Abstract
Background
Asthma is a chronic inflammatory disease of the airways but recent studies have shown that alveoli are also subject to pathophysiological changes. This study was undertaken to compare hydrogen peroxide (H2O2) concentrations in different parts of the lung using a new technique of fractioned breath condensate sampling.
Methods
In 52 children (9-17 years, 32 asthmatic patients, 20 controls) measurements of exhaled nitric oxide (FENO), lung function, H2O2 in exhaled breath condensate (EBC) and the asthma control test (ACT) were performed. Exhaled breath condensate was collected in two different fractions, representing mainly either the airways or the alveoli. H2O2 was analysed in the airway and alveolar fractions and compared to clinical parameters.
Results
The exhaled H2O2 concentration was significantly higher in the airway fraction than in the alveolar fraction comparing each single pair (p = 0.003, 0.032 and 0.040 for the whole study group, the asthmatic group and the control group, respectively). Asthma control, measured by the asthma control test (ACT), correlated significantly with the H2O2 concentrations in the alveolar fraction (r = 0.606, p = 0.004) but not with those in the airway fraction in the group of children above 12 years. FENO values and lung function parameters did not correlate to the H2O2 concentrations of each fraction.
Conclusion
The new technique of fractionated H2O2 measurement may differentiate H2O2 concentrations in different parts of the lung in asthmatic and control children. H2O2 concentrations of the alveolar fraction may be related to the asthma control test in children.
Keywords: Paediatric asthma, exhaled airway markers, oxidative stress
Background
Asthma is a chronic inflammatory disease that is predominantly characterised by inflammatory processes of the airways. However, studies have shown that alveoli are also subject to pathophysiological changes and might play a central role for asthma control and severity of the disease [1-4]. In childhood, inflammation is mostly caused by allergic and eosinophilic changes [5], and so far, alveolar involvement has been demonstrated mainly by measurement of alveolar nitric oxide, a marker of eosinophilic inflammation. However, reactive oxygen species (ROS) like hydrogen peroxide (H2O2) seem to play a role in the pathophysiology of childhood asthma [6-8]. Jöbsis et al. demonstrated that in children with asthma, overall exhaled H2O2 is elevated compared to controls, but to date there is no account of the contribution of the alveoli to these elevated H2O2 concentrations [9].
Exhaled breath condensate (EBC) is a well-known method to collect inflammation mediators and other soluble particles in exhaled breath [10]. Until recently it has only been possible to detect markers like H2O2 in unfractionated breath condensate, which did not allow detecting the origin of production. A new method of fractionated sampling now provides the ability to collect condensate from different parts of the lung. By measuring ROS like H2O2, it may be possible to locate the origin of active inflammation and therefore weigh the contribution of the alveoli to the severity of asthma and asthma control. Whereas results for fractionated H2O2 measurements in adults with COPD are published, use of this technique in asthma and in children has not yet been described [11].
One of the aims of this study was to apply the new technique of fractionated breath condensate sampling in children. Through the new technique, the principal aim was to compare H2O2 in different condensate fractions in asthma. Our primary hypothesis was that airway H2O2 concentrations are significantly higher than alveolar concentrations. In addition we aimed to correlate these results with data of exhaled nitric oxide (FENO), lung function measurements and the asthma control test (ACT).
Methods
Subjects
Asthmatic patients (aged 9-17 years) were recruited from the asthma clinic of the Children's University Hospital Halle. Asthma was diagnosed clinically when children had episodic cough, breathlessness and wheeze responsive to bronchodilators according to International and American Thoracic Society (ATS) criteria.
Healthy non-atopic controls with no history of chronic cough, wheezing or other pulmonary symptoms and without any chronic disease involving the immune system (e.g. Crohn's disease, Diabetes and rheumatic diseases) were recruited in various outpatient clinics.
Subjects who were active smoking or had an acute respiratory infection during the previous two weeks were excluded.
Study design
First, subjects underwent clinical examination, an asthma questionnaire was filled out and atopic sensibilisation was tested. Atopic sensibilisation was diagnosed by RAST or prick test, atopy was defined by a serum-specific IgE > 0.34 kU/L or a positive skin prick test (wheal > 2 mm larger than negative control) to at least one antigen (D. pteronyssinus, D. farinae, cat, dog, grass pollen, birch pollen, Aspergillus fumigatus). Afterwards subjects completed FENO measurement, lung function testing, and collection of fractionated EBC in this chronological order. Subjects completed the study protocol within 4 hours.
