF combined with maternal HAART to reduce postnatal transmission (additional 0.8% postnatal transmission in the BF group with HAART), but without a FF comparison group at the same period of time[2].
The results with FF were also encouraging, showing an overall mortality lower than in infants born to HIV-uninfected mothers in Rwanda[32]. The total mortality of children followed in the AMATA trial was 4.7% at 9 months, without statistically significant differences between the BF and FF groups. This is in contrast with some studies that have shown an overall increased mortality in FF infants and we suggest that this is because both studies (DREAM and AMATA) were strongly focused on patients’ counseling, education, and good quality of care and adaptation of the feeding option to the mother’s choice. These data also confirm the importance of postnatal follow-up for exposed children, very often neglected when PCR-testing is not available, resulting in limited access to care and information.
We chose a cohort design instead of a randomized clinical trial to avoid the ethical problems associated with a fixed allocation of the infant feeding practices[33]. In addition, this design helped to assess the mother’s choice of feeding method with FF with a close follow-up and education for the preparation of milk. We believe this was a key factor in encouraging good adherence[34]. To be able to offer women a culturally acceptable method of feeding, that matched HIV transmission rates using FF, resulted in very important public health implications.
This study had several limitations. First, while adjusting for the cohort design our findings remained essentially unchanged in multivariate analysis, residual confounding may still be possible. Second, these outcomes were obtained within a specific study research setting with high quality of care and follow-up. Consequently, these findings could not be generalized to the country level. Third, even though the overall tolerance of HAART was very good in this study, toxicity of HAART could be problematic in settings with fewer resources for follow-up.
Finally, this study did not have the power to detect small differences in postnatal HIV-1 infection or mortality between the two approaches.
There are some public health caveats, as well. In most low-income countries, access to HAART services is still limited and particularly challenging for pregnant women during the last trimester of pregnancy. Even if accessible everywhere, cost implications of dissemination of this PMTCT model will have to be considered in each country. There is also the potential of an increased risk of infection with resistant viruses for those newborns infected while breastfed from mothers taking HAART[35]. This issue may become less problematic now since, based on a recent pediatric study, WHO currently recommends to treat all young HIV infected children with protease inhibitors in case of recent exposure to NVP[36–37].
Several relevant questions remain to be addressed. How will mixed feeding affect the postnatal transmission rate, even if HAART is taken? If HAART does protect against transmission during mixed feeding, then could HAART be continued for longer periods (e.g. up to 1 year) or during all the BF period? It also remains to be seen whether sufficient levels of adherence to prophylactic HAART can be achieved to avoid emergence of drug-resistance, especially when HAART is given for a longer period.
There may be several alternatives to replacement feedings such as diluted, boiled cow’s milk or heated expressed breast milk[38] that might be more easily implemented in remote places where provision of HAART or FF can be problematic.
We conclude that BF when combined with maternal HAART can be associated with a minimal risk of postnatal transmission, similar to the FF one in our cohort, and with HIV transmission rates as low as those in high-income countries. A key implication of this study is that women can be offered a choice in infant feeding options, both of which could be safe and effective, given regular postnatal follow-up and counseling. This information would be useful in guiding recommendations on the safest and best infant feeding modalities according to the different African contexts combined with the full spectrum of antiretroviral strategies, including ART for those in need.
Acknowledgments
We thank all families of children enrolled in the study. We appreciated the constant collaboration of Ministry of health of Rwanda. We thank Joseph Viankandondera for his advice and for sharing with us his valuable experience in PMTCT clinical trials in Rwanda. We also thank Tony Reid for reviewing the manuscript in detail.
Funding: This study was funded by the ministry of foreign affairs of Grand-Duché of Luxembourg (Lux-Development, projects RWA 021, INT 107, INT108 ESTHER Phase 2 Luxembourg)
Footnotes
Contributors: Cécile Alexandra Peltier (CAP), Gilles François Ndayisaba (GFN), Philippe Lepage (PL), Johan van Griensven (JvG), Valériane Leroy (VL), Christine Omes (CO), Patrick Cyaga Ndimubanzi (PCN), Olivier Courteille (OC), Vic Arendt (VA).
