Abstract
The molecular mechanisms leading to neurodegeneration in Parkinson’s disease remain elusive. Deletion and mutations of DJ-1 (PARK7) have been reported to cause autosomal recessive familial Parkinson’s disease. Wildtype DJ-1 scavenges H2O2 by cysteine oxidation in response to oxidative stress, and thus confers neuroprotection. Activation of the transcription factor NF-E2 related factor-2 (Nrf2) has also been shown to be important for protection against oxidative stress in many models of neurodegenerative diseases. Previous data indicate that DJ-1 affects the transcriptional functions and stability of Nrf2. However, this observation has not been confirmed. In the current study, the role of DJ-1 in the regulation of Nrf2 is examined in primary cultured neurons, astrocytes and in vivo. The prototypical Nrf2 activator, tBHQ, protected primary cortical neurons derived from DJ-1 knockout (KO) as well as DJ-1 wildtype mice by activation of Nrf2-ARE pathway. Nrf2 nuclear translocation, robust increases of canonical Nrf2-driven genes and proteins, and dramatic activation of the ARE reporter gene, hPAP, were observed after tBHQ treatment. These results were further confirmed by siRNA mediated DJ-1 knockdown in primary cortical astrocytes from ARE-hPAP mice and tBHQ administration into the striatum of mouse brain. In addition, over-expression of Nrf2 with adenovirus preferentially in astrocytes from DJ-1 KO mice enhanced survival of neurons under oxidative insults. These findings indicate that activation of the Nrf2-ARE pathway is independent of DJ-1, and Nrf2 activation is a potential therapeutic target to prevent neurodegeneration in sporadic and DJ-1 familial Parkinson’s disease.
Keywords: Nrf2, DJ-1, tBHQ, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, affecting 1% of the population over 65 years of age (Dawson & Dawson, 2003). PD is a multifactorial disease caused by both genetic and environmental factors. Although most patients suffer from sporadic PD, linkage analyses in large families have identified an increasing number of genes that cause inherited forms of parkinsonism. The study of these genes has provided new insights into the pathogenesis of the disease. For example, DJ-1 (PARK7) deletion and point mutations have been found to cause autosomal recessive PD (Bonifati et al., 2003). DJ-1 mutations are the second most frequent identifiable genetic cause of PD after parkin (Abou-Sleiman et al., 2003; Hedrich et al., 2004).
DJ-1 is a 189-amino-acid protein and belongs to the Thi/PfpI protein superfamily (Wilson et al., 2004). DJ-1 has been reported to have multiple functions associated with PD pathogenesis. Wildtype DJ-1, not mutated DJ-1, confers neuroprotection by scavenging H2O2 through oxidation of Cys106-sulfinic acid in response to oxidative stress with a shift of pI from 6.2 to 5.8 (Mitsumoto et al., 2001; Canet-Aviles et al., 2004). In human PD brain, there is an accumulation of oxidized DJ-1 (Bandopadhyay et al., 2004).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an antioxidant transcription master regulator, which belongs to the cap “n” collar family of transcription factors. Small Maf proteins (MafF, MafG and MafK) possess the region-leucine zipper (L-Zip) domain that is required for homodimer or heterodimer complex formation with other basic L-Zip transcription factors like Nrf2 (Motohashi et al., 2002). Nrf2 is sequestered in cytoplasm by its repressor Keap1, released and translocated into nucleus under oxidative stress (Itoh et al., 1999). In nucleus, the heterodimer complex of Nrf2 and small Maf proteins binds to the antioxidant responsive element (ARE) sequence leading to transcriptional activation of downstream genes encoding phase II detoxifying enzymes and antioxidants (Itoh et al., 1997). The ARE is a cis-acting regulatory element in promoter regions of Nrf2-regulated genes (Rushmore et al., 1991). Activation of Nrf2-ARE pathway has been shown to play very important roles in protection against oxidative stressors in many models of neurodegenerative diseases (Johnson et al., 2008). Numerous Nrf2 activators including tBHQ (tert-Butylhydroquinone) have been shown to protect neurons from oxidative stress induced toxicity (Lee et al., 2003; Shih et al., 2005).
Recently published data suggests that DJ-1 is necessary for transcriptional function and stability of Nrf2 in primary mouse embryonic fibroblasts cultures (MEFs) derived from DJ-1 knockout (KO) mice (Clements et al., 2006). However, the modulation of Nrf2 via DJ-1 or the direct interaction between them has not been fully described. Considering that MEFs may not reflect the characteristics of neurons or neuronal pathology in neurodegenerative disease, here we used primary cortical neuronal and astrocyte cultures derived from DJ-1 KO and wildtype (WT) mice as well as ARE-driven human placental alkaline phosphatase (ARE-hPAP) transgenic reporter mice to evaluate the relationship between DJ-1 and Nrf2 in vivo.
Materials and Methods
Animals
ARE human placental alkaline phosphatase (ARE-hPAP) transgenic mice were created using the NAD(P)H:quinone oxidoreductase (NQO1) promoter upstream of an hPAP reporter construct (Johnson et al., 2002). DJ-1(−/−) mice (KO) were graciously provided by Dr. Ted M Dawson and Valina L. Dawson (School of Medicine, Johns Hopkins University, Baltimore, MD). DJ-1 was disrupted by partial deletion of exon 2, deletion of exon 3, and the introduction of a stop codon and a neo selection cassette (Andres-Mateos et al., 2007). Heterozygous male and female mice were bred to generate homozygous mice. Littermate DJ-1(+/+) mice (WT) were used as controls. DJ-1 mice were maintained on C57BL/6J background. ARE hPAP mice were maintained on B6/SJL mice (The Jackson Laboratory, Bar Harbor, Maine, USA). DJ-1(−/−)/ARE-hPAP(+) mice (KO-ARE) and DJ-1(+/+)/ARE-hPAP(+) mice (WT-ARE) were generated by a two-step breeding process. First, ARE-hPAP(+) mice were crossed with DJ-1(−/−) mice resulting in DJ-1(+/−)/ARE-hPAP(+) and DJ-1(+/−)/ARE-hPAP(−) mice. These two genotype mice were then bred to generate the KO-ARE and WT-ARE for the experiments. Thus, the background of these mice was a mixed B6/SJL. All animal procedures were approved by University of Wisconsin-Madison Institutional Animal Care and Use Committee (IACUC). All experiments were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Chemicals
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Fisher Scientific (Fair Lawn, NJ, USA).