This cross-sectional study was approved by the local ethics committee of the Martin-Luther-University Halle-Wittenberg. Written consent was obtained from the participant's parents and age-appropriate consent from the children themselves.
Power analysis: We assumed a probability of 70% achieving higher values of airway concentrations compared to alveolar concentrations as clinical relevant. With this probability and a significant level of α = 0.05 a sample size of 44 subjects is sufficient to achieve a power of 90% in a two-sided Wilcoxon signed-rank test.
Asthma questionnaire
To evaluate disease control for children 12 years or older the Asthma Control Test (ACT) was used, for children younger than 12 years the Childhood Asthma Control Test was used [12,13]. Due to different scoring systems, these tests were not comparable. Parents and subjects were asked for passive smoking histories.
Lung function tests
Bodyplethysmography and spirometry (Masterlab, Jaeger, Würzburg, Germany) were performed for measurement of FEV1 (forced expiratory volume in 1 second), VC (vital capacity), MEF25 (maximal expiratory flow at 25% of VC), MEF50 (maximal expiratory flow at 50% of VC), MEF75 (maximal expiratory flow at 75% of VC), ITV (intrathoracic volume), TLC (total lung capacity), RV%TLC (residual volume- total lung capacity ratio) and sRaw, tot (specific airway resistance) as previously reported for all subjects [14].
FENO -Measurement
FENO was measured for each subject using NiOX MINO® (Aerocrine, Sweden), at a flow rate of 50 mL/s, according to the manufacturer's instructions.
Collection of fractionated exhaled breath condensate
Exhaled breath condensate was collected according to the current ATS/ERS guidelines using ECoScreen 2® (Filt GmbH, Germany). This new system, constructed to collect fractionated breath condensate, passes captured air through two different chambers depending on the settings for the single exhaled breath volume and the threshold between the airway and alveolar fraction. The collector includes a spirometer for measuring exhaled volumes and peak flows.
For this study, the separation threshold has been set at one third of the exhaled breath volume based on the results of Möller et al. [11] which showed, based on exhaled CO2 profiles, the starting point of the alveolar plateau is at approximately one third of the exhaled breath volume. Exhaled breath volume was set to 1200 mL for subjects ≥ 60 kg, leading to sampling volumes of 400 mL for fraction 1 and 800 mL for fraction 2. Fraction 1 is thought to represent the airway fraction and fraction 2 the alveolar fraction. In children less than 60 kg the exhaled breath volume was proportionately weight-adjusted, with 60 kg and 1200 mL set as 100%. Subjects wore a nose clip to allow only orally exhaled breath condensate to be collected. Patients were asked to breathe slowly and regularly with an increased tidal breathing. Standardised breathing patterns were enforced by asking patients to breathe out until they heard a beep from the collecting machine.
The sampling period was ended after the total gas sampling volume was 300 L or 200 L depending on the subjects' tolerance. Sampling times and mean peak flow values were recorded. Breath condensate volumes for each fraction were measured and condensate immediately transferred for further analysis.
Analysis of H2O2 concentrations
For further analysis of H2O2 concentration, ECoCheck (Filt GmbH, Germany) was used as described by Gerritsen et al. [15]. With this device, the H2O2 value is obtained by a specific reaction of the substance with oxidase that is followed by amperometric detection. Values can be measured in a range of 15 to 10 000 nmol/L according to the manufacturer's instructions.
Data analysis
Data was processed using SPSS (version 12.0). Values are expressed as median and interquartile range (IQR). Correlations were measured using the Spearman rank correlation. Comparisons between groups were made using the Mann-Whitney-U test. Differences of H2O2 concentration between the fractions were analysed by the Wilcoxon signed-rank test, comparing each single related sample pair (paired difference test). Results with p < 0.05 were considered statistically significant.
Results
Patient characteristics
32 asthmatic and 20 non-asthmatic children were included in this study. Patient characteristics are shown in table 1. Approximately half of the asthmatic patients were prescribed inhaled corticosteroids (ICS) with a median dosage of 200 μg (IQR 0-400 μg).
Table 1.