Role of contributors: VA, CO and CAP designed and conceived the study; GFN, PCN, OC, CO and CAP implemented the study. JvG and GFN analyzed the data; VL, PL, CAP, JvG wrote the paper, OC coordinated laboratory collection and quality of analysis, PCN and GFN coordinated data collection and conceived the database, VL, PL and VA also checked the methodology and results of statistical analysis. All authors read and approved the final version of the report.
Conflict of interest: None
Reference List
- 1.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of Highly Active Antiretroviral Therapy. Clin Infect Dis. 2005;40:458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 2.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):65–S71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 3.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 4.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 5.Magoni M, Bassani L, Okong P, Kituuka P, Germinario EP, Giuliano M, et al. Mode of infant feeding and HIV infection in children in a program for prevention of mother-to-child transmission in Uganda. AIDS. 2005;19:433–7. doi: 10.1097/01.aids.0000161773.29029.c0. [DOI] [PubMed] [Google Scholar]
- 6.Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 7.Mbori-Ngacha D, Nduati R, John G, Reilly M, Richardson B, Mwatha, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: A randomized clinical trial. JAMA. 2001;286:2413–20. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leroy V, Karon JM, Alioum A, Epkini ER, van de Perre P, Greeberg AE, et al. Postnatal transmission of HIV-1 after a maternal short-course zidovudine peripartum regimen in West Africa. AIDS. 2003;17:1493–501. doi: 10.1097/00002030-200307040-00010. [DOI] [PubMed] [Google Scholar]
- 9.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 10.Leroy V, Ekouevi DK, Becquet R, Viho I, Dequae-Merchadou L, Tonwe-Gold B, et al. ANRS 1201/1202 DITRAME PLUS Study Group. 18-month effectiveness of short-course antiretroviral regimens combined with alternatives to breastfeeding to prevent HIV mother-to-child transmission. PLoS ONE. 2008;3:e1645. doi: 10.1371/journal.pone.0001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagaayi J, Gray RH, Brahmbhatt H, Kigozi G, Nalugoda F, Wabwire-Mangen F, et al. Survival of infants born to HIV-positive mothers, by feeding modality, in Rakai, Uganda. PLoS ONE. 2008;3:e3877. doi: 10.1371/journal.pone.0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollins NC, Becquet R, Bland RM, Coutsoudis A, Coovadia HM, Newell ML. Infant feeding, HIV transmission and mortality at 18 months: the need for appropriate choices by mothers and prioritization within programmes. AIDS. 2008;22(17):2349–57. doi: 10.1097/QAD.0b013e328312c740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organisation (WHO) HIV and infant feeding technical consultation held on behalf of the inter agency task team (IATT) on prevention of HIV infections in pregnant women, mothers, and their infants. Consensus statement; Geneva, Switzerland. October 25–27, 2006; www.who.int/reproductive-health/stis/mtct/infantfeedingconsensusstatement.pdf. [Google Scholar]
- 14.Kilewo C, Karlsson K, Massawe A, Lyamuya E, Swai A, Mhalu F, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: The Mitra Study. J Acquir Immune Defic Syndr. 2008;48:315–23. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 15.Mofenson LM. Antiretroviral prophylaxis to reduce breast milk transmission of HIV type 1: New data but still questions. J Acquir Immune Defic Syndr. 2008;48:237–40. doi: 10.1097/QAI.0b013e31817dc89e. [DOI] [PubMed] [Google Scholar]
- 16.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, Mipando L, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 17.Arendt V, Ndimubanzi CP, Vyankandondera J, Ndayisaba G, Muganda J, Courteille O, et al. Effectiveness of antiretroviral therapy in breastfeeding mothers to prevent post-natal vertical transmission in Rwanda [Oral]. 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 22–25 July 2007; Sydney, Australia. p. Abstract TUAX102. [Google Scholar]
- 18.Tonwe-Gold B, Ekouevi DK, Viho I, Amani-Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano M, Guidotti G, Andreotti M, Pirillo MF, Villani P, Liotta G, et al. Triple antiretroviral prophylaxis administered during pregnancy and after delivery significantly reduces breast milk viral load: a study within the Drug Resource Enhancement Against AIDS and Malnutrition Program. J Acquir Immune Defic Syndr. 2007;44:286–91. doi: 10.1097/QAI.0b013e31802c5441. [DOI] [PubMed] [Google Scholar]
- 20.Lehman DA, Chung MH, John-Stewart GC, Richardson BA, Kiarie J, Kinuthia J, et al. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS. 2008;22:1475–85. doi: 10.1097/QAD.0b013e328302cc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro RL, Ndung’u T, Lockman S, Smeaton LM, Thior I, Wester C, et al. Highly active antiretroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA, but not DNA, in breast milk. J Infect Dis. 2005;192:713–9. doi: 10.1086/432489. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Antiretroviral drugs and the prevention of mother to child transmision of HIV infection in resource-limited settings. Recommendation for a public health approach. (2005 Revision); Geneva, Switzerland. 28–29 June 2005. [Google Scholar]
- 23.CDC Growth Charts for the United States : Methods and Development. Vital and Health Statistics Series Report 11. 2000;246:30–37. [PubMed] [Google Scholar]
- 24.WHO. New international Child Growth Standards for infants and young children. [Accessed 27 April 2006]. www.who.int/mediacentre/news/releases/2006/pr21/en/index.