Mouse primary cortical neuronal culture
The pregnant female mouse was anaesthetized using isoflurane. The cortices of E15–16 embryos were dissected and removed to ice-cold Hanks’ balance salt solution (HBSS). The individual cortex was dissociated with 0.05% trypsin in HBSS for 15min at 37°C, and then filtered through a 70μm cell strainer (BD Bioscience, San Jose, CA, USA). The embryo tails were kept for genotyping. Cells were plated on poly-D-lysine-coated plates at 3.2×105 cells/cm2 in minimum essential medium with Earle’s salt (Mediatech, Inc., Manassas, VA, USA) containing heat-inactivated (55°C, 30min) 10% fetal bovine serum and 10% horse serum (Atlanta Biologicals, Inc., Lawrenceville, GA, USA), and 1% penicillin/streptomycin (CEMEM) in a 37°C humidified tri-gas incubator (Forma Scientific, Inc., Waltham, MA, USA) to maintain normoxic conditions (5% O2, 5% CO2, 90% N2). Fresh CEMEM were changed after 45min. The cells were cultured in CEMEM for 48hr before a media change to Neurobasal medium (Invitrogen Corporation, Carlsbad, CA, USA) plus B27 supplement containing antioxidants (NB plus AO). Half the medium was replaced every two days. Unless otherwise specified, tBHQ treatment began on the fourth day in vitro (4DIV). After incubation with the treatment for 48hr, the media was changed to Neurobasal medium containing B27 supplement without antioxidants (NB minus AO) before the toxins were applied for an additional 24hr.
Mouse primary cortical astrocyte culture
Cerebral cortices from newborn pups (P1–2) were removed, pooled and placed in ice-cold HBSS (Invitrogen Corporation), centrifuged at 300g for 2min, and digested with 0.05% trypsin in HBSS for 25min at 37°C. Tissues were washed twice with HBSS, resuspended in CEMEM, sieved through 70μm cell strainers and plated at 3 ×104 cells/cm2. The medium was changed after 24hr of initial plating and every 3 days thereafter. Cultures were maintained at 37°C in a humidified three-gas incubator (5% O2, 5% CO2, 90% N2). Cells were allowed to grow to confluence prior to treatment.
Real-time PCR
Total RNA was isolated using TRIZol reagent (Invitrogen Corporation). RNA quality was assessed with the 2100 Bioanalyzer (Agilent Technologies, Foster City, CA) and 1μg of RNA (RIN number ≥ 8.0) was reverse transcribed with oligo {dT}15 primers using Reverse Transcription System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol. Minus reverse transcriptase controls were included in each assay. PCR was performed in a 20 μl reaction with 1× Light-Cycler480 SYBR Green I Master in a LightCycler480 Real-time PCR System (Roche Applied Science, Indianapolis, IN, USA). PCR product quantification was subject to relative standard curves. The efficiencies of amplification are limited between 1.8 and 2.2, with error < 0.2. The cycling parameters were as follows: 95°C, 10s; 55°C, 10s; 72°C, 15s. Specific primers were:
DJ-1 forward (5′-TTGCACTAGCCATTGTGGAG-35′),
DJ-1 reverse (55′-ACATACAGACCCGGGATGAG-35′);
Keap1 forward (55′-AAGGACCTTGTGGAAGACCA-35′),
Keap1 reverse (55′-CCCTGTCCACTGGAATTGAT-35′);
Cullin3 forward (55′-TGGAAAGCCTGAAGCTCAAT-35′),
Cullin3 reverse (55′-TATTTGGCACGAACACCTGA-35′). The rest of primer sequences were described previously (Johnson et al., 2002; Vargas et al., 2008).
Gel electrophoresis and western blot
Protein was quanti ed using a BCA Protein Assay Kit (Pierce, Rockfold, IL, USA) and 20 50μg protein per lane was loaded on to a 10–12% sodium dodecyl sulfate polyacrylamide gel. PVDF membranes were blocked with 10% milk/Tris Buffered Saline-Tween 20 (TBST) at room temperature (RT) for 1–2hr, and then incubated with primary antibodies in 5% BSA in TBST overnight at 4°C at the following dilutions: rabbit anti-DJ-1, 1:6000 (Chemicon, Billerica, MA, USA); rabbit anti-Nrf2 (c-20), 1:500 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); goat anti-NQO1, 1:1000 (Abcam, Cambridge, MA, USA); goat anti-Keap1, 1:500 (Santa Cruz Biotechnology, Inc.); rabbit anti-GCLC, 1:20000 and rabbit anti-GCLM, 1:20000 (provided generously by Dr. T. Kavanagh); mouse anti-beta actin (AC-15), 1:10000 (Abcam). The membrane was then washed three times in TBST followed by incubation for 1–2hr in a 1:5000 dilution of horseradish peroxidase-labeled anti-rabbit or anti-mouse (Amersham, Piscataway, NJ, USA) or horseradish peroxidase-labeled anti-goat (Santa Cruz Biotechnology, Inc.) secondary antibody. After washing in TBST, bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Nuclear Extraction
Nuclear fractions were isolated as described previously (Lee et al., 2001) with modifications. Briefly, cells were washed with cold PBS and resuspended in cold buffer A (lysis buffer: 10mM HEPES, pH 8.0, 1mM EDTA, 1.5mM MgCl2, 10mM KCl, 1mM DTT, 0.6% NP-40, 1mM sodium orthovanadate, 1mM NaF, Roche cocktail protease inhibitors). Cells were mixed vigorously for 10s by vortex, and then allowed to swell on ice for 15min. The homogenate was centrifuged for 50s at 16,000g. The supernatant was removed for cytoplasmic fraction and the nuclear pellet was washed 2–3 times with buffer A. The pellet was then resuspended in cold buffer B (extraction buffer: 20mM HEPES, pH 8.0, 2mM EDTA, 1.5mM MgCl2, 420mM NaCl, 20% glycerol, 1mM DTT, 1mM sodium orthovanadate, Roche cocktail protease inhibitors), and resuspended by sonication, then centrifuged for 10min at 16,000g. The supernatant was taken as the nuclear extract and salts were removed with microcons (MW 3000, Millipore, Billerica, MA, USA).
hPAP enzyme activity assay
Cells were harvested in TMNC lysis buffer (0.05M Tris, 0.005M MgCl2, 0.1M NaCl, 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate [CHAPS]) and freeze-thawed at −80°C. Tissues were homogenized in TMNC lysis buffer. Endogenous phosphatase activity was heat inactivated at 65°C for 30 min in 0.2M diethanolamine buffer. hPAP activity was quantitated by addition of the Applied Biosystems (Bedford, MA, USA) substrates CSPD and Emerald (Johnson et al., 2002). A Berthold Orion microplate luminometer was used to measure the samples at various times after substrate addition. Values were corrected to ARE hPAP negative mice and normalized by protein concentration.
Cytotoxicity
After 48hrs of tBHQ pretreatments, medium was changed before toxin exposure. Cells were incubated in either hydrogen peroxide (H2O2) or tert-butyl hydroperoxide (tBOOH) in fresh NB minus AO for 24hr. Cell viabilities were then measured with the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl) 2H tetrazolium, inner salt (MTS, Promega) assay.
For virus pretreatments, cultures were incubated with replication-deficient adenovirus (Provided by Adenovirus Core Facility, University of Ottawa, Canada) in MEM for 45 min at 37°C, after which the conditioned medium was added back to the cells and incubated for 48hr. Medium was changed before cells were exposed to toxins for 24hr. Cell viabilities were then measured by MTS assay.
