Abstract
Objective
Along with impaired discrimination, patients with Alzheimer’s disease (AD) often show an abnormally liberal response bias (greater tendency to respond “old”). Previously we matched discrimination by varying study-test list length and found that participants’ usual bias is maintained, such that patients with AD were more liberal than healthy controls. However, this pattern could be a result of the way in which discrimination was matched. In this experiment, we examined whether matching discrimination with the use of a delay would lead to a liberal response bias in healthy younger and older adults as it might lead to the use of more similar memorial processing to the patients with AD.
Method
Younger adults, older adults, and patients with AD were run in two study-test sessions, with study and recognition test separated by either a one-minute or one-day delay.
Results
With the one-minute delay, both younger adults and healthy older adults showed a conservative response bias, while patients with AD showed a liberal response bias. When discrimination was matched between patients with AD and controls by the use of a delay, response bias was also matched, with all participants showing a more liberal response bias.
Conclusions
The current study suggests that how discrimination is matched between patients with AD and controls matters greatly. Potentially, this liberal bias is a result of healthy younger and older adults relying primarily on familiarity at the longer delay, thus using more similar memorial processes to patients with AD who are dependent on familiarity at any delay.
Keywords: recognition memory, Alzheimer’s disease, response bias, familiarity, recollection
Introduction
Even more frequently than healthy older adults, patients with Alzheimer’s disease (AD) suffer from distortions of memory, in addition to failing to retrieve desired information. Although sometimes these distortions can be extreme, as in confabulation (Dalla Barba, Nedjam, & Dubois, 1999; Tallberg & Almkist, 2001), distortions may also be more mundane and common. For example, patients who suffer only from a failure to retrieve information and cannot remember whether they have turned off the stove, can check it again. Similarly, patients who have difficulty remembering whether they have taken their medications can use organizational strategies (e.g., a pill box) to aid their memory. However, reminders and organizational strategies will be of little help for patients who falsely remember that they have turned off the stove or taken their medications, and these patients will need additional supervision.
In examining false recognition experimentally, the focus has mainly been on a specific type of false recognition which occurs when subjects falsely recognize items because they share the general meaning, idea, or gist conveyed by a collection of items (gist information or gist memory; cf., Reyna & Brainerd, 1995; Schacter, Norman, & Koutstaal, 1998). Using a modification of the Deese/Roediger-McDermott (DRM) paradigm, Budson, Daffner, Desikan, and Schacter (2000) found that compared to healthy younger and older adults, patients with AD showed similar levels of uncorrected false recognition of semantic associates after a single study-test trial, but higher levels of false recognition across five trials. These results suggested that over the study-test trials, the patients with AD built up gist memories that led to more false alarms while the healthy younger and older adults were able to reduce their false memories with increased item-specific memory.
In addition to this gist-based false recognition, however, we and other investigators have found that patients with AD also show high levels of false recognition of unrelated items (Balota, Burgess, Cortese, & Adams, 2002; Bartok et a., 1997; Budson, Wolk, Chong, & Waring, 2006; Snodgrass & Corwin, 1988). False alarms to unrelated items are often used as the baseline false alarm rate in recognition memory tests and are usually subtracted from the hit rate to calculate “corrected” recognition scores. Few studies, however, have specifically examined this high rate of baseline false alarms in AD. Most researchers have typically considered this high rate of baseline false alarms to non-studied unrelated items observed in AD to be a “nuisance”—something to be analytically subtracted away—rather than investigated.
One way to further examine these false alarms to unrelated items is to look at measures of response bias. Response bias quantifies the tendency to either respond in a predominantly liberal (i.e., responding “old” frequently) or conservative (i.e., responding “old” infrequently) direction. Several studies have investigated changes in response bias with aging. Some investigations have demonstrated a more conservative response bias with age (although only at highest education levels; Marcuie & Baracat, 2000), while others have found that in older participants, increasing age correlated with increasing liberal response bias (Huh, Kramer, Gazzaley, & Delis, 2006). Whereas findings with healthy older adults have been mixed, many studies have found that patients with AD demonstrate a significant liberal response bias (Balota et al., 2002; Bartok et al., 1997; Snodgrass & Corwin, 1988).
An outstanding question was whether this liberal response bias in patients with AD was simply a result of their poor discrimination. Response bias and discrimination performance in patients with AD was dissociated in a study that equated discrimination levels between patients with AD and healthy older controls (Budson et al., 2006). In this study, discrimination was equated by presenting study-test lists of increasing length. When healthy older adults studied 160 words and then were tested on 320 words (160 old, 160 new), their discrimination did not significantly differ from patients with AD when they studied 10 words and were tested on 20 words (10 old, 10 new). At this discrimination level, the patients with AD showed a liberal response bias while the healthy older adults showed a conservative response bias. The healthy older adults still showed a conservative response bias even with poor discrimination. Importantly, response bias remained constant across the discrimination levels for both patients with AD and the older controls indicating that while list length had a significant effect on discrimination, it was not related to bias. Following up on the results of Budson et al. (2006), Beth, Waring, Budson, & Ally (2009) examined whether improving discrimination by using pictures as stimuli would result in a more conservative response bias for the patients with AD. Although the patients’ discrimination was better for the pictures than for the words, their bias remained equally liberal for words and pictures.
