Abstract
In the field of nanomedicine there is a great demand for technologies that allow the creation of self-assembled structures of which the size and morphology can be accurately controlled. In the current study, we report a nanoparticle platform that is composed of a paramagnetic lipid and a fluorescently labeled lipopeptide. By judiciously controlling the ratio of the aforementioned amphiphilic molecules a variety of well-defined nano-sized supramolecular structures with different sizes and morphologies could be created. The hydrodynamic radii of the different structures were determined by dynamic light scattering. Cryo-TEM revealed the aggregate morphology to vary from small micellar structures to plate-like and even full grown ribbons of which the aspect ratios varied from a diameter of 5–8 nm to structures with a width of up to 25 nm and infinite length. Interestingly, nuclear magnetic resonance dispersion profiling revealed excellent properties for MRI and also showed that the relaxivity of the structures was tunable and morphology dependent. Finally, macrophage cells were treated with two selected nanoparticles and were shown to be avidly taken up.
In conclusion we demonstrate a methodology to create structures that are (1) paramagnetic to enable their detection with MRI, (2) exhibit fluorescent properties, (3) can be tuned to defined sizes and shapes and (4) are efficiently taken up by macrophage cells in vitro.
In the field of molecular imaging nanoparticulate structures are becoming increasingly important, since they can carry high payloads of contrast generating materials while their surface can be functionalized to improve bio-compatibility or to introduce specificity. 1,2 In addition to inorganic nanocrystals, such as quantum dots (QDs)3 and iron oxide4 or gold nanoparticles,5 self-assembled organic structures including liposomes6, micelles7 and microemulsions8 have shown great potential for in vivo imaging. In the field of targeted imaging and therapy the pharmacokinetic profile as well as the tissue penetration potential of the nanovehicles is of paramount importance. Hence, there is a great demand for nanostructures of which the final morphology and size can be judiciously controlled. To this aim, we created a variety of well-defined nano-sized supramolecular structures based on two amphiphilic molecules: Gd-DTPA-DSA, a Gd3+ chelating lipid which exhibits paramagnetic properties for MRI,6,9 and P2fA2 a fluorescein labeled apolipoprotein E derived lipopeptide that has been shown to enhance nanoparticle uptake into rat brain capillary endothelial cells in vitro.10
Here we show how we can control the morphology and size of the imaging probes by varying the ratio of the two amphiphiles and thereby optimizing the molecular relaxivity of the resulting lipidic aggregates. The two most effective morphologies were selected for in vitro studies and were demonstrated to be effectively internalized by macrophage cells.
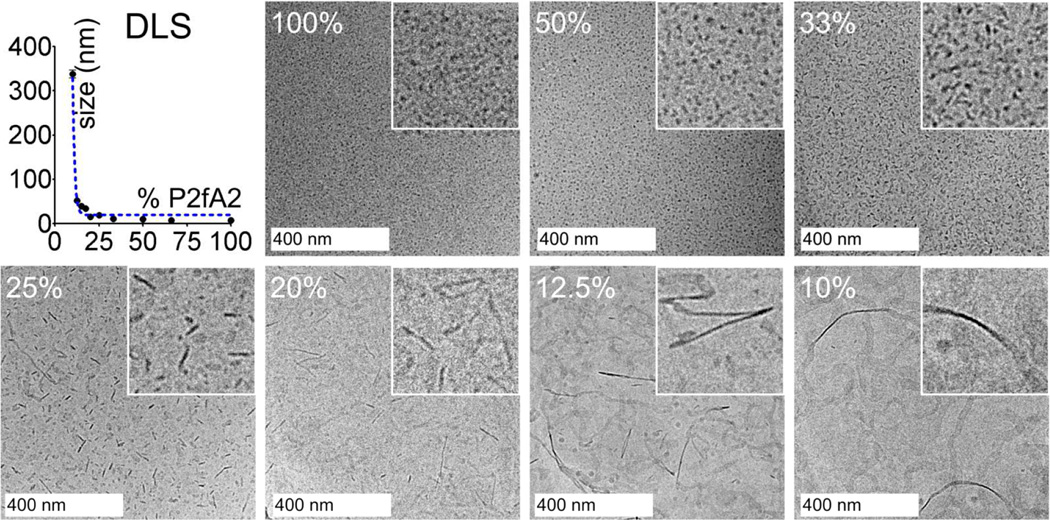
Aqueous dispersions of mixtures of P2fA2 and Gd-DTPA-DSA were prepared (Table 1 Supporting Information) and dynamic light scattering (DLS) revealed that the aggregates had narrow size distributions (Supporting Information Figure S1) and that their mean hydrodynamic diameters increased with decreasing P2fA2/Gd-DTPA-DSA ratios (Figure 1). At high P2fA2 contents of 50 mol% or more small structures with a mean diameter below 10 nm were observed, whereas a transition to larger aggregates (8–15 nm) was observed in case the P2fA2 content was lower than 33 mol%. Below 20 mol% P2fA2 the mean hydrodynamic diameter increased to ~60 nm for 12.5 mol% P2fA2 and to more than 300 nm for a formulation that contained 10 mol% P2fA2.