Immunofluorescence and histochemistry
Cells in permanox chamber slides (Fisher Scientific, Pittsburgh, PA) were fixed with 4% paraformaldehyde (PFA) in PBS at RT for 20min, then permeabilized and blocked with 10% goat serum, 1% bovine serum albumin, 0.1% Triton X-100 diluted in PBS for 1hr at RT. Cells were incubated with primary antibodies diluted in blocking solution for 1hr at RT. Primary antibodies were used at the following dilutions: anti-β(III)-tubulin, 1:500 (Promega); anti-GFAP, 1:1000 (Millipore); anti-GFP, 1:1000 (Santa Cruz Biotechnology). Secondary antibodies diluted in blocking solution were incubated for 1hr at RT. Secondary antibodies conjugated to either fluorescein or Texas Red were used at a dilution of 1:200, and appropriate IgG were used for controls simultaneously. Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining was performed using the Cell Death Detection kit (Roche Applied Science) according to the manufacturer’s instructions. Incubation of slides in 0.1mM Hoechst 33258 in PBS was used to stain nuclear DNA. Images were taken using a Zeiss (Oberkochen, Germany) photomicroscope and analyzed using Axiovision software. For hPAP histochemical staining, mice were killed with CO2 and perfused with PBS followed by 4% PFA. Tissues were postfixed approximately 2hr at 4°C and cryoprotected in 30% sucrose in PBS overnight at 4°C. Adjacent coronal 20μm sections were prepared using a cryostat (Leica, Deerfield, IL), and stained with NBT/BCIP (Johnson et al., 2002).
Total intracellular glutathione assay
Total glutathione [γ-l-glutamyl-l-cysteinylglycine (GSH + GSSG)] levels were determined using the modified Tietze method as previously described (Vargas et al., 2006). Cells in 6 well plates were harvested in 100μl ice-cold 3% perchloric acid. Glutathione content was standardized to protein concentration determined by BCA protein assay (Pierce).
siRNA Transfection
siRNA transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, confluent primary cortical astrocytes were transfected with 50nM DJ-1 siRNA (D-050181-01, Dharmacon, Inc., Lafayette, CO, USA), Keap1 siRNA (D-041104-02, Dharmacon) and non-targeting siRNA (D-001210-02–05, Dharmacon). Medium was changed after 24hr and then every 2–3 days.
tBHQ Injection
Two to three-month old KO-ARE mice and littermate WT-ARE mice were exposed to tBHQ by intrastriatal stereotaxic injection with contralateral vehicle injections. Mice were anesthetized with isoflurane. One microliter tBHQ (50mM in PBS, pH 7.4, 10% ethanol vehicle) was injected 0.5mm anterior to bregma, 2.1mm lateral to midline, and 3.3mm ventral to dura. The solution was administered at the speed of 0.2ul/min for 5min. After finishing the injection, the needle was left undisturbed for 5min, then withdrawn halfway and left for an additional 5min before being removed.
Statistical analysis
Each experiment was repeated with at least three different litters. All data were represented as mean±SEM. Statistical analysis was performed using one-way or two-way ANOVA followed by Bonferroni post test and unpaired two-tailed Student’s t test (GraphPad prism 4), and differences were declared statistically significant if p<0.05.
Results
Nrf2 and downstream genes are activated by tBHQ in primary cortical cultures derived from DJ-1 KO mice
Gene and protein levels of DJ-1 were measured in primary cortical neuronal cultures of individual pups derived from DJ-1(+/−) crosses. The results from quantitative real time PCR and western blot showed that DJ-1 gene expression is decreased ≥99% and no DJ-1 protein was detected in cultures derived from KO mice (Data not shown).
Nrf2 and its regulated genes were evaluated in WT and KO primary cortical cultures. Real time PCR analysis demonstrated that, compared to WT cultures, basal levels of Nrf2 expression decreased by 45% in KO cultures (Fig. 1A). This reduction in Nrf2, however, was reflected in a modest decrease in basal levels of Nrf2-driven NQO1, glutamate-cysteine ligase catalytic subunit (GCLC), modifier (GCLM) and heme oxygenase (HO-1). Keap1 mRNA displayed equivalent basal levels in WT and KO cultures.
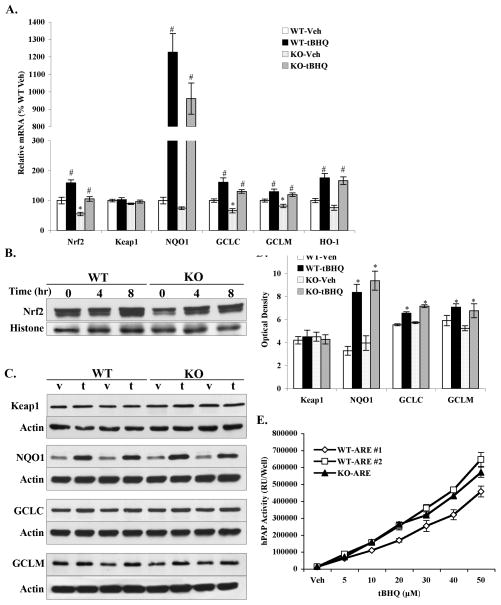
Figure 1. tBHQ activated Nrf2 and downstream genes in primary cortical neurons from both DJ-1 WT and DJ-1 KO mice.
A. Primary cortical neurons (4DIV) were treated with 20μM tBHQ or 0.1% ethanol vehicle (Veh) for 24hr before harvesting for real time PCR. Keap1, Nrf2 and Nrf2-modulated phase II detoxifying genes including NQO1, GCLC, GCLM, HO-1 were evaluated. All data were standardized by β-actin mRNA level, and compared to WT-Veh. Mean±SEM, n=5–7. *p<0.05, significantly different from WT-Veh sample; #p<0.05, significantly different from corresponding Veh sample (two-way ANOVA). B. Primary cortical neurons (4DIV) were treated with 20μM tBHQ for various times (0, 4, 8hr) before harvesting and nuclear extractions were prepared. Western blots were performed to detect protein levels of Nrf2 in these nuclear fractions. Representative blots were shown (n=3). C. Primary cortical neurons (4DIV) were treated with 20μM tBHQ or vehicle for 48hr before harvesting for western blotting. Keap1, NQO1, GCLC and GCLM were measured. Representative blots were shown. v-vehicle, t-tBHQ. D. The optical densities of western blots in C were quantified using ImageJ. Representative blots were shown (n=4), Mean±SEM. *Significantly different from corresponding Vehicle treated sample (two-way ANOVA, p<0.05). E. Primary cortical cultures from individual pups were prepared. Cultures (4DIV) from DJ-1 WT-ARE and DJ-1 KO-ARE mice were treated with increasing concentrations of tBHQ or Vehicle for 48hr and hPAP activity was measured. Cells from ARE hPAP negative animals were used to subtract background luminescence. Mean±SEM, n=5 (one-way ANOVA).