Although we found that changes in study-test list length and stimulus type did not alter response bias, it is still unclear whether other ways of matching performance, such as a delay, would lead to different results. Giovanello and Verfaellie (2001) suggested that it was essential to consider the method of matching performance between patient groups and healthy controls. In their experiments, they matched recognition between a group of patients with amnesia (mixed etiology) and healthy controls in order to examine differences in free recall performance. Recognition performance was matched either by providing the amnesic patients with additional study time or by testing healthy controls after a long delay. They found that free recall performance differed depending on the method of matching recognition. The healthy older controls performed more similarly to the patients with amnesia after the long delay and it was proposed that after this delay the controls were relying on memorial processes more similar to the patient group. Similar to free recall performance, response bias might be influenced by the type of memorial processes used by controls and, thus, a delay might result in different patterns of response bias than manipulating list length or stimulus type.
An important issue is whether the method of matching discrimination between the groups could change the response bias patterns. In the current experiment, we explored whether matching discrimination between patients with AD and healthy older controls using a delay between study and test would show the same response bias differences between groups as when manipulating list length. To investigate this question, participants were given two study-test sessions with the study and test phases separated by either a short (one minute) or long (one day) delay. Discrimination performance and response bias was compared between the two delay conditions. We predicted that at the short delay healthy younger and older adults would show a more conservative response bias than patients with AD, consistent with prior work. Potentially a delay between study and test will force healthy younger and older adults to rely on more similar memorial processes as the patients with AD and thus show a similar liberal response bias at the long delay.
Method
Participants
Sixteen healthy younger adults (5 male), 16 healthy older adults (5 male), and 12 patients (9 male) with a clinical diagnosis of probable AD were recruited for this study. Healthy younger and older adults were recruited from online and community postings in the Boston area. In addition, some of the healthy older adults were also spouses and friends (but not blood relatives) of the AD patients who participated in the study. Patients with probable mild AD met criteria described by the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (McKahn et al., 1984) and were recruited from the Boston University Alzheimer’s Disease Center (BU ADC). These patients were each assessed and diagnosed by a neurologist and neuropsychologist and were otherwise healthy. Participants were screened for clinically significant depression, alcohol or drug use, past stroke, traumatic brain injury, or other neurologic disorder. All participants were native English speakers and had normal or corrected to normal vision. The study was approved by the human studies committees of the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA, and Boston University, Boston, MA. Written informed consents were obtained from all participants and from their caregivers when appropriate. Participants were paid $10/hour for their participation.
The healthy older adults and patients with AD completed a brief neuropsychological battery in a 45-minute session either directly following the experimental session or on a separate date. This battery included the MMSE (Folstein, Folstein, & McHugh, 1975), CERAD Word List Memory Test (Morris et al., 1989), Trail Making Test Part B (Adjutant General’s Office, 1944), Verbal fluency to letters and categories (Monsch et al., 1992), and the short form Boston Naming Test (Mack, Freed, Williams, & Henderson, 1992). Table 1 presents demographic and neuropsychological data for the participants.
Table 1.