Patients characteristics
| Asthmatic patients | Controls | p | |
|---|---|---|---|
| n | 32 | 20 | |
| Age (yr) | 12.5 (11.0-15.0) | 13.5 (12.0-15.8) | ns |
| Female (n) | 11 | 12 | ns |
| Atopy (n) | 30 | 0 | |
| Passive smoking | 9 | 7 | ns |
| Treatment | |||
| ICS (n) | 17 | ||
| Beclomethasone | |||
| equivalent dosage (μg) | 200 (0-400) | ||
| Budesonide (n) | 13 | ||
| Fluticasone (n) | 4 | ||
| Bronchodilator (n) | 12 | ||
| Salbutamol | 4 | ||
| Formeterol | 8 | ||
| Montelukast (n) | 3 | ||
| FEV1 (% pred.) | 105 (97-118) | 107 (101-116) | ns |
| VC (% pred.) | 103 (96-112) | 101 (89-99) | ns |
| MEF 25 (% pred.) | 104 (83-115) | 100 (79-113) | ns |
| MEF 50 (% pred.) | 94 (76-126) | 104 (84-124) | ns |
| MEF 75 (% pred.) | 92 (75-117) | 125 (91-158) | ns |
| TLC (% pred.) | 98 (85-104) | 92 (87-100) | ns |
| ITV (% pred.) | 101 (82-113) | 101 (86-121) | ns |
| RV/TLC (% pred.) | 88 (71-98) | 92 (73-99) | ns |
| sRaw, tot (kPa*s) | 0.67 (0.50-0.91) | 0.50 (0.44-0.63) | ns |
| FENO (ppb)* | 17.0 (8.0-39.3) | 9.0 (7.0-11.0) | 0.001 |
| ACT (points) | |||
| < 12 years (n = 11) | 25 (22-26) | ||
| ≥ 12 years (n = 21) | 23 (21-25) | ||
| H2O2 concentrations airway fraction (nmol/L) | 290 (155-505) | 310 (115-555) | ns |
| H2O2 concentrations alveolar fraction (nmol/L) | 220 (140-460) | 180 (120-320) | ns |
Values as medians (IQR) or numbers, as appropriate; ns: not significant
Asthmatic patients had significantly higher FENO values than controls (median 17.0 ppm (8.0-39.3) vs. 9.0 ppm (7.0-11.0), p = 0.001). There were also significant lower FENO values in asthmatic patients using ICS than in asthmatic patients without regular usage of ICS (median (IQR) 12.0 ppb (6.5-21.5 ppb) vs. 28.0 ppb (17.0-44.0 ppb), p = 0.015).
Exhaled H2O2 concentrations and sampling parameters
14 of 32 asthmatic patients and 10 of 20 control subjects succeeded in collecting breath condensate with 300 litres of total gas volume, 18 of 32 patients and 10 of 20 controls only succeeded in collecting 200 litres. Details of the sampling times, peak flow values and sampled condensate volumes are presented in table 2. There was no significant difference in the alveolar and airway H2O2 concentrations whether the total gas sampling volume was 200 l or 300 l. Therefore the following statistical calculations were done for asthmatic patients and controls irrespective of the total gas sampling volumes achieved.
Table 2.
Results of the fractionated exhaled breath condensate sampling parameters
| Asthmatic patients | Controls | |||||
|---|---|---|---|---|---|---|
| Total gas sampling volume (L) | 200 | 300 | p | 200 | 300 | p |
| n | 18 | 14 | 10 | 10 | ||
| Sampling time (min) | 8.6 (6.9-11.7) | 12.8 (9.3-16.1) | 0.01 | 9.2 (7.9-10.6) | 9.7 (8.1-11.1) | ns |
| Peak flow (L/s) | 2.2 (1.5-2.7) | 2.0 (1.4-2.2) | ns | 2.1 (1.8-2.6) | 2.0 (1.5-2.4) | ns |
|
Condensate volume airway fraction (μL) |
410 (293-625) | 825 (450-1050) | 0.02 | 400 (303-540) | 600 (488-763) | 0.007 |
|
Condensate volume alveolar fraction (μL) |
1275 (988-1650) | 2000 (1400-2400) | 0.07 | 1200 (925-1838) | 2150 (1670-2400) | 0.009 |
| H2O2 concentration fraction 1 (nmol/L) | 290 (95-515) | 310 (160-640) | ns | 250 (95-545) | 320 (155-615) | ns |
| H2O2 concentration fraction 2 (nmol/L) | 270 (155-475) | 190 (115-405) | ns | 140 (120-290) | 250 (135-450) | ns |
Values as medians (IQR) or numbers, as appropriate; ns: not significant
H2O2 concentrations were below the detection limit (15 nmol/L) in three samples of fraction 1 (airway fraction) and in one sample of fraction 2 (alveolar fraction). In these 4 cases we used values of 0 nmol/L for statistical analysis, but only performed nonparametric tests. Furthermore H2O2 concentrations could not be obtained in 2 samples of the airway fraction because sample analysis curves were not acceptable.