Following a 24hr tBHQ treatment, Nrf2 (tBHQ, F1,19 = 31.48, P < 0.0001; Gene, F1,19 = 25.70, P <0.0001; tBHQ × Gene, F1,19 = 0.21, P = 0.65) and its regulated genes increased significantly and showed similar fold changes in WT and KO cultures relative to corresponding vehicle groups (Fig. 1A). Over a 12-fold increase was observed in both WT (12.3-fold) and KO cultures (12.9-fold) for NQO1 (tBHQ, F1,20 = 113.97, P < 0.0001; Gene, F1,20 = 2.60, P = 0.12; tBHQ × Gene, F1,20 = 1.73, P = 0.20). tBHQ treatment did not affect the Nrf2 repressor Keap1 (tBHQ, F1,20 = 1.75, P = 0.20; Gene, F1,20 = 3.79, P = 0.07; tBHQ × Gene, F1,20 = 0.76, P = 0.39). Nuclear translocations of Nrf2 and protein levels of its regulated phase II enzymes were also measured in primary cortical cultures by western blot. As previously published data describes, Nrf2 migrates at an approximate molecular weight of 100 kDa (Chan et al., 1993; Moi et al., 1994; Lee et al., 2001). Nrf2 protein in the nuclear fraction increased in WT and KO cultures after tBHQ treatment for 4hr and 8hr (Fig. 1B). The trends in basal Nrf2 protein levels are consistent with quantitative gene expression analysis described above, however, the extent of nuclear translocation was unaffected by the lack of DJ-1. In contrast to the modest reduction in mRNA levels, there was no statistically significant difference in the baseline protein levels of Keap1, NQO1, GCLC, GCLM between WT and KO cultures (Fig. 1C, D). Compared to corresponding vehicle groups, protein level of NQO1 increased more than 2-fold in WT and KO cultures following a 48hr tBHQ treatment (tBHQ, F1,12 = 60.03, P < 0.0001; Gene, F1,12 = 1.34, P = 0.27; tBHQ × Gene, F1,12 = 0.00, P = 0.96). Significant increases were also observed in the protein levels of GCLC (tBHQ, F1,12 = 60.31, P < 0.0001; Gene, F1,12 = 5.07, P = 0.05; tBHQ × Gene, F1,12 = 3.64, P = 0.08) and GCLM (tBHQ, F1,12 = 10.07, P = 0.0080; Gene, F1,12 = 1.36, P = 0.27; tBHQ × Gene, F1,12 = 0.17, P = 0.68). Keap1 protein expression was not affected by tBHQ treatment (tBHQ, F1,12 = 0.01, P = 0.94; Gene, F1,12 = 0.01, P = 0.93; tBHQ × Gene, F1,12 = 0.37, P = 0.56).
Finally, hPAP activity in primary individual cortical cultures from WT-ARE and KO-ARE mice was measured. tBHQ treatment led to a dramatic dose-dependent increase in hPAP activity in both WT and KO cultures (Fig. 1E). hPAP activity was increased 16.5- and 22.3-fold at 20μM, 45.0- and 53.4-fold at 50μM in WT-ARE #1 and #2 cultures, respectively. Similarly, hPAP activity increased 20.9-fold at 20μM and 45.3-fold at 50μM in KO-ARE culture (F = 0.36, P = 0.70). As seen with the other Nrf2-dependent genes, no significant difference in basal hPAP activity was observed. These results demonstrate that the presence of DJ-1 is not required for Nrf2-ARE activation by tBHQ.
tBHQ pretreatment protects primary cortical neurons against oxidative stress-induced cell death
tBHQ protects neurons from oxidative stress by activating Nrf2 and is unable to confer protection in Nrf2-KO mice (Lee et al., 2003; Kraft et al., 2004). To determine if the presence of DJ-1 was required to tBHQ-mediated protection, DJ-1 WT and KO primary cortical neuronal cultures were treated with tBHQ and then exposed to hydrogen peroxide (H2O2) and tert-butylhydroperoxide (tBOOH). Both toxins induced significant cell death that was attenuated by pretreatment with 20μM tBHQ pretreatment in DJ-1 WT (H2O2, F1,48 = 219.17, P < 0.0001; tBOOH, F1,48 = 218.12, P < 0.0001) as well as KO cultures (H2O2, F1,48 = 128.22, P < 0.0001; tBOOH, F1,48 = 251.09, P < 0.0001, Fig. 2A, B). In addition, H2O2 (50μM) led to an increased TUNEL-positive cell staining that was attenuated by 20μM tBHQ pretreatment for 48hr in both WT and KO cultures (Fig. 2C). As increased glutathione is a major component of the protection conferred by Nrf2-ARE activation in astrocyte-motor neuron co-cultures (Vargas et al., 2008), total glutathione levels were measured and no baseline difference between KO and WT cultures was observed (Fig. 2D). In addition, tBHQ treatment enhanced GSH levels by 40% in both WT and KO cultures (tBHQ, F1,24 = 78.02, P < 0.0001; Gene, F1,24 = 0.21, P = 0.65; tBHQ × Gene, F1,24 = 0.42, P = 0.52). Finally, consistent with other groups (Kim et al., 2005), neuronal cultures from KO mice were more sensitive oxidative stress mediated toxicity compared to neurons from WT mice (Fig. 2A, B). This is probably associated with the recent finding that DJ-1 has atypical peroxidase activity (Andres-Mateos et al., 2007) that protects cells from oxidative stress.
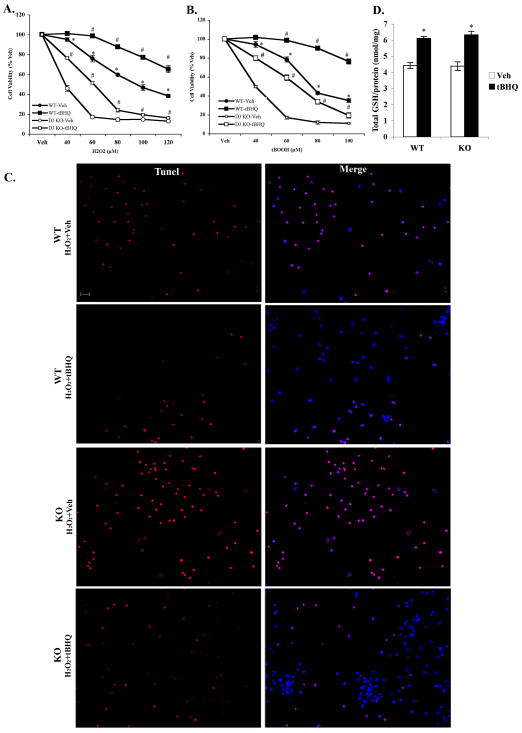
Figure 2. Nrf2 activation by tBHQ treatment protected both DJ-1 WT and DJ-1 KO cortical neuronal cultures from oxidative stress-induced cell death.