Demographic and Neuropsychological data for participant groups
| Test | Younger Adults Mean (SD) |
Older Adults Mean (SD) |
Patients with AD Mean (SD) |
|---|---|---|---|
| Age | 22.9 (2.39) | 75.1 (6.16) | 78.3 (4.90) |
| Years of Education | 15.4 (2.13) | 16.1 (2.66) | 14.7 (3.28) |
| MMSE | 29.6 (.62) | 26.4 (2.43) | |
| CERAD | |||
| Immediate | 20.8 (4.06) | 11.8 (3.22) | |
| Delayed | 6.6 (2.23) | 1.83 (1.70) | |
| Recognition | 9.2 (1.64) | 6.75 (2.41) | |
| Trails-B | 102.4 (46.08) | 195.9 (89.25) | |
| FAS | 48.2 (12.51) | 33.6 (16.02) | |
| CAT | 45.6 (9.32) | 30.9 (8.98) | |
| BNT-15 | |||
| No cue | 13.7 (2.40) | 12.83 (3.13) | |
| Semantic cue | 0.07 (0.27) | 0.08 (0.29) | |
| Phonemic cue | 1.07 (0.45) | 1.5 (1.98) | |
Trails B was unavailable for one healthy older adult, Verbal Fluency was unavailable for two healthy older adults, Boston Naming Test was unavailable for two healthy older adults
Although the average age of the AD patients was 3 years greater than the older adult controls, this difference was not significant (F(1,26) = 2.0, p = .168). There was also no significant difference in years of education reported between patients with AD, older adult controls, and younger adults (ps > .3). Patients with AD were in the very mild or mild stage of the disease based upon their performance on the MMSE (mean = 26.4, range = 22–30). Older controls scored significantly higher than AD patients on the MMSE (F(1,26) = 25.97, p < .001), CERAD immediate recall (F(1,26) = 39.30, p < .001), CERAD delayed recall (F(1,26) = 37.41, p < .001), CERAD recognition (F(1,26) = 10.12, p < .01), lexical fluency (FAS: F(1,24) = 6.84, p < .05), and categorical fluency (CAT: F(1,24) = 16.68, p < .001). Older adults were also faster to complete the Trail Making Test Part B (F(1,25) = 12.43, p < .01). There were no significant differences between AD patients and older controls on the Boston Naming Test measures.
Materials
The stimuli were 640 words, three to eight letters in length with Kucera-Francis frequencies between 10–700, generated from the University of Western Australia MRC Psycholinguistic Database (http://www.psy.uwa.edu.au/MRCDataBase/uwa.mrc.htm). The words were then divided into four lists of 160 counterbalanced by word length and frequency. These four lists were used as study and test lists for the short delay (one minute) and long delay (one day) conditions. Assignment of lists to experimental conditions was counterbalanced across participants. Words were presented in 40 point Chicago font in the center of the screen at both study and test.
Procedure
The experiment consisted of two sessions on consecutive days. Each participant completed two study-test blocks: one block separated by a short delay (one minute) and one block separated by a long delay (one day). For half of the participants, the first session consisted of a study phase immediately followed by a test phase after a one-minute delay and then concluding with a second study phase. After a one-day delay, the second session consisted of the second test phase. For the other half of the participants, the first session consisted only of a study phase. In the second session (after one day), these participants completed the first test phase, then a second study phase followed by a second test phase after a one-minute delay.
Each study phase consisted of 160 words and each test phase consisted of 320 words (half studied, half new). Because response bias values (C) become more constrained with increasing performance (Snodgrass & Corwin, 1988), we wanted to assure that our ability to determine response bias in healthy subjects including young adults was not hampered by near ceiling performance. There was no stimuli overlap between the two study-test blocks. In the study phases, each word was presented for 2500 ms with a 400 ms interstimulus interval. Participants were instructed to read each word aloud and to remember them for a subsequent memory test. For the short delay condition, participants worked on a number search for one minute after completing the study phase. In the test phases, participants were instructed to respond “old” if the word had been presented before or “new” if the word had not been presented previously. The test word remained on the screen until participants responded.
Results
In all analyses, younger adults and older adults were compared to examine the effects of aging and then the older adults were compared to the patients with AD to examine the effects of disease.
Hits and false alarms
Younger adults versus older adults
A repeated-measures ANOVA was conducted using between-subjects factors of group (younger versus older adults) and test order (short first versus long first) and a within-subjects factor of delay condition (short versus long) for hit rates. This analysis resulted in no significant main effects or interactions.
An analogous repeated-measures ANOVA was conducted for false alarm rates. Participants demonstrated a main effect of delay (F(1,28) = 53.72, p < .001, η2 = .657) with no interaction with group. Both younger and older adults made more false alarms after the long delay than the short delay (see Figure 1). There was also a main effect of test order (F(1,28) = 4.50, p < .05, η2 = .138) which was a result of a higher false alarm rate when the long delay condition was first (40.3%) than when the short delay condition was first (31.3%). Importantly, this main effect did not interact with delay or group.
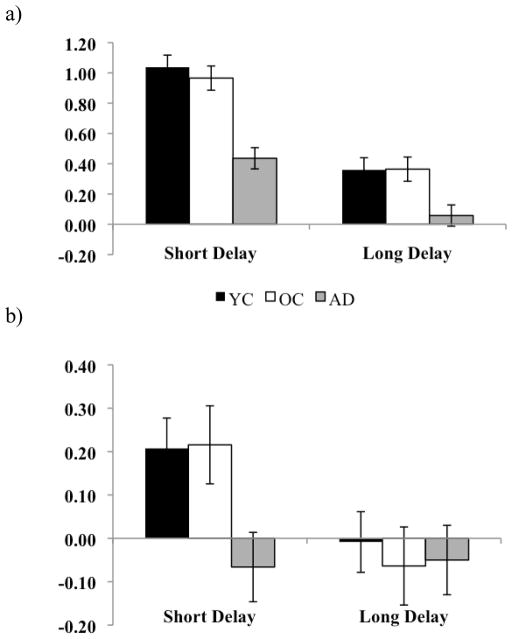
Figure 1.
a) Proportion of hits for healthy young adults (YC), healthy older adults (OC), and patients with AD at both the short and long delay. b) Proportion of false alarms for young adults, older adults, and patients with AD at the short and long delay.