No significant correlation was found between H2O2 concentrations of both fractions and the peak flow values during sampling breath condensate.
Comparison of H2O2 concentrations between the airway and the alveolar fraction
Comparing H2O2 concentrations between the two fractions for each related single pair we found that H2O2 concentrations were significantly higher in the airway fraction than in the alveolar fraction. P-values using the Wilcoxon signed-rank test were 0.003, 0.032 and 0.040 for the whole study group, the asthmatic group and the control group, respectively. Medians and IQRs are shown in tables 1 and 2, detailed values of each single patient are shown in Figure 1. P-values were 0.034 for the subgroup of patients with a sampling volume of 200 L and 0.053 for patients with a sampling time of 300 L.
Figure 1.
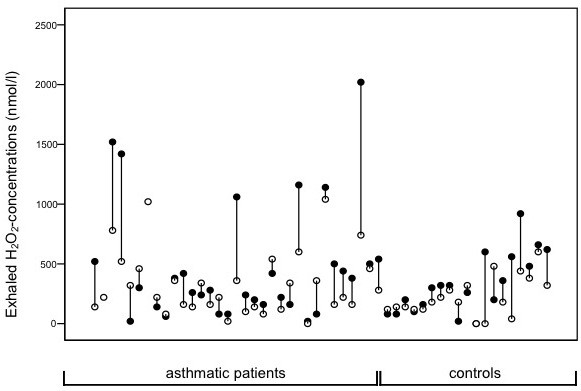
Paired exhaled H202 concentrations (● = airway fraction, ○ = alveolar fraction) of asthmatic children and controls. The exhaled H2O2 concentration was significantly higher in the airway fraction than in the alveolar fraction comparing each single pair (p = 0.003).
There was no significant difference in H2O2 concentrations between asthmatic patients and controls. However, extremely high values above 1000 nmol/L were only present in asthmatic patients (n = 6) and not in controls (Figure 1).
Correlation of exhaled H2O2 concentrations to passive smoking
There was no significant difference in H2O2 concentrations whether the patients or their parents reported second hand smoke exposure.
Correlation of exhaled H2O2 concentrations to asthma questionnaire
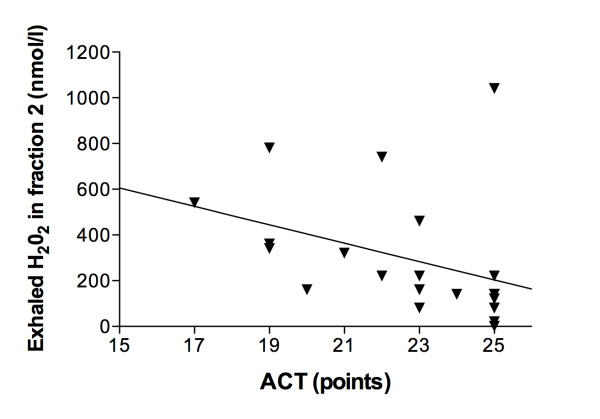
H2O2 concentrations in the alveolar fraction, but not in the airway fraction, were negatively correlated to the asthma control test for children 12 years or older (r = -0.606, p = 0.004, Figure 2). One outlier showed very high H2O2 concentrations with a perfect ACT score. There was no correlation with the asthma control test for younger children.
Figure 2.
Spearman's correlation between the asthma control test (ACT) in points and H202 concentrations in the alveolar fraction of asthmatic children 12 years and older. The asthma control test correlated significantly with the H2O2 concentrations in the alveolar fraction (r = -0.606, p = 0.004).
Furthermore H2O2 concentration of both fractions did not differ based on prescribed ICS use.