Cultures (4DIV) were treated with 20μM tBHQ or Vehicle for 48hr and then treated with increasing concentrations of A. hydrogen peroxide (H2O2) or B. tert-butyl hydroperoxide (tBOOH) for an addition 24hr. Cell viability was assessed using the soluble MTS assay. Mean±SEM, n=5. *p<0.05, significantly different from the corresponding values for DJ-1 KO vehicle treated samples; #p<0.05, significantly different from corresponding values Vehicle treated samples (two-way ANOVA). C. TUNEL staining of cells displaying fragmented DNA (red) overlaid with Hoechst 33258 (blue) (scale bar 20μm). D. Total intracellular glutathione was determined in primary cortical cultures treated with either 20μM tBHQ or Vehicle for 48hr. Mean±SEM, n=7. *Significantly different from corresponding Vehicle treated sample (two-way ANOVA, p<0.05).
Overexpression of Nrf2 selectively in astrocytes protects neighboring neurons in primary cortical cultures
The above data showed that tBHQ displayed neuroprotection against oxidative stress by activating Nrf2-driven genes in DJ-1 KO cultures as well as WT cultures. Adenovirus-Nrf2 was used to transduce Nrf2 into DJ-1 KO cultures to determine if overexpressing Nrf2 could rescue sensitive neurons. MTS assay results revealed that adenovirus-Nrf2 dramatically attenuated toxicity from H2O2 and tBOOH compared to adenovirus-eGFP control (F1,48 = 463.37, P < 0.0001 and F1,48 = 693.42, P < 0.0001) Fig. 3A, B). In addition, overexpression of Nrf2 resulted in a 36% increase in total intracellular GSH level similar to that seen with tBHQ treatment (P = 0.0004, Fig. 3C). Fluorescent staining showed that most GFAP (red) positive astrocytes colocalized with GFP (green), whereas β(III)-tubulin (red) positive neurons rarely colocalized with GFP (Fig. 3D), which is consistent with previous data that adenovirus-Nrf2 preferentially transduces astrocytes (Kraft et al., 2004).
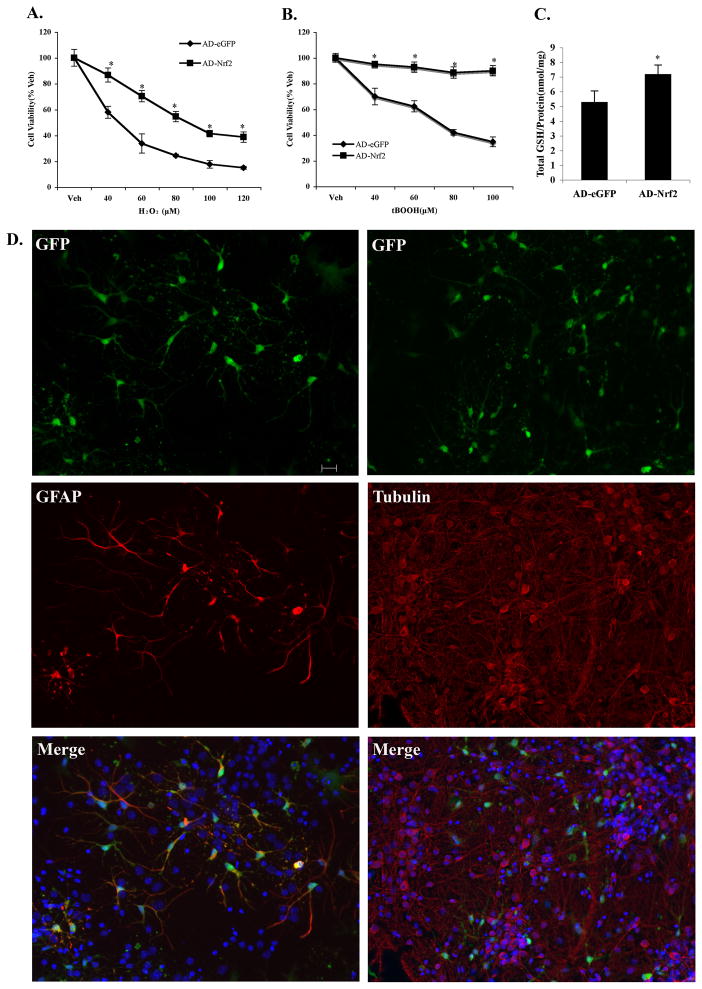
Figure 3. Overexpression of Nrf2 selectively in astrocytes protected neighboring neurons against toxins in DJ-1 KO primary cortical neuronal cultures.
Cells were transduced with 50 MOI (multiplicity of infection) adenovirus-eGFP-Nrf2 (AD-Nrf2) or adenovirus-eGFP (AD-eGFP). Forty-eight hr later cultures were treated with either A. H2O2 or B. tBOOH. Mean±SEM, n=5. *Significantly different from corresponding AD-eGFP value (two way ANOVA, p<0.05). C. Total intracellular glutathione levels in primary cortical cells were measured after a 48hr infection of 50 MOI AD-Nrf2 and AD-eGFP. Mean±SEM, n=7. *AD-Nrf2 vs AD-eGFP (two-tailed t test, p<0.05). D. Infection of primary cortical cultures with 50 MOI of adenovirus showed adenovirus-infected cells colocalized with GFAP-positive astrocytes and, rarely with β(III)-tubulin-positive neurons (100 MOI) (scale bar 20μm). Blue: Hoechst 33258 staining.
DJ-1 knockdown by siRNA does not affect Nrf2-ARE activation in primary cortical astrocytes from ARE-hPAP mice
Since KO mice lack DJ-1 throughout the developmental process and there is the potential for compensatory pathways to mask DJ-1-dependent Nrf2 activation, DJ-1 was knocked down with an anti-mouse DJ-1 siRNA (siDJ). Primary cortical astrocytes from ARE-hPAP mice were transfected with DJ-1 siRNA. Neurons were not used for these studies because the lipofectamine was toxic to neurons making data interpretation impossible. Real time PCR analysis revealed that the gene levels of DJ-1 were reduced 70% by siRNA at D1 and 60% at D8 as compared to non-targeting siRNA control (siNT) (P = 0.0017 and P = 0.0005, Fig. 4A). Cullin3 is part of E3 ubiqintin ligase and binds to BTB domain of Keap1 to mediate Nrf2-ubiquitinated degradation (Kobayashi et al., 2004). DJ-1 knockdown did not affect gene levels of Nrf2, Keap1 and Cullin3 at these two time points. Western blot confirmed the functional deletion of DJ-1 protein by siRNA. DJ-1 protein decreased 50% of baseline at 2 days (D2) post-transfection with siDJ and was suppressed to 20% at 6 days (D6) in comparison to siNT (Fig. 4B). Nrf2 protein was unchanged by siDJ transfection (Fig. 4B).