Older adults and AD patients
A repeated-measures ANOVA was conducted using between-subjects factors of group (older versus patients with AD) and test order (short first versus long first) and a within-subjects factor of delay condition (short versus long) for hit rates. Participants demonstrated a main effect of test order (F(1,24) = 5.8, p < .05, η2 = .195). Participants had an increased hit rate when the long delay occurred first (64.1% versus 52%) but this did not interact with delay or group.
An analogous repeated-measures ANOVA was conducted for false alarm rates. This ANOVA for false alarms demonstrated a significant effect of delay (F(1,24) = 26.95, p < .001, η2 = .529) as well as a significant interaction between delay and group (F(1,24) = 6.85, p < .05, η2 = .222). Older adults demonstrated a significant increase in false alarms after the long delay compared to after the short (t(15) = 5.48, p < .001) while patients with AD showed a marginal increase in false alarms with the longer delay (t(11) = 2.18, p < .06). The interaction is a result of a larger magnitude of change in the false alarm rate for the healthy older controls (average FA difference = .20) than for AD (average FA difference = .07, t(26) = 2.70, p < .05).
d′ and C
To examine discrimination and response bias, we calculated d′ and C (Snodgrass & Corwin, 1988). High values of d′ indicate greater discrimination, while a d′ value of zero indicates chance performance. Response bias, measured by C, can be either conservative (less likely to respond old; indicated by positive values) or liberal (more likely to respond old; indicated by negative values).
d′ data
Younger adults versus older adults
A repeated-measures ANOVA was conducted using between-subjects factors of group (younger versus older adults) and test order (short first versus long first) and within-subjects factor of delay condition (short versus long) examining discrimination (d′). Participants demonstrated a main effect of delay (F(1,28) = 62.44, p < .001, η2 = .69) but this did not interact with group or test order (see Figure 2a). Both younger and older adults performed better after the short delay than after the long delay.
Figure 2.
a) Discrimination (d′) for healthy young adults (YC), healthy older adults (OC), and patients with AD at both the short and long delay. b) Response bias (C) for young adults, older adults, and patients with AD at the short and long delay.
Older adults and AD patients
A repeated-measures ANOVA was conducted using between-subjects factors of group (older versus patients with AD) and test order (short first versus long first) and a within-subjects factor of delay condition (short versus long) examining discrimination (d′). Participants demonstrated decreased discrimination after the longer delay (F(1,24) = 94.91, p < .001, η2 = .798) which also showed an interaction with group (F(1,24) = 4.72, p < .05, η2 = .164; see Figure 2a). This interaction is likely due to the fact that while the difference between delays were significant for both groups (OC: t(15) = 8.08, p < .001; AD: t(11) = 7.32, p < .001), the decrease in discrimination at the longer delay was larger for the healthy older controls (average d′ difference = .60) than for AD (average d′ difference = .38, t(26) = 2.29, p < .05). Importantly, there was no difference in discrimination between the healthy older controls at the short delay (d′ = .36) and the patients with AD at the long delay (d′ = .43; t(26) < 1, ns).
C data
Younger adults versus older adults
A repeated-measures ANOVA was conducted using between-subjects factors of group (younger versus older adults) and test order (short first versus long first) and a within-subjects factor of delay condition (short versus long) examining response bias (C). Participants demonstrated a main effect of delay (F(1,28) = 15.53, p < .01, η2 = .357) indicating that response bias became more liberal with the delay but this did not interact with group or test order (see Figure 2b).
Older adults and AD patients
A repeated-measures ANOVA was conducted using between-subjects factors of group (older versus patients with AD) and test order (short first versus long first) and within-subjects factor of delay condition (short versus long) examining response bias (C). Participants demonstrated a marginal main effect of delay (F(1,24) = 3.67, p < .07, η2 = .133) which did show a significant interaction with group (F(1,24) = 4.43, p < .05, η2 = .156; see Figure 2b). Additional analyses were performed to further characterize the interactions between delay and participant group. Older adults showed a shift to a more liberal response bias with the long delay (t(15) = 2.93, p < .05) while the patients with AD remained liberal at both delays (t(11) < 1, ns). When discrimination was matched (comparing the short delay performance for the patients with AD to the long delay performance of the healthy controls), response bias was also matched between the two groups (AD: C = −.07, OC: C = −.06; t(26) < 0.1, ns).