Correlation of exhaled H2O2 concentrations to lung function parameters
We found no correlation between H2O2 concentrations and lung function parameters as described above. In particular, there was no correlation between H2O2 concentrations in the alveolar fraction and lung function parameters, which suggest an involvement of the distal airways including MEF75 (% pred.) and RV/TLC (% pred.).
Correlation of exhaled H2O2 concentrations to FENO values
There was no positive correlation detected between H2O2 concentrations and FENO values in asthmatic patients, regardless of which fraction was measured. Furthermore, in 5 out of 6 patients with very high airway H2O2 concentrations (above 1000 nmol/L), low NO values (< 20 ppb) were measured. Looking at alveolar H2O2 concentrations, the results were similar; both of the two patients who had H2O2 values above 1000 nmol/L had low NO values (< 20 ppb).
Discussion
In this study we showed for the first time a significant difference between H2O2 concentrations in two different lung compartments of asthmatic children. The H2O2 concentration in the airway fraction was significantly higher than in the alveolar fraction. Additionally, we demonstrated a significant correlation of asthma control and H2O2 concentrations in the alveolar fraction.
Whereas the role of alveolar NO as a non-invasive marker of inflammation in asthma has been shown, this study contributes to the assessment of fractionated H2O2 in this setting. Whereas results of fractionated H2O2 detection in COPD are published [11], results in asthma and in children are still missing. The new technique of fractionated H2O2 measurement allows a differentiation of H2O2 concentrations in different parts of the lung. Our findings demonstrate a significantly lower concentration of H2O2 in the alveolar fraction than in the airway fraction of asthmatic patients. These results are in accordance to those of Möller et al. who found lower H2O2 concentrations in the alveolar fraction of adult COPD patients [11]. The distribution pattern of H2O2, showing lower values in the alveolar fraction than in the airway fraction, is consistent with the distribution of other inflammation markers like NO and IL-4 [3,16].
For children 12 years and older, we showed for the first time that worse asthma control correlates with higher H2O2 concentrations in the alveolar fraction. There were outliers, but the results still show a significant trend towards higher concentrations in suboptimally controlled asthma. The relationship between lung inflammation in general and an increased production of reactive oxygen species is well known [8,17]. However, our findings support the assumption that inflammation in the alveoli may play an important role in asthma control.
Growing evidence shows the importance of distribution patterns of inflammation rather than total values for a more complex understanding of asthma. Eosinophils as a marker for inflammation have been found in all parts of the airways, but the amount of eosinophils in peripheral airways seems to correlate with asthma severity [1]. Exhaled NO has been frequently linked to eosinophilic inflammation, and several studies show that high alveolar NO values correlate with worse asthma control [3,17].
There have been studies that tried to link elevated alveolar inflammation to peripheral lung function parameters. Van Veen et al. could demonstrate a correlation between alveolar NO and RV/TLC (% pred.), a marker for distal air trapping [18]. Also TLC and TGV have been positively correlated to distal lung inflammation measured by eosinophilic alveolar inflammation [19]. Correlations of distal inflammation to other lung function parameters like FEV1 and MEF25-75 are more conflicting [19]. In our study, we could not find a correlation between the mentioned lung function parameters and the alveolar fraction of H2O2. However, lung function parameters in children are mostly within normal values and do not correlate with asthma severity [20], especially since we excluded patients with acute infections.
We did not find any significant relation between H2O2 concentrations of the airway fraction and FENO, mainly representing the NO deriving from the airway fraction [3]. A similar discordant behavior between FENO and EBC 8-isoprostane, another marker of airway oxidative stress [21], has been observed in asthmatic children with exercise-induced bronchoconstriction [22]. This may be due to different types of airway inflammation, which are represented by the two values. As shown by us in a former study, FENO correlates strongly with the amount of bronchoalveolar lavage (BAL) eosinophils and is generally thought of as a marker for eosinophilic inflammation [23]. Sources of H2O2 are thought to be more diverse compared to those of FENO, since the producing superoxide dismutase can be found in a range of cells, i.e. macrophages, alveolar type II cells and the amount of H2O2 might be amplified by neutrophilic and eosinophilic peroxidases [24-26]. Since we found a significant correlation between alveolar H2O2 and asthma control, this implies the importance of measuring an additional biomarker, not only representing eosinophilic inflammation but oxidative stress. This assumption is strengthened by our findings that patients with very high H2O2 concentrations (above 1000 nmol/L) have mostly low FENO values (< 20 ppb). Different phenotypes of inflammation may be measurable in paediatric asthma, providing additional information for assessing asthma control. Studies aiming at assessing other non-eosinophilic exhaled markers of airway inflammation including LTB4 [27] and volatile organic compounds [28] are required to reinforce the present data.