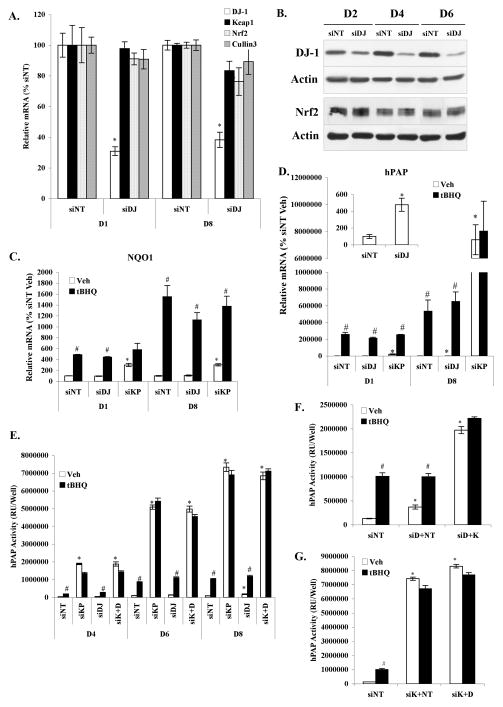
Figure 4. Knockdown of DJ-1 by siRNA did not affect Nrf2-dependent activation of the ARE by tBHQ in primary cortical astrocytes.
Cultures were transfected with siRNA targeting DJ-1 (siDJ) or non-targeting siRNA (siNT) for 24hr. A. mRNA levels of DJ-1, Keap1, Nrf2, and Cullin3 were determined 1 (D1) and 8 days (D8) after siRNA transfection. All data were standardized by β-actin mRNA levels, and compared to non-targeting siRNA (siNT) vehicle. Mean±SEM, n=3. *Significantly different from corresponding siNT value (two-tailed t test, p<0.05). B. Western blots were performed at different time points (D2, D4, D6) following siRNA transfection. C. and D. siRNA transfected cultures were treated for 24hr with 50μM tBHQ or vehicle on D1 or D8 post-transfection. NQO1 (C.) and hPAP (D.) mRNA levels were determined. Insert in D. showed basal levels of hPAP gene at D8 after siNT and siDJ transfection. Mean±SEM, n=3. All data were standardized by β-actin mRNA level, and compared to siNT vehicle. *p<0.05, significantly different from siNT vehicle treated sample; #p<0.05, significantly different from corresponding Vehicle treated sample (two-way ANOVA and two-tailed t test). E. hPAP activity in primary cortical astrocytes transfected with siRNAs was measured at different time points after a 24hr treatment with 50μM tBHQ or Vehicle. siK+D: cotransfection with the same amount of Keap1 and DJ-1 siRNA at the same time. Mean±SEM, n=6. *p<0.05, significantly different from corresponding Vehicle treated siNT value; #p<0.05, significantly different from corresponding Vehicle treated sample (two-way ANOVA and two-tailed t test). F. hPAP activities in primary cortical astrocytes transfected with siRNAs were measured at D8 after a 24hr treatment with 50μM tBHQ or Vehicle. siD+NT: astrocytes were transfected with DJ-1 siRNA on D0 and then with non-targeting siRNA on D6; siD+K: astrocytes transfected with DJ-1 siRNA on D0 and then with Keap1 siRNA on D6. Mean±SEM, n=6. *p<0.05, significantly different from Vehicle treated siNT. #p<0.05, significantly different from corresponding Vehicle treated sample (two-way ANOVA and two-tailed t test). G. hPAP activities in primary cortical astrocytes transfected with siRNAs were measured at D8 after a 24hr treatment with 50μM tBHQ or Vehicle. siK+NT: astrocytes transfected with Keap1 siRNA on D0 and then with non-targeting siRNA on D2; siK+D: astrocytes transfected with Keap1 siRNA on D0 and then with DJ-1 siRNA on D2. Mean±SEM, n=6. *p<0.05, significantly different from Vehicle treated siNT; #p<0.05, significantly different from corresponding Vehicle treated sample (two-way ANOVA and two-tailed t test).
The Nrf2-regulated gene NQO1 was evaluated after siRNA transfection in the absence and presence of tBHQ. Knockdown of the Nrf2 repressor Keap1 (siKP) was used as a positive control for Nrf2 activation (Wakabayashi et al., 2003). As shown in Figure 4C, baseline mRNA levels of NQO1 did not show significant change after siDJ transfection; however, it significantly increased by 3-fold after siKP transfection at both D1 and D8. NQO1 mRNA was also increased by tBHQ treatment with similar fold changes in siNT (D1, 4.9-fold; D8, 15.5-fold) and siDJ (D1, 4.4-fold; D8, 11.2-fold) groups compared to vehicle control at D1 and D8. tBHQ also caused an additional increase of NQO1 in the siKP transfected cells (Fig. 4C).
Similar results were observed for ARE reporter gene hPAP (Fig. 4D). Basal expression of hPAP was not significantly change after siDJ transfection at D1, but by D8 baseline mRNA levels were significantly increased (4.8-fold; P = 0.0099, Fig. 4D insert). Dramatic increases in basal expression of hPAP were noted in the siKP transfected astrocytes (D1, 200-fold; D8, 73940-fold). hPAP gene was also increased by tBHQ treatment with the exception of D8 siKP astrocytes suggesting that the Nrf2 activation/binding to the ARE is saturated after 8 days of Keap1 knockdown. As observed at the mRNA level, basal hPAP activity was actually increased by DJ-1 knockdown (D8, 1.8-fold, P = 0.0090, Fig. 4E). Furthermore, hPAP enzymatic activity was significantly increased by both tBHQ and Keap1 knockdown (Fig. 4E). However, neither the tBHQ-mediated or siKP-mediated increase in hPAP activity was affected by DJ-1 knockdown (Fig. 4E). Finally, because of the longer half-life of DJ-1 (33hr) than that of Keap1 (6hr), two additional siRNA knockdown experiments were performed. The first transfected siDJ 6 days prior to transfecting with siKP and hPAP activity was measured (Fig. 4F). The second transfected siKP 2 days prior to transfecting with siDJ and hPAP activity was measured (Fig. 4G). Again, the ability of tBHQ or siKP to activate Nrf2 in both experiments was completely unaffected by DJ-1 knockdown (Figs. 4F, G).