Discussion
Supporting our initial predictions, we found that healthy younger and older adults showed a more liberal response bias with a longer study-test delay, similar to the patients with AD, who remained consistently liberal. Although the hit rates did not differ drastically across the delay or between groups, the false alarm rates for healthy younger and older adults increased with the long delay, while the patients with AD had a large false alarm rate at both delays. Healthy younger and older adults showed a lower d′ at the long delay than at the short delay. The patients with AD also showed a decrease in d′ at the longer delay, but not as large a decrease as for the healthy older adults potentially because patients were impaired at both delays.
Unlike our prior study that matched discrimination by varying study-test list length (Budson et al., 2006), when discrimination was matched between patients with AD and healthy controls by inserting a study-test delay, both groups showed a liberal response bias. Our results therefore suggest that the method used to match discrimination performance can influence the memorial processes used and that the results of using different methods can be informative in understanding underlying mechanisms of memory and response bias in various populations.
Our results are consistent with prior studies that examined criterion shifting across differing delays in younger adults (Singer & Wixted, 2006; Singer, Gagnon, & Richards, 2002). In Singer and Wixted (2006), no criterion shifts were found between items studied immediately before the test and items studied 20–40 minutes before, but participants shifted to a more liberal response bias for items studied 2-days before. Singer and Wixted proposed that a single process could underlie this effect as participants are forced to rely on weaker memory traces with a longer delay. They also suggested that at the longer delay, healthy younger adults are aware that their memory is not as reliable as at the shorter delay and consciously adjust their criterion accordingly.
Although a single process theory could explain our results, they could also be explained by dual process theory, which suggests that two independent processes contribute to accurate recognition decisions: recollection and familiarity (Jacoby & Dallas, 1981; Yonelinas, 2002). Recollection is specific recall of an event/item that brings to mind particular details. Familiarity is a more general sense of having encountered an event or item before without recall of the specific context. Prior research suggests that a longer delay reduces recollection and thus memorial judgments in this test phase may be based more on familiarity (Gardiner & Java, 1991; Giovanello & Verfaellie, 2001; Hockley & Consoli, 1999; Yonelinas, 2002). Since recollection is severely impaired in patients with AD, they are forced to rely mainly on familiarity (Balota et al., 2002; Budson et al., 2000; Gallo, Sullivan, Daffner, Schacter, & Budson, 2004; Knight, 1998; Koivisto, Portin, Seinela, & Rinne, 1998; Smith & Knight, 2002; Wolk et al., 2005; Wolk, Dickerson et al., 2011; Wolk, Dunfee, et al., 2011). While familiarity is not entirely spared in patients with AD, it is better preserved than recollection (Ally, Gold, & Budson, 2009; Ally, McKeever, Waring, & Budson, 2009; Westerberg et al., 2006). This reliance on familiarity may contribute to the abnormally liberal response bias shown by AD patients. By introducing a day delay, we potentially caused increased reliance upon familiarity that in turn may have led to a more liberal response bias in younger and older adults—forcing them to use memorial processes resembling those of the patients at any delay.
If our hypotheses are correct, the results of the present study suggest that relying on familiarity alone may lead to a more liberal response bias than if memorial judgments are based on both recollection and familiarity. After a short delay, younger and older adults with no memory impairments will likely rely more on recollection to make memorial judgments and may show a conservative bias. For example, if you vividly remember studying the word “cat” because you recall thinking of your own cat and also the word that came after it in the list, then you are experiencing strong feelings of recollection. If this subjective sense of recollection occurs for several items, then you might demand this type of recollection for each item before responding old. When memory strength is weaker and judgments are no longer based on recollection, then these judgments are likely determined by the familiarity strength (Yonelinas, 2002). When memorial decisions are based more on familiarity than recollection, such as after a long delay, you may show a liberal response bias since you are not experiencing strong recollection for many items. If you do not experience the sense of vivid recollection (as when you saw the word “cat” at the short delay) for many test items, then you might endorse items as old based on less evidence. For example, you might be more likely to respond old to “coffee” because it is familiar to you even though you are not certain whether the feeling of familiarity is present because you studied it previously or because you had a cup this morning. Thus, manipulations such as list length and delay might not lead to the same memorial expectations. Although increasing list length boosts difficulty, healthy older adults are likely still relying on recollection (along with the use of some familiarity) to make recognition decisions and are thus relying on different memorial processes than those available to the patients with AD that may result in a more conservative response bias. After a delay, healthy participants may not expect to rely on the same memorial processes as they did after one minute and thus may rely more on familiarity than recollection (similar to patients with AD) resulting in a more liberal response bias. These ideas are consistent with Hudon, Belleville, and Gauthier (2009) who used a Remember-Know paradigm (similar but not identical to asking for Recollection versus Familiarity judgments) and found that all groups were more conservative for “remember” than “know” responses, and patients with AD were more liberal than both healthy older adults and patients with mild cognitive impairment in their response bias for “know” responses.