In our study there was a significant difference in FENO values but not in H2O2 concentrations between steroid naïve asthmatic patients and asthmatic patients taking ICS, leading to the assumption that FENO values, but not H2O2 concentrations may be suppressed by corticosteroids. This may be in accordance to the fact that Horvath et al. mostly found elevated H2O2 concentrations but normal FENO values in their steroid-treated, unstable asthmatic group [29]. Unfortunately, to our knowledge no follow-up studies exist looking at H2O2 concentrations before and after corticosteroid prescription to support our findings. Also leukotriene receptor antagonists, which are widely used for asthma treatment [30], reduce FENO concentrations in asthmatic children [31], but their effect on EBC hydrogen peroxide in asthmatic children is unknown and should be clarified. Likewise, future studies should establish whether measurement of hydrogen peroxide in the alveolar fraction of EBC might be useful for choosing the best pharmacological strategy in children with mild asthma [32].
We did not find any correlation between asthma control and H2O2 concentration in the group of children < 12 years. This may be due to the much smaller group of children (n = 11 vs. n = 21). Furthermore, assessment of asthma control for children < 12 years might be more difficult compared to children ≥ 12 years, because the integration of the parents' perception might cause a bias.
In this study we were the first to apply the new technique of fractionated breath condensate sampling to measure H2O2 concentrations in asthmatics and in children. We admit that the sampling technique in this study differed in between our study group, which might be a weakness of our study. Finally about half of the children succeeded in collecting 300 litres of gas volume, whereas the other half only reached 200 litres. However, there were no significant differences between H2O2 concentrations in both collecting groups (table 2).
A very difficult issue in collecting fractionated breath condensate sampling is the determination of the threshold between the alveolar and the airway fraction. In our study, we applied the threshold according to the mean gas sampling volumes of both fractions measured in the study of Möller et al. [11]. We admit that the one third/two third ratio we chose represents the volume relations in adult airways and may not be applied to the growing lungs of children. However, imaging studies show that the airway surface length/area ratio was linearly associated to alveolar surface/volume ratio in CT scans of 50 children from 0-17.2 years of age [33]. In the imaging based study by de Jong and colleagues, there is no over proportional growth of the alveolar volume, suggesting that the growth of the airways and the alveoli is closely linked.
Another problematic issue in separating airway and alveolar compartments is the possible inhomogeneous narrowing of the asthmatic airways. This could potentially lower alveolar volume values in children with less controlled asthma. We admit that the accuracy of the single test might be limited in our young subjects, since the threshold was not determined individually for each patient. Whether a capnograph based method instead of a volume based method for differentiation between both compartments may be applied in children and will reveal different results needs further investigations.
We were unable to include a flow restrictor into the experimental design of the machine to keep the flow constant during the exhalation. Schleiss et al. found out that H2O2 concentrations negatively correlated to the expiratory flow [34]. Concurrent with this, a recent publication by Gajdocsi et al. showed that H2O2 concentrations are lower during increased tidal breathing compared to tidal breathing [35]. In our study, patients had an increased tidal volume and therefore an increased flow during the first part of the exhalation. The measured H2O2 concentration could be falsely decreased since the expiratory flow was higher during collecting the airway fraction. We measured the expiratory peak flow in our study, but could not find any significant correlation of this value to the H2O2 concentrations of either fraction.
Conclusion
In summary, this study showed for the first time that H2O2 concentrations in exhaled breath condensate were significantly higher in the airway fraction than in the alveolar fraction in asthmatic children and young adolescents. Only the H2O2 concentrations of the alveolar fraction correlated with asthma control in children 12 years and older suggesting that alveolar H2O2 plays a role in asthma control. However, whether fractionated exhaled H2O2 may be used as a non-invasive marker of alveolar involvement in asthmatics needs to be further investigated.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CL conceived and coordinated the study and revised the manuscript. JT and NM carried out interpretation of the data, drafted the manuscript and participated in the study design. SK and CMM carried out the acquisition and analysis of the data. SU contributed as statistical advisor. All authors have read and approved the final manuscript.