- 25.WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants, towards universal access: recommendations for a public health approach. 2006. www.who.int/hiv/pub/guidelines/pmtct/en/ [PubMed]
- 26.WHO, UNAIDS, UNICEF. HIV transmission through breastfeeding: a review of available evidence- an update from 2001 to 2007. update.whqlibdoc.who.int/publications/2008/9789241596596_eng.pdf.
- 27.Alioum A, Cortina-Borja M, Dabis F, Dequae-Merchadou L, Haverkamp G, Hughes J, et al. Estimating the efficacy of interventions to prevent mother-to-child transmission of human immunodeficiency virus in breastfeeding populations: comparing statistical methods. Am J Epidemiol. 2003;158:596–605. doi: 10.1093/aje/kwg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. In: Colton T, et al., editors. Statistics in Medicine. Vol. 1. UK: 1982. pp. 121–129. [DOI] [PubMed] [Google Scholar]
- 29.Timothy TR, Masaba R, Ndivo Zeh C, Borkowf C, Thigpen M, et al. Prevention of mother-to-child transmission of HIV-1 among breastfeeding mothers using HAART: The Kisumu breastfeeding study, Kisumu, Kenya, 2003–2007[Oral] CROI; Boston, USA: Feb 3–6, 2008. p. Abstract 45aLB. [Google Scholar]
- 30.Becquet R, Bequet L, Ekouevi DK, Viho I, Sakarovitch C, Fassinou P, et al. Two-year morbidity-mortality and alternatives to prolonged breast-feeding among children born to HIV-infected mothers in Cote d’Ivoire. PLoS Med. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips ML. Options for breastfeeding mothers with HIV. Lancet Infect Dis. 2007;7:183. doi: 10.1016/s1473-3099(07)70038-5. [DOI] [PubMed] [Google Scholar]
- 32.Institut National de la Statistique du Rwanda (INSR), ORC Macro. Rwanda Demographic and Health Survey 2005. Vol. 2005 Calverton, Maryland, USA: INSR and ORC Macro; 2006. [Google Scholar]
- 33.Nataraj S. Ethical considerations in research on preventing mother-to-child HIV transmission. Monash Bioeth Rev. 2005;24:28–39. doi: 10.1007/BF03351440. [DOI] [PubMed] [Google Scholar]
- 34.Stulac SN, Franke MF, Rugira IH, Uwamuhoro L, Bucyibaruta B, Iyamungo, et al. Successful implementation of replacement feeding for HIV exposed infants. [Oral]. HIV/AIDS Implementers’ meeting; Kigali, Rwanda. 16–19 June 2007; p. Abstract 251. [Google Scholar]
- 35.WHO. Expert Consultation on New and emerging evidence on the use of antiretroviral drugs for the prevention of mother-to-child transmission of HIV. Geneva, Switzerland: Nov 17–19, 2008. [Google Scholar]
- 36.WHO Technical Reference Group Paediatric. HIV Care. ART guideline review meeting; Geneva, Switzerland. 10–11 April 2008. [Google Scholar]
- 37.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. CHER Study Team. Collaborators. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KA, Abrams BF, Coutsoudis A, Sibeko LN, Cheryk LA, Chantry CJ. Vitamin content of breast milk from HIV-1-infected mothers before and after flash-heat treatment. J Acquir Immune Defic Syndr. 2008;48:444–9. doi: 10.1097/QAI.0b013e31817beb8d. [DOI] [PMC free article] [PubMed] [Google Scholar]