One potential objection to the argument above is that patients with medial temporal amnesia due to other causes—despite their dependence upon familiarity—typically do not show an abnormally liberal response bias (Snodgrass & Corwin, 1988). But, unlike patients with AD, amnesic patients often have an awareness of their memory impairment and potentially are cautious of attributing feelings of familiarity to prior experience (Verfaellie, Giovanello, & Keane, 2001). Verfaellie and colleagues showed that amnesic patients improved their discrimination when encouraged to use a more liberal criterion, likely due to an increased reliance on familiarity. When this same paradigm was used with patients with AD, both healthy older controls and patients were able to shift their response bias accordingly to the instructions (Waring, Chong, Wolk, & Budson, 2008). Unlike the amnesic patients, the patients with AD did not show improved discrimination when encouraged to use a more liberal criterion, perhaps because they are already relying primarily on familiarity. The liberal response bias is related not to the familiarity of each item per se, but to the individual’s reliance on familiarity and how that individual adjusts his or her criterion as a result of expectations. It may be that patients with AD and amnesic patients have different expectations or strategies for responding when only familiarity is available.
There are several other possible explanations for the liberal response bias observed in patients with AD but not in amnesic patients. Patients with AD have disrupted semantic networks that lead to atypical associations between items (Chan, Butters, & Salmon, 1997) that may, in turn, create a false sense of familiarity to items that are either unrelated or weakly related to the studied items.
Another possibility, one that was an original prediction of the Budson et al. (2006) study, is that the liberal response bias in patients with AD might be due to their frontal dysfunction. Damage to the frontal lobes has been associated with high levels of false recognition (Parkin et al., 1996; Schacter, Curran, Galluccio, Milberg, & Bates, 1996) although this is not always the case (Verfaellie, Rapcsak, Keane, & Alexander, 2004). In a prior experiment, we also found that frontal lesion patients were unable to decrease false recognition across trials in a repeated trials DRM paradigm (Budson et al., 2002). Kramer and colleagues (2005) correlated brain volume with AD patients’ performance on a variety of aspects of the California verbal learning test-short form (CVLT-SF; Delis et al., 2000). They found that whereas hippocampal volumes were the best predictor of delayed recall and recognition, frontal volumes were the best predictor of semantic clustering and response bias. In Budson et al. (2006), however, no significant correlations were found between response bias and measures of frontal lobe functioning. In the current experiment, we performed correlations between response bias at both short and long delays versus our frontal lobe measures (word fluency to letters and the Trailmaking Test Part B) in patients with AD. After correction for multiple comparisons, none of the correlations reached significance. This finding replicates the lack of correlation found by Budson et al. (2006) and further suggests that impaired frontal functioning is not entirely responsible for the liberal response bias in patients with AD.
Parietal dysfunction may also contribute to the liberal response bias in patients with AD. Ally et al. (2008) and others have suggested that the parietal cortex is not essential to accurate recognition but may be involved in the subjective episodic experience (Davidson et al., 2008; Drowos, Berryhill, Andre, & Olson, 2010; Simons et al., 2008; Simons and Mayes, 2008; Simons, Peers, Mazuz, Berryhill, & Olson, 2010). Neuroimaging evidence suggests the importance of the parietal cortex to response bias (Miller, Handy, Cutler, Inati, & Wolford, 2001; O’Connor, Han, & Dobbins, 2010). Miller and colleagues (2001) found that changes in response bias were reflected in changes in activations in lateral cerebellum, lateral parietal cortex, and dorsolateral prefrontal cortex. O’Connor et al. (2010) found that regions of the inferior parietal cortex were positively correlated with an individual’s response bias (and not correlated with discrimination). In fact, the posterior association cortex may be one of the earliest areas of the cortex to be affected by AD pathology (McKee et al., 2006). Dysfunction in parietal cortex may therefore lead, in part, to the liberal response bias in patients with AD and should be further investigated.
Damage to only one area related to memory judgments does not seem to result in the same abnormal response bias as is seen in patients with AD. Patients with frontal lesions, parietal lesions, or medial temporal amnesia do not seem to consistently show an abnormally liberal response bias when compared to control populations (e.g., Davidson et al. 2008; Drowos et al., 2010; Snodgrass & Corwin, 1988; Verfaellie et al., 2001; Verfaellie et al., 2004). Thus, the liberal response bias in patients with AD might reflect a combination of factors.