Injection of tBHQ into striatum activates hPAP in KO-ARE and WT-ARE mice in vivo
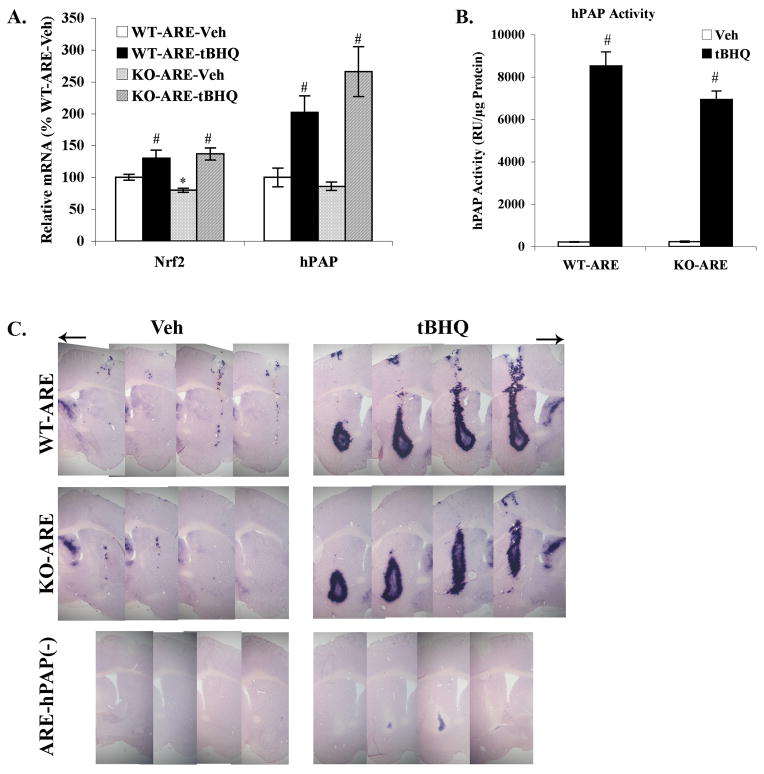
To confirm these in vitro data, tBHQ was directly administrated into the striatum of hPAP reporter mice on a wildtype or DJ-1 knockout background. Seven days later tissue was harvested and analyzed using real time PCR (Fig. 5A), hPAP activity assay (Fig. 5B) and histochemistry (Fig. 5C). The basal level of Nrf2 mRNA was modestly but significantly reduced in DJ-1 KO mice (P = 0.0078, Fig. 5A), however, this small reduction in Nrf2 mRNA was not reflected by a significant decrease in hPAP mRNA. Both Nrf2 (tBHQ, F1,16 = 25.32, P = 0.0001; tBHQ × Gene, F1,16 = 2.46, P = 0.14) and hPAP (tBHQ, F1,12 = 31.94, P = 0.0001; Gene, F1,12 = 1.00, P = 0.34; tBHQ × Gene, F1,12 = 2.45, P = 0.14) mRNA levels were significantly increased after tBHQ injection and a similar increase was observed in both WT and KO mice (Fig. 5A). Furthermore, hPAP activity was increased more than 30-fold after tBHQ injection in both WT-ARE and KO-ARE mice (tBHQ, F1,9 = 453.31, P < 0.0001; Gene, F1,9 = 5.02, P = 0.0518; tBHQ × Gene, F1,9 = 5.14, P = 0.05, Fig. 5B). Finally, histochemical detection of hPAP activity confirmed that there was similar Nrf2 activation following injection of tBHQ in both WT-ARE and KO-ARE mice (Fig. 5C).
Figure 5. Injection of tBHQ into striatum activated hPAP in both DJ-1 WT and DJ-1 KO mice.
tBHQ (50mM) was stereotactically injected into the right striatum with a contralateral control of 10% ethanol in PBS. Tissues were harvested after 7 days. A. mRNA levels of Nrf2 and hPAP after tBHQ administration. All data were standardized by β-actin mRNA level, and compared to WT-ARE vehicle. Mean±SEM, n=4–5. *p<0.05, significantly different from WT-ARE Vehicle sample; #p<0.05, significantly different from corresponding Vehicle treated sample (two-way ANOVA and two-tailed t test). B. hPAP activity was measured after tBHQ injection. ARE hPAP negative mice [ARE-hPAP(−)] were used to subtract background luminescence. Mean±SEM, n=4. #p<0.05, significantly different from corresponding Vehicle treated sample (two-way ANOVA). C. Immunohistochemical staining for hPAP activity were performed on WT-ARE, KO-ARE and ARE-hPAP(−) mice. Representative sections of mice with hPAP activation are shown (n=3, 20-μm sections).
Discussion
DJ-1 was first identified as a mitogen-dependent oncogene that enhanced cell transformation ability in the presence of c-myc or h-ras (Nagakubo et al., 1997). Subsequently, mutations and deletion of DJ-1 have been found to cause recessive early onset Parkinson’s disease in several families (Bonifati et al., 2003). Although nigral pathology in parkinsonian patients has been shown to be associated with oxidative stress, mitochondrial dysfunction, excitotoxicity, dysfuntion of the ubiquitin-proteasome system (Biskup et al., 2008), the clear mechanism(s) underlying DJ-1-mediated PD is still under investigation. More recently, Clements et al. (2006) suggested that DJ-1 was necessary for tBHQ-mediated Nrf2 activation and stabilization based on data generated in primary mouse embryonic fibroblasts (MEFs) from DJ-1 KO mice. These data suggest that reduced Nrf2 protein and activity may contribute to a diminished capacity to handle oxidative stress in patients lacking functional DJ-1. The data presented herein, however, clearly show that Nrf2 activation, Nrf2-dependent gene induction, and Nrf2-mediated neuroprotection are not dependent on the presence of DJ-1 in brain or primary cultures derived from the brain.
The most probable explanation for the difference in these data and that of Clements et al. (2006) is the different cell types used in each study. A liver cell line (Huh7) and MEFs are distinctly different from primary cortical neurons and astrocytes, not to mention brain in vivo. Another study also concluded a link exists between DJ-1 and Nrf2 in DJ-1 knockout MEFs, the human lung epithelial cell line Baes2B and mouse lung in vivo (Malhotra et al., 2008). In these studies, the lack of DJ-1 attenuated cigarette smoke-induced changes mediated through Nrf2. Nrf2-dependent gene changes were moderately affected by the lack of DJ-1 in immortalized MEFs and Baes2B cells under oxidative stress condition. Contrary to Clements and coworker’s data, basal levels of Nrf2 and Nrf2-dependent genes were not affected by DJ-1 disruption. Nrf2 protein did not degrade, and sulforaphane as well as Keap1 siRNA still induced Nrf2 and Nrf2-dependent genes in the absence of DJ-1 (Malhotra et al., 2008). It is obvious that different results have been observed in different cells strongly suggesting that the relationship between DJ-1 and Nrf2 is cell type-specific.
We did find that basal levels of Nrf2 mRNA and protein in primary cortical neurons and in striatum from DJ-1 KO mice decreased modestly compared to DJ-1 WT mice (Fig. 1, 5A). This decrease in Nrf2 mRNA correlated with small declines in Nrf2-dependent gene mRNA levels, but did not translate to reduced Nrf2-dependent protein levels or basal hPAP activity. In addition, the DJ-1 knockdown experiments showed no association between reduced DJ-1 and compromised Nrf2 function.