Figure 1.
Dynamic light scattering (top, left) and cryo-TEM images of different P2fA2/Gd-DTPA-DSA preparations. The insets show a close up of the cryo-TEM images and represent 200 by 200 nm. The percentages refer to the amount of P2fA2 in the formulations.
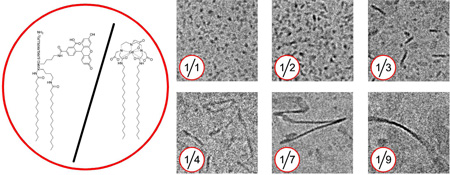
In line with the DLS measurements cryo-TEM revealed aggregates of which the size increased with decreasing P2fA2 concentration (Figure 1). At P2fA2 concentrations ≥ 50 mol% small micellar structures with diameters of 5–8 nm were observed. By decreasing the P2fA2 concentration plate-like morphologies appeared with a constant thickness of 5–8 nm of which the aspect ratios increased, from by 10 × 15–25 nm (33 mol% P2fA2) to 10–15 × 150 nm (25 mol% P2fA2) and up to 10–15 × 250 nm (20 mol% P2fA2). In the preparations that contained 10–12.5 mol% P2fA2 fully grown ribbons were present of up to 25 nm in width and with infinite lengths.
Previously, in a study by Johnsson and Edwards it was demonstrated that by carefully mixing regular phospholipids and poly(ethylene glycol) (PEG) conjugated phospholipids a variety of structures, ranging from vesicular, discoidal and micellar, could be created. 11 The morphological changes were ascribed to the differences in geometry of the amphilphiles used. In our study Gd-DTPA-DSA tends to form bilayers with little curvature, while P2fA2 acts like a strong detergent and curves such that it forms micelles. Therefore aggregates of mixtures of such molecules can have morphologies that are more spherical, plate-like and even long elongated structures (Supporting Information Figure S2), depending on the ratio of both components.