Contributor Information
Jordis Trischler, Email: jordis.trischler@medizin.uni-halle.de.
Nick Merkel, Email: nick.merkel@medizin.uni-halle.de.
Stephanie Könitzer, Email: stephanie.koenitzer@student.uni-halle.de.
Christina-Maria Müller, Email: christina-maria.mueller@medizin.uni-halle.de.
Susanne Unverzagt, Email: susanne.unverzagt@medizin.uni-halle.de.
Christiane Lex, Email: christiane.lex@uk-halle.de.
Acknowledgements
We thank Ilka Becker and Susann Wolff for their competent technical help.
References
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P. et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Kraft M. The distal airways: are they important in asthma? European Respiratory Journal (Official scientific journal of the European Respiratory Society) 1999;14:1403–1417. doi: 10.1183/09031936.99.14614039. [DOI] [PubMed] [Google Scholar]
- Paraskakis E, Brindicci C, Fleming L, Krol R, Kharitonov SA, Wilson NM, Barnes PJ, Bush A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med. 2006;174:260–267. doi: 10.1164/rccm.200506-962OC. [DOI] [PubMed] [Google Scholar]
- Puckett JL, Taylor RW, Leu SY, Guijon OL, Aledia AS, Galant SP, George SC. Clinical patterns in asthma based on proximal and distal airway nitric oxide categories. Respir Res. 2010;11:47. doi: 10.1186/1465-9921-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Li F, Gibson PG. Different inflammatory phenotypes in adults and children with acute asthma. European Respiratory Journal (Official scientific journal of the European Respiratory Society) 2011;38:567–574. doi: 10.1183/09031936.00170110. [DOI] [PubMed] [Google Scholar]
- Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- Dohlman AW, Black HR, Royall JA. Expired breath hydrogen peroxide is a marker of acute airway inflammation in pediatric patients with asthma. Am Rev Respir Dis. 1993;148:955–960. doi: 10.1164/ajrccm/148.4_Pt_1.955. [DOI] [PubMed] [Google Scholar]
- Ueno T, Kataoka M, Hirano A, Iio K, Tanimoto Y, Kanehiro A, Okada C, Soda R, Takahashi K, Tanimoto M. Inflammatory markers in exhaled breath condensate from patients with asthma. Respirology. 2008;13:654–663. doi: 10.1111/j.1440-1843.2008.01315.x. [DOI] [PubMed] [Google Scholar]
- Jobsis Q, Raatgeep HC, Hermans PW, de Jongste JC. Hydrogen peroxide in exhaled air is increased in stable asthmatic children. European Respiratory Journal (Official scientific journal of the European Respiratory Society) 1997;10:519–521. [PubMed] [Google Scholar]
- Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jobsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P. et al. Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal (Official scientific journal of the European Respiratory Society) 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- Moller W, Heimbeck I, Weber N, Khadem Saba G, Korner B, Neiswirth M, Kohlhaufl M. Fractionated exhaled breath condensate collection shows high hydrogen peroxide release in the airways. J Aerosol Med Pulm Drug Deliv. 2010;23:129–135. doi: 10.1089/jamp.2009.0764. [DOI] [PubMed] [Google Scholar]
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- Lex C, Dymek S, Heying R, Kovacevic A, Kramm CM, Schuster A. Value of surrogate tests to predict exercise-induced bronchoconstriction in atopic childhood asthma. Pediatr Pulmonol. 2007;42:225–230. doi: 10.1002/ppul.20556. [DOI] [PubMed] [Google Scholar]
- Gerritsen WB, Zanen P, Bauwens AA, van den Bosch JM, Haas FJ. Validation of a new method to measure hydrogen peroxide in exhaled breath condensate. Respir Med. 2005;99:1132–1137. doi: 10.1016/j.rmed.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Minshall EM, Hogg JC, Hamid QA. Cytokine mRNA expression in asthma is not restricted to the large airways. J Allergy Clin Immunol. 1998;101:386–390. doi: 10.1016/S0091-6749(98)70252-0. [DOI] [PubMed] [Google Scholar]