Future studies could further examine reliance on familiarity and recollection and the effect on response bias. For example, using a remember/know paradigm in combination with different delays we would expect that patients with AD would report more “know” judgments at both delays while the healthy younger and older adults might only show this pattern at the longer delay. Examining neural correlates would provide insight into the specific brain regions (and by inference, processes) that are active across the delay and further evaluate the claim that after a one-day delay the healthy younger and older adults are using more similar memorial processes to the patients with AD. These studies would also help investigate differences between healthy younger versus older adults. Interestingly, we found no differences in response bias between these groups despite older adults having previously shown differences in response bias (Macruie & Baracat, 2000; Huh et al., 2006), recollection and familiarity (Yonelinas, 2002), and deterioration of brain areas related to response bias (Raz & Rodrigue, 2006).
In summary, the current study suggests that how discrimination is matched between patients with AD and controls matters greatly. If matching is achieved by manipulating study-test list length, subjects’ usual bias is maintained, such that patients with AD are more liberal than healthy controls. If discrimination is matched by the use of a delay, response bias is also matched with all participants showing a more liberal response bias. Potentially, this liberal bias is a result of healthy younger and older adults relying primarily on familiarity at the longer delay, thus using more similar memorial processes to patients with AD who are dependent on familiarity at any delay. In addition to helping us to understand the cognitive neuropsychology of patients with AD, we have also proposed a potential relationship between response bias and the memorial processes of recollection and familiarity in healthy memory, and speculated possible cortical regions important for this relationship. Future studies will be able to prove or disprove these hypotheses. In better understanding the underlying cause of the liberal response bias in patients with AD, we have helped to elucidate the underlying cause of their false memories, which in turn may lead to the development of methods to reduce these false memories, allowing patients with mild AD to require less supervision and live more independent lives.
Acknowledgments
This research was supported by National Institute on Aging grants R01 AG025815 (AEB), K23 AG031925 (BAA), P30 AG13846 (AEB), and a Department of Veterans Affairs, Veterans Health Administration, VISN 1 Early Career Development Award to RGD. This material is also the result of work supported with resources and the use of facilities at the Bedford VA Hospital in Bedford, MA and the VA Boston Healthcare System, Boston, MA.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Adjutant General’s Office. Army Individual Test Battery: Manual of Directions and Scoring. Washington, D.C: War Department; 1944. [Google Scholar]
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. Brain Cogn. 2009;69:504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, McKeever JD, Waring JD, Budson AE. Preserved frontal memorial processes for pictures in patients with mild cognitive impairment. Neuropsychologia. 2009;47:2044–2055. doi: 10.1016/j.neuropsychologia.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Burgess GC, Cortese MJ, Adams DR. The word-frequency mirror effect in young, old, and early-stage Alzheimer’s disease: Evidence for two processes in episodic recognition performance. Journal of Memory and Language. 2002;46:199–226. [Google Scholar]
- Bartok JA, Wilson CS, Giordani B, Keys BA, Persad CC, Foster NL, Berent S. Varying patterns of verbal recall, recognition, and response bias with progression of Alzheimer’s disease. Aging Neuropsychology & Cognition. 1997;4:266–272. doi: 10.1080/13825589708256651. [DOI] [PubMed] [Google Scholar]
- Beth EH, Waring JD, Budson AE, Ally BA. Response bias for picture recognition in patients with Alzheimer’s disease. Cogn Behav Neurol. 2009;22:229–235. doi: 10.1097/WNN.0b013e3181b7f3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, Schacter DL. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer’s disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sullivan AL, Mayer E, Daffner KR, Black PM, Schacter DL. Suppression of false recognition in Alzheimer’s disease and in patients with frontal lobe lesions. Brain. 2002;125:2750–2765. doi: 10.1093/brain/awf277. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer’s disease: Separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Chan AS, Butters N, Salmon DP. The deterioration of semantic networks in patients with Alzheimer’s disease: A cross-sectional study. Neuropsychologia. 1997;35:241–248. doi: 10.1016/s0028-3932(96)00067-x. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G. Recognition memory and recollective experience in Alzheimer’s disease. Memory. 1997;5:657– 672. doi: 10.1080/741941546. [DOI] [PubMed] [Google Scholar]
- Dalla Barba G, Nedjam Z, Dubois B. Confabulation, Executive Functions and Source Memory in Alzheimer’s Disease. Cognitive Neuropsychology. 1999;16:385–398. [Google Scholar]
- Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, et al. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test: Manual. 2. San Antonio: Psychological Corporation; 2000. [Google Scholar]