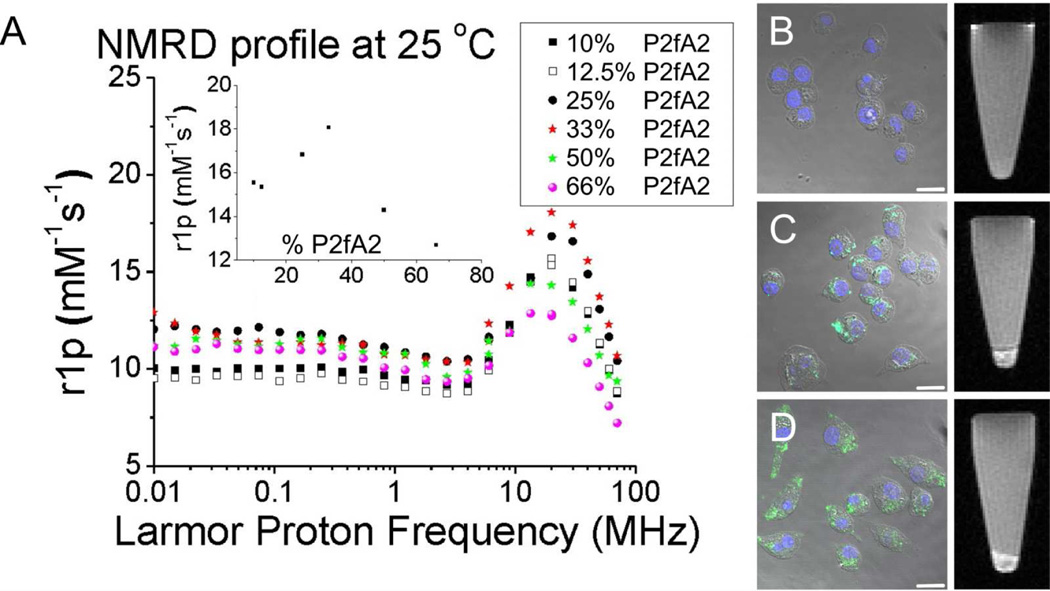
To assess the applicability of the different nanostructures for magnetic resonance imaging and to characterize their paramagnetic properties nuclear magnetic resonance dispersion profiles (NMRD-profiles) were obtained (Figure 2). Typical so-called macromolecular profiles with maximum intensities around 20 to 30 MHz were observed. These indicate a diminished mobility of the Gd3+ chelates in the lipidic aggregates which causes their tumbling rate τR to decrease. This result is typical for supramolecular and macromolecular contrast agents12 at these moderate, but clinically relevant field strengths and is not observed for low molecular weight Gd3+ chelates. Moreover, these results further support the incorporation of Gd-DTPA-DSA in the lipidic aggregates. Our approach allowed the formation of structures with tunable ionic relaxivities, varying between 13 to 18 mM−1s−1 at 20 MHz (inset Figure 2), which is 3 to 5 times higher than commercially available Gd-DTPA at this field strength. Interestingly, the highest ionic relaxivity of 18 mM−1s−1 was observed for the formulation containing 33% P2fA2, the preparation with predominately small plate-like aggregates.
Figure 2.
(A) NMRD-profiles of the different structures. The inset displays the relaxivity as function of %P2fA2 at 20 MHz. Confocal microscopy (left) and MRI of loosely packed cell pellets (right) of macrophage cells that were (B) left untreated, incubated with 33% (C) and (D) 50% P2fA2 nanoparticles. Scale bar: 20 µm.
In general such differences in relaxivity are largely determined by the variations in the interplay between the rotation correlation time (τR) and the exchange of water molecules coordinated to a single Gd3+ chelate. At high P2fA2 concentrations, i.e. at low local concentrations of Gd-DTPA-DSA, it is unlikely that the water exchange rate is the limiting factor. Therefore the observed sub-optimal relaxivity at this composition is most likely due to the high τR, related to the small aggregate dimensions. Although the aggregate size increases with decreasing P2fA2 concentrations, a reduction in the relaxivity is observed upon further lowering of the P2fA2 content past the optimum value of 33 mol%. In this regime, the reduction in ionic relaxivity cannot be related to τR, as this parameter is expected to decrease further with the increasing dimensions of the aggregates. Rather, at the concomitant higher Gd-DTPA-DSA concentrations, the water exchange will become a limiting factor and the amount of water that is relaxed per Gd3+ chelate will be limiting the ionic relaxivity. Clearly at 33 mol% we find the morphology that optimally benefits from a low rotation correlation time associated with the aggregate dimensions and a Gd3+ surface concentration that allows a high enough water exchange rate.
Based on these results we set out to test these P2fA2 containing nanostructures for their suitability as MR imaging probes for extravascular targets, such as macrophages. Since small nanoparticles are more likely to escape from the circulation and cross the diseased and permeable endothelium, the two formulations with a high relaxivity and small size, i.e. those with 33 mol% (small platelets) and 50 mol% (micelles) P2fA2 were selected for further in vitro experiments with cultured mouse macrophage cells (J774A1). The incorporation of P2fA2 and Gd-DTPA-DSA allowed the visualization of nanoparticle uptake using fluorescence confocal laser scanning microscopy and MRI, respectively (Figure 2). The CLSM images show nuclei in blue (DAPI) and P2fA2 in green, merged with bright field images of the macrophage cells. While control cells revealed no nanoparticle-associated fluorescence or MRI contrast (Figure 2B), significant amounts of intracellular fluorescence as well as MRI signal enhancement were observed after 30 min of incubation for cells that were incubated with 50% (Figure 2C) and 33% (Figure 2D) P2fA2 nanoparticles. In addition, we performed quantitative uptake experiments under inhibitory conditions (Supporting Information Figure S3). The results revealed the particle uptake to be specific and further confirmed their multifunctional character.