Malhotra et al. (2008) also used DJ-1 siRNA in vivo through intratracheal administration followed by cigarette smoke exposure. Cigarette smoke increased DJ-1, Nrf2, NQO1 and GCLM mRNA levels in mouse lungs. DJ-1 knockdown blocked the increase of DJ-1, NQO1 and GCLM but did not attenuate the increase in Nrf2. Furthermore, DJ-1 knockdown did not change the basal level of Nrf2 and its regulated genes in mouse lung. Another report also showed that there was no difference in basal level of Nrf2 protein between DJ-1 KO mice and DJ-1 WT mice (Yang et al., 2007). Paraquat decreased Nrf2 protein in ventral midbrain, not in striatum of DJ-1 KO mice. Our data in vivo show that the small decrease basal levels of Nrf2 gene expression do not correlate with reduced basal levels in hPAP expression, or hPAP activity. Finally, the lack of DJ-1 has no effect on Nrf2-mediated increases in hPAP mRNA and activity following in vivo administration of tBHQ. Taken together, these data support the conclusion that no co-dependence between DJ-1 and Nrf2 exists in primary neural cells or tissue.
It has been demonstrated that there is no spontaneous dopaminergic neuron degeneration in DJ-1 KO mice (Kim et al., 2005; Andres-Mateos et al., 2007; Yamaguchi & Shen, 2007), but sensitivity to the dopaminergic neuronal toxin MPTP has been inconsistent. Kim and coworkers showed increased nigral dopaminergic neuron loss and striatal denervation in DJ-1 KO mice after MPTP exposure (Kim et al., 2005). Using the same MPTP subchronic model, Manning-Bog et al. found that there was only increased striatal terminal depletion and no difference in dopaminergic nigral cell loss (Manning-Bog et al., 2007). We did not see increased sensitivity of the DJ-1 KO mice to subchronic MPTP administration (data not shown). Small but significantly decreased mRNA level of Nrf2 in DJ-1 KO mice may or may not contribute to differences in sensitivity to neurotoxins, but the preserved ability to activate Nrf2 in the absence of DJ-1 is probably important for minimizing dopaminergic neuronal damage. Similar to previously published data from our laboratory (Chen et al., 2009), we observed increases in Nrf2 and Nrf2-modulated genes in substantial nigra of DJ-1 knockout mice following MPTP exposure (data not shown). If DJ-1 deletion led to Nrf2 degradation, one would predict that DJ-1 KO mice should respond similar to Nrf2 KO mice. Indeed, the Nrf2 KO mice are more sensitive to MPTP exposure (Burton et al., 2006; Chen et al., 2009); yet most studies discussed above, including our own data, shows that DJ-1 KO mice do not have increased MPTP sensitivity.
In this study, we crossed DJ-1 KO mouse with ARE-hPAP transgenic mouse to reveal the relationship between DJ-1 and the Nrf2-ARE pathway in brain. The data do support the concept that DJ-1 is neuroprotective in vitro, however, the data also clearly show that Nrf2 activation is not dependent on the presence of DJ-1. This clarification of the relationship between DJ-1 and Nrf2 in neural cells implies that targeting Nrf2 in familial DJ-1 associated PD remains a viable therapeutic approach for treatment.
Acknowledgments
We especially thank Dr. Neal C. Burton for editing this manuscript and providing valuable discussions. This work was supported by National Institute of Environment Health Sciences grant (NIEHS ES10042).
Abbreviations
- ARE
antioxidant responsive element
- ARE-hPAP
ARE-driven hPAP
- 4DIV
fourth day in vitro
- GCLC
glutamate-cysteine ligase catalytic subunit
- GCLM
glutamate-cysteine ligase modifier subunit
- HO-1
heme oxygenase
- hPAP
human placental alkaline phosphatase
- KO
DJ-1(−/−) mice
- KO-ARE
DJ-1(−/−)/ARE-hPAP(+) mice
- MEFs
mouse embryonic fibroblasts
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl) 2H tetrazolium, inner salt
- NQO1
NAD(P)H: quinone oxidoreductase
- Nrf2
Nuclear factor erythroid 2-related factor 2
- PD
Parkinson’s disease
- siDJ
anti-mouse DJ-1 siRNA
- siKP
anti-mouse Keap1 siRNA
- siNT
non-targeting siRNA control
- tBHQ
tert-Butylhydroquinone
- tBOOH
tert-butyl hydroperoxide
- WT
wildtype littermate of KO
- WT-ARE
littermate DJ-1(+/+)/ARE-hPAP(+) mice
References
- Abou-Sleiman PM, Healy DG, Quinn N, Lees AJ, Wood NW. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Annals of neurology. 2003;54:283–286. doi: 10.1002/ana.10675. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Biskup S, Gerlach M, Kupsch A, Reichmann H, Riederer P, Vieregge P, Wullner U, Gasser T. Genes associated with Parkinson syndrome. Journal of neurology. 2008;255(Suppl 5):8–17. doi: 10.1007/s00415-008-5005-2. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science (New York, NY. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Han XL, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science (New York, NY. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Hedrich K, Djarmati A, Schafer N, Hering R, Wellenbrock C, Weiss PH, Hilker R, Vieregge P, Ozelius LJ, Heutink P, Bonifati V, Schwinger E, Lang AE, Noth J, Bressman SB, Pramstaller PP, Riess O, Klein C. DJ-1 (PARK7) mutations are less frequent than Parkin (PARK2) mutations in early-onset Parkinson disease. Neurology. 2004;62:389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and biophysical research communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & development. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. Journal of neurochemistry. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Annals of the New York Academy of Sciences. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and cellular biology. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. The Journal of biological chemistry. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Moehlenkamp JD, Hanson JM, Johnson JA. Nrf2-dependent activation of the antioxidant responsive element by tert-butylhydroquinone is independent of oxidative stress in IMR-32 human neuroblastoma cells. Biochemical and biophysical research communications. 2001;280:286–292. doi: 10.1006/bbrc.2000.4106. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. American journal of respiratory and critical care medicine. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Manning-Bog AB, Caudle WM, Perez XA, Reaney SH, Paletzki R, Isla MZ, Chou VP, McCormack AL, Miller GW, Langston JW, Gerfen CR, Dimonte DA. Increased vulnerability of nigrostriatal terminals in DJ-1-deficient mice is mediated by the dopamine transporter. Neurobiology of disease. 2007;27:141–150. doi: 10.1016/j.nbd.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free radical research. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochemical and biophysical research communications. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. The Journal of biological chemistry. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. The Journal of biological chemistry. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. Journal of neurochemistry. 2006;97:687–696. doi: 10.1111/j.1471-4159.2006.03742.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nature genetics. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wilson MA, St Amour CV, Collins JL, Ringe D, Petsko GA. The 1.8-A resolution crystal structure of YDR533Cp from Saccharomyces cerevisiae: a member of the DJ-1/ThiJ/PfpI superfamily. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1531–1536. doi: 10.1073/pnas.0308089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Shen J. Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-deficient mice. Molecular neurodegeneration. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen L, Ding Y, Zhuang X, Kang UJ. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Human molecular genetics. 2007;16:2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]