- Mahut B, Delacourt C, Zerah-Lancner F, De Blic J, Harf A, Delclaux C. Increase in alveolar nitric oxide in the presence of symptoms in childhood asthma. Chest. 2004;125:1012–1018. doi: 10.1378/chest.125.3.1012. [DOI] [PubMed] [Google Scholar]
- van Veen IH, Sterk PJ, Schot R, Gauw SA, Rabe KF, Bel EH. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. European Respiratory Journal (Official scientific journal of the European Respiratory Society) 2006;27:951–956. doi: 10.1183/09031936.06.00087905. [DOI] [PubMed] [Google Scholar]
- Sutherland ER, Martin RJ, Bowler RP, Zhang Y, Rex MD, Kraft M. Physiologic correlates of distal lung inflammation in asthma. J Allergy Clin Immunol. 2004;113:1046–1050. doi: 10.1016/j.jaci.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Curro D, Ragazzoni E, Preziosi P, Ciabattoni G. Anaphylaxis increases 8-iso-prostaglandin F2alpha release from guinea-pig lung in vitro. Eur J Pharmacol. 1999;365:59–64. doi: 10.1016/S0014-2999(98)00859-0. [DOI] [PubMed] [Google Scholar]
- Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009;135:66–73. doi: 10.1378/chest.08-0722. [DOI] [PubMed] [Google Scholar]
- Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006;174:1286–1291. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ, Hamon CB. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972;12:170–196. [PubMed] [Google Scholar]
- Kinnula VL, Everitt JI, Whorton AR, Crapo JD. Hydrogen peroxide production by alveolar type II cells, alveolar macrophages, and endothelial cells. Am J Physiol. 1991;261:L84–91. doi: 10.1152/ajplung.1991.261.2.L84. [DOI] [PubMed] [Google Scholar]
- Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P, Martello S, Felli M, Mondino C, Chiarotti M. Ion trap liquid chromatography/tandem mass spectrometry analysis of leukotriene B4 in exhaled breath condensate. Rapid Commun Mass Spectrom. 2004;18:2723–2729. doi: 10.1002/rcm.1682. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Santonico M, Mondino C, Pennazza G, Mantini G, Martinelli E, Capuano R, Ciabattoni G, Paolesse R, Di Natale C, Barnes PJ, D'Amico A. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest. 2010;137:790–796. doi: 10.1378/chest.09-1836. [DOI] [PubMed] [Google Scholar]
- Horvath I, Donnelly LE, Kiss A, Kharitonov SA, Lim S, Chung KF, Barnes PJ. Combined use of exhaled hydrogen peroxide and nitric oxide in monitoring asthma. Am J Respir Crit Care Med. 1998;158:1042–1046. doi: 10.1164/ajrccm.158.4.9710091. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clin Exp Allergy. 2010;40:1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Mondino C, Koch P, Ciabattoni G, Barnes PJ, Baviera G. Effects of montelukast treatment and withdrawal on fractional exhaled nitric oxide and lung function in children with asthma. Chest. 2007;132:1876–1881. doi: 10.1378/chest.07-1587. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ. New perspectives in pharmacological treatment of mild persistent asthma. Drug Discov Today. 2011;16:1084–1091. doi: 10.1016/j.drudis.2011.09.005. [DOI] [PubMed] [Google Scholar]
- de Jong PA, Long FR, Wong JC, Merkus PJ, Tiddens HA, Hogg JC, Coxson HO. Computed tomographic estimation of lung dimensions throughout the growth period. Eur Respir J. 2006;27:261–267. doi: 10.1183/09031936.06.00070805. [DOI] [PubMed] [Google Scholar]
- Schleiss MB, Holz O, Behnke M, Richter K, Magnussen H, Jorres RA. The concentration of hydrogen peroxide in exhaled air depends on expiratory flow rate. European Respiratory Journal (Official scientific journal of the European Respiratory Society) 2000;16:1115–1118. doi: 10.1034/j.1399-3003.2000.16f16.x. [DOI] [PubMed] [Google Scholar]
- Gajdocsi R, Bikov A, Antus B, Horvath I, Barnes PJ, Kharitonov SA. Assessment of Reproducibility of Exhaled Hydrogen Peroxide Concentration and the Effect of Breathing Pattern in Healthy Subjects. J Aerosol Med Pulm Drug Deliv. 2011. [DOI] [PubMed]