In summary we have demonstrated that by carefully controlling the ratio of two functional amphiphiles Gd-DTPA-DSA and P2fA2, it was possible to create a variety of well-defined supramolecular structures, containing both fluorescein and Gd3+ chelates. Moreover, we showed that by changing the morphology we could optimize the relaxivity of the imaging probes, since NMRD-profiling disclosed excellent and tunable MRI properties for the different formulations, especially at clinically relevant field strengths.
We envision the application of these contrast agents for pathologies in which macrophage inflammation plays a key role, such as atherosclerosis, rheumatoid arthritis, or cancer.13
Supplementary Material
Acknowledgement
Partial support was provided by NIH/NHLBI R01 HL71021, NIH/ NHLBI R01 HL78667 (ZAF) and the DFG (DA 324/5-2 and FG 463, E.L.). David P. Cormode and Heike Nikolenko are gratefully acknowledged for their input.
Footnotes
Supporting Information Available: Figure S1–S3 Table S1 and detailed experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Nat.Rev.Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Nat.Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 4.Bulte JW, Kraitchman DL. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 5.Alric C, Taleb J, Le Duc G, Mandon C, Billotey C, Meur-Herland A, Brochard T, Vocanson F, Janier M, Perriat P, Roux S, Tillement O. J.Am.Chem.Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- 6.Mulder WJ, Strijkers GJ, Griffioen AW, van Bloois L, Molema G, Storm G, Koning GA, Nicolay K. Bioconjug.Chem. 2004;15:799–806. doi: 10.1021/bc049949r. [DOI] [PubMed] [Google Scholar]
- 7.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JG, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, Fisher EA, Fayad ZA. Proc.Natl.Acad.Sci.U.S.A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanza GM, Winter PM, Caruthers SD, Hughes MS, Cyrus T, Marsh JN, Neubauer AM, Partlow KC, Wickline SA. Nanomedicine. 2006;1:321–329. doi: 10.2217/17435889.1.3.321. [DOI] [PubMed] [Google Scholar]
- 9.(a) Mulder WJ, Koole R, Brandwijk RJ, Storm G, Chin PT, Strijkers GJ, de Mello DC, Nicolay K, Griffioen AW. Nano.Lett. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]; (b) van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, Tang CY, Gordon RE, Nicolay K, Meijerink A, Fayad ZA, Mulder WJ. Nano.Lett. 2008;8:2517–2525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 10.(a) Keller S, Sauer I, Strauss H, Gast K, Dathe M, Bienert M. Angew.Chem.Int.Ed Engl. 2005;44:5252–5255. doi: 10.1002/anie.200500519. [DOI] [PubMed] [Google Scholar]; (b) Sauer I, Nikolenko H, Keller S, Abu AK, Bienert M, Dathe M. Biochim.Biophys.Acta. 2006;1758:552–561. doi: 10.1016/j.bbamem.2006.03.017. [DOI] [PubMed] [Google Scholar]; (c) Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M. Biochemistry. 2005;44:2021–2029. doi: 10.1021/bi048080x. [DOI] [PubMed] [Google Scholar]
- 11.Johnsson M, Edwards K. Biophys.J. 2003;85:3839–3847. doi: 10.1016/S0006-3495(03)74798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aime S, Botta M, Terreno E. Advances in Inorganic Chemistry - Including Bioinorganic Studies, Vol 57. 2005;57:173–237. [Google Scholar]
- 13.(a) Libby P, Ridker PM, Maseri A. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]; (b) Firestein GS. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]; (c) Mantovani A, Allavena P, Sica A, Balkwill F. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.