- Drowos DB, Berryhill M, Andre JM, Olson IR. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24:465–475. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, Budson AE. Associative recognition in Alzheimer’s disease: evidence for impaired recall-to-reject. Neuropsychology. 2004;18:556–563. doi: 10.1037/0894-4105.18.3.556. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI. Forgetting in recognition memory with and without recollective experience. Memory and Cognition. 1991;19:617–623. doi: 10.3758/bf03197157. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M. The relationship between recall and recognition in amnesia: Effects of matching recognition between patients with amnesia and controls. Neuropsychology. 2001;15:444–451. doi: 10.1037//0894-4105.15.4.444. [DOI] [PubMed] [Google Scholar]
- Hockley WE, Consoli A. Familiarity and recollection in item and associative recognition. Mem Cognit. 1999;27:657–664. doi: 10.3758/bf03211559. [DOI] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the Remember/Know procedure in amnestic mild cognitive impairment and probable Alzheimer’s disease. Brain Cogn. 2009;70:171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Huh TJ, Kramer JH, Gazzaley A, Delis DC. Response bias and aging on a recognition memory task. J Int Neuropsychol Soc. 2006;12:1–7. doi: 10.1017/S1355617706060024. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. J Exp Psychol Gen. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Knight RG. Controlled and automatic memory processes in Alzheimer’s disease. Cortex. 1998;34:427– 435. doi: 10.1016/s0010-9452(08)70765-2. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Portin R, Seinela A, Rinne J. Automatic in uences of memory in Alzheimer’s disease. Cortex. 1998;34:209–219. doi: 10.1016/s0010-9452(08)70748-2. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, et al. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19:799–805. doi: 10.1037/0894-4105.19.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. Journal of Gerontology. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health. 1984. [DOI] [PubMed] [Google Scholar]
- McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- Miller MB, Handy TC, Cutler J, Inati S, Wolford GL. Brain activations associated with shifts in response criterion on a recognition test. Can J Exp Psychol. 2001;55:162–173. doi: 10.1037/h0087363. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Bindschaedler C, Harsent L, Metzler C. Pathological false alarm rates following damage to the left frontal cortex. Brain Cogn. 1996;32:14–27. doi: 10.1006/brcg.1996.0055. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Piolino P, Mezenge F, Landeau B, Lalevee C, Pelerin A, Viader F, de lS V, Eustache F, Desgranges B. Autonoetic consciousness in Alzheimer’s disease: neuropsychological and PET findings using an episodic learning and recognition task. Neurobiol Aging. 2007;28:1410–1420. doi: 10.1016/j.neurobiolaging.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;6:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Brainerd CJ. Fuzzy-trace theory: An interim synthesis. Learning and Individual Differences. 1995;7:1–75. [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: a case study. Neuropsychologia. 1996;34:793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Simons JS, Mayes AR. What is the parietal lobe contribution to human memory? Neuropsychologia. 2008;46:1739–1742. doi: 10.1016/j.neuropsychologia.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex. 2010;20:479–85. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Gagnon N, Richards E. Strategies of text retrieval: a criterion shift account. Can J Exp Psychol. 2002;56:41–57. doi: 10.1037/h0087384. [DOI] [PubMed] [Google Scholar]
- Singer M, Wixted JT. Effect of delay on recognition decisions: evidence for a criterion shift. Mem Cognit. 2006;34:125–137. doi: 10.3758/bf03193392. [DOI] [PubMed] [Google Scholar]
- Smith JA, Knight RG. Memory processing in Alzheimer’s disease. Neuropsychologia. 2002;40:666–682. doi: 10.1016/s0028-3932(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Tallberg IM, Almkvist O. Confabulation and memory in patients with Alzheimer’s disease. J Clin Exp Neuropsychol. 2001;23:172–84. doi: 10.1076/jcen.23.2.172.1215. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Verfaellie M, Giovanello KS, Keane MM. Recognition memory in amnesia: effects of relaxing response criteria. Cogn Affect Behav Neurosci. 2001;1:3–9. doi: 10.3758/cabn.1.1.3. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Rapcsak SZ, Keane MM, Alexander MP. Elevated false recognition in patients with frontal lobe damage is neither a general nor a unitary phenomenon. Neuropsychology. 2004;18:94–103. doi: 10.1037/0894-4105.18.1.94. [DOI] [PubMed] [Google Scholar]
- Waring JD, Chong H, Wolk DA, Budson AE. Preserved metamemorial ability in patients with Alzheimer’s disease: Shifting response bias. Brain & Cognition. 2008;66:32–39. doi: 10.1016/j.bandc.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer’s diseases. Neuropsychology. 2006;20:193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with mild Alzheimer’s disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC Alzheimer’s Disease Neuroimaging Initiative. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54:1530–9. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, Dekosky ST. A medial temporal lobe division of labor: Insights from memory in aging and early Alzheimer disease. Hippocampus. 2011;21:461–6. doi: 10.1002/hipo